Abstract
Purpose
We prospectively examined the development of depressive symptoms and fatigue among men with locally advanced prostate cancer receiving hormone therapy.
Methods
Fifty-two men with advanced or recurrent prostate cancer were randomly assigned to receive either parenteral leuprolide or oral bicalutamide. Patients completed the Beck Depression Inventory (BDI) and Fatigue Severity Scale (FSS) at pretreatment baseline, 6 months, and 12 months.
Results
Rates of at least mild depression ranged from 10.4 to 16.3% over the 12 months and were not significantly different at each time point. Mean change in BDI scores from baseline to 6 months for the entire sample was 0.91 (SE = 0.73), and from baseline to 12 months was 0.35 (SE = 0.67). Mean FSS scores increased significantly from baseline (M = 24.43; SD = 11.75) to 6 months (M = 27.93; SD = 13.52) and remained steady at 12 months (M = 27.80; SD = 14.44). There were no significant differences in depression between the two types of hormone therapy.
Conclusion
Hormone therapy does not appear to cause clinically significant changes in depression among men with locally advanced prostate cancer. However, fatigue increased significantly over the study period.
Keywords: depression, fatigue, prostate cancer treatment
Introduction
Hormone therapy is used in the treatment of advanced-stage prostate cancer and, based on evidence for improved outcomes, hormone therapy is now also being used in the treatment of patients without distant metastases including men with locally advanced, node positive, or biochemically recurrent disease [1,2]. Because these patients typically have a life expectancy of 10 years or more on these treatments, the impact on quality of life has become an important treatment consideration [3–6].
Fatigue is a common side effect of hormone therapy [7,8]. Other side effects include hot flashes, loss of sexual interest, anemia, decreased muscle mass, and osteoporosis [9]. Nonsteroidal antiandrogen compounds such as bicalutamide may have a better side effect profile in monotherapy with decreased incidence of osteoporosis, anemia, fatigue, and vasomotor flushing compared to leuprolide, a GnRH agonist [10].
Little is known about the neuropsychiatric effects of hormone therapy even though research on hypogonadism in men not being treated for prostate cancer suggests that low testosterone states may be associated with depression [11–13]. Case reports of men developing depression on hormone therapy for prostate cancer have been published, and a recent study on the effect of short-term leuprolide administration on mood in healthy men demonstrated clinically significant increases in depressive symptoms in 9.7% of participants [14–16]. In a previous study, our group found major depressive disorder present in 12.8% of men receiving hormone therapy at an ambulatory prostate oncology clinic [17]. While this rate falls within estimates of major depression in other studies of men with prostate cancer, those studies did not stratify by cancer treatment and likely overestimate the rate by using scales of depressive symptoms [18,19]. Because hormone therapy has been shown to cause worse emotional functioning than other approaches such as watchful waiting, it remains unclear if it might cause major depressive disorder as is seen in men without prostate cancer who have testosterone deficiency [11–13,20].
Fatigue and major depression share many over-lapping features and can be difficult to tease apart in people with cancer [21]. Cancer-related fatigue appears to be more prevalent than depression, with estimates of 60–90% of patients experiencing fatigue [22]. Because fatigue is an already established side effect of hormone therapy, depression and fatigue must be studied simultaneously to limit confounding as well as illustrate their relationship. Depression can cause fatigue and fatigue can lead to depression. However, it is important to try to distinguish them because depression can be a serious complication during cancer treatment and is associated with poor adherence, increased hospital stays, increased morbidity, greater desire for death, and, perhaps, mortality [23–26].
To understand better the effects of hormone therapy in men being treated for prostate cancer, this prospective study longitudinally assessed men for depression and fatigue over 12 months after starting hormone therapy. This study was a substudy of a clinical trial comparing bicalutamide monotherapy versus leuprolide monotherapy on bone mineral density and body composition [10]. The framework of the primary study allowed us to also compare the effects of two types of hormone therapies on mood. Given our prior cross-sectional data, we hypothesized that hormone therapy would lead to increased depressive symptoms in men with advanced prostate cancer in addition to increases in fatigue. Because bicalutamide does not cross the blood–brain barrier and does not decrease serum testosterone, we had a secondary hypothesis that the treatment group receiving leuprolide would develop greater depressive symptoms and fatigue.
Methods
Fifty-two men with locally advanced, lymph-node positive or recurrent prostate cancer and no bone metastases on radionucleotide bone scan were followed for 12 months after starting hormone therapy. Men with Karnofsky performance status less than 90, history of hypogonadism, history of growth hormone or anabolic steroid use, Paget’s disease, hypothyroidism, hyperthyroidism, Cushing’s disease, hyperprolactinemia, chronic liver disease, corrected serum calcium <8.4 or >10.6 mg/dL, or serum creatinine concentration >2.0 mg/dL (177 μmol/L) were excluded. Men with prior neoadjuvant or adjuvant hormone therapy were included if the interval between completion of treatment and the study entry was greater than 1 year. Men were excluded if they had received bisphosphonate, calcitonin, or glucocorticoid therapy, or suppressive doses of thyroxine within 1 year.
Participants were randomly assigned to receive either leuprolide depot 22.5 mg intramuscularly every 3 months or bicalutamide 150 mg by mouth daily for 12 months. Men assigned to leuprolide treatment also received bicalutamide (50 mg by mouth daily) for 1 month to prevent the potential disease flare associated with initial leuprolide administration.
The main outcomes for this study were changes in depressive symptoms and fatigue from baseline to 12 months. Only at baseline, each participant was given the depression module of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID) to assess for an existing pre-treatment major depressive episode [27]. Men who were found to have major depressive disorder were referred for psychiatric evaluation and were not included in the longitudinal analyses.
Depressive symptoms were measured by the Beck Depression Inventory (BDI), a 21-item self-report scale that has been widely used in cancer patients. The BDI was chosen because its greater range of scores would increase the sensitivity to detect differences from baseline to 12 months as well as differences between the two hormone therapies. The BDI has established cut-off scores for mild (10–18), moderate (19–29), and severe (30–63) depression [28]. A cut-off score of 11 or above was selected to identify men with probable cases of major depressive disorder based on previous research on its ability to predict major depressive disorder in people with cancer [29].
Men with BDI scores of 11 or greater were considered probable cases of major depressive disorder and were referred for psychiatric evaluation. Because of the small sample size, it was decided to include men who were referred for psychiatric evaluation at 6 months in the 12 month analyses unless they were diagnosed with major depressive disorder and received treatment with either anti-depressant medications or psychotherapy.
Fatigue was assessed with the Fatigue Severity Scale (FSS). The FSS is a 9-item self-report instrument that assesses the severity of fatigue and its interference with functioning [30]. It has been used in prior studies of fatigue in men with prostate cancer receiving hormone therapy [8].
The BDI and FSS were completed at baseline, 6 months, and 12 months. Changes in BDI and FSS scores were examined as continuous variables using repeated measures ANOVA. BDI scores were also analyzed as a categorical variable (probable case of major depressive disorder) with chi-square analyses to examine change in rates of depression over the three study time points. Independent samples t-tests were also calculated comparing BDI and FSS scores between the two hormone therapy treatment conditions at each of the three time points. Because the primary study was powered for different endpoints and potential changes in BDI and FSS scores might not reach statistical significance in this sample, effect sizes (Cohen’s d) were also calculated. Finally, to address the possible confounding of changes in depressive symptoms by fatigue, differences in BDI scores were analyzed using repeated measures ANCOVA controlling for FSS scores.
This study was reviewed and approved by the Dana-Farber/Harvard Cancer Center internal review board. Personnel involved in acquisition, analysis, or review of the primary data were blinded to treatment assignments.
Results
Sample
Fifty participants completed the longitudinal assessments of mood. The patients ranged from 46 to 84 years of age (M = 62.0, SD = 9.0). Ninety percent of the participants were white, 6% were African-American, 2% were Latino/Hispanic, and 2% were Asian. Twenty-two percent of participants had prior treatment with hormone therapy.
Depression
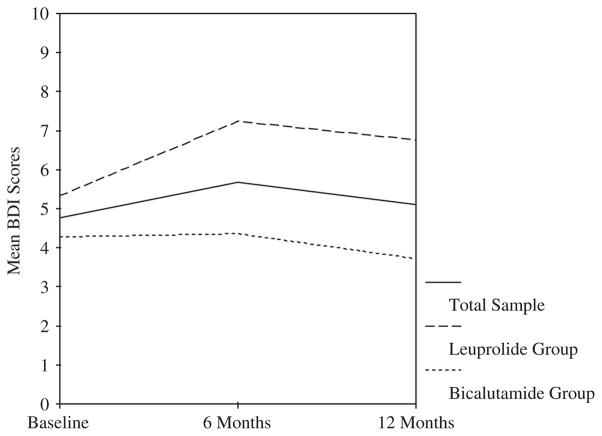
Two men (4%), both in the leuprolide group, met SCID criteria for major depressive disorder at baseline and their scores were excluded from further analyses. As shown in Figure 1, BDI scores did not differ significantly across the three time points. Specifically, the mean change in BDI scores from baseline (M = 4.76, SD = 3.86) to 6 months for the entire sample was 0.91 (SE = 0.73), and from baseline to 12 months was 0.35 (SE = 0.67). Similarly, within each hormone therapy treatment group, the BDI scores did not change significantly from baseline to 6 or 12 months. Comparing the two types of hormone therapy using independent samples t-tests, there was no significant difference in mean BDI scores at baseline, but the leuprolide group had higher mean BDI scores than the bicalutamide group at both 6 months (M = 7.36 versus 4.36, t(45) = 2.16, SE = 1.39, p = 0.04) and 12 months (M = 6.76 versus 3.72, t(44) = 2.40, SE = 1.27, p = 0.02).
Figure 1.
Beck Depression Inventory (BDI) scores across time for total sample and by treatment group. Effect size estimates (Cohen’s d) for 6- and 12-month change in BDI scores compared to pretreatment baseline: total sample =0.21 at 6 months and 0.08 at 12 months; leuprolide group = 0.42 at 6 months and 0.32 at 12 months; bicalutamide group = 0.02 at 6 months and −0.15 at 12 months
Cross-sectionally, depression scores varied within each time point. A notable number of men reported depressive symptoms at least in the mild range, defined by BDI score of 11 or greater. Five men (10.4%) had at least mild depressive symptoms at baseline, eight (16.7%) at 6 months, and five (10.4%) at 12 months. Differences among these rates were not significant at the three time points overall or when sorted by treatment group (see Table 1). Of the men who had BDI scores of less than 11 at baseline, 16.3% (7/43) developed at least mild depression during the 12-month follow-up period. Comparing the two types of hormone therapies, more men receiving leuprolide developed BDI scores meeting criteria for mild depression (25.0%, 5/20) at either 6 or 12 months after starting treatment compared to the men receiving bicalutamide (8.7%, 2/23), though this difference was not statistically significant (χ2(1) = 2.09, p = 0.15).
Table 1.
Rates of patients meeting criteria for at least mild depression
| Baseline % (N) | 6 months total cases % (N) | 6 months new casesa % (N) | 12 months total cases % (N) | 12 months cumulative new casesb % (N) | |
|---|---|---|---|---|---|
| BDI≥ 11, combined | 10.4% (5/48) | 16.7% (8/48) | 14.0% (6/43) | 10.4% (5/48) | 16.3% (7/43) |
| BDI≥ 11, leuprolide | 13.0% (3/23) | 26.0% (6/23) | 20.0% (4/20) | 19.0% (4/21) | 25.0% (5/20) |
| BDI≥ 11, bicalutamide | 8.0% (2/25) | 8.0% (2/25) | 8.7% (2/23) | 4.0% (1/25) | 8.7% (2/23) |
Includes new cases at 6 months among participants not depressed at baseline.
Includes all new cases by 12 months among participants not depressed at baseline.
Only two of the men with elevated BDI scores during the study completed psychiatric evaluations and both met Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV) criteria for major depressive disorder [31]. Both were referred at the 12-month assessment. One of these two men had a past history of depression. Based on these data, which are limited by the low rate of psychiatric evaluations of the men with BDI scores greater or equal to 11, the lowest possible incidence rate for major depressive disorder in this population is 4.7% per year.
Fatigue
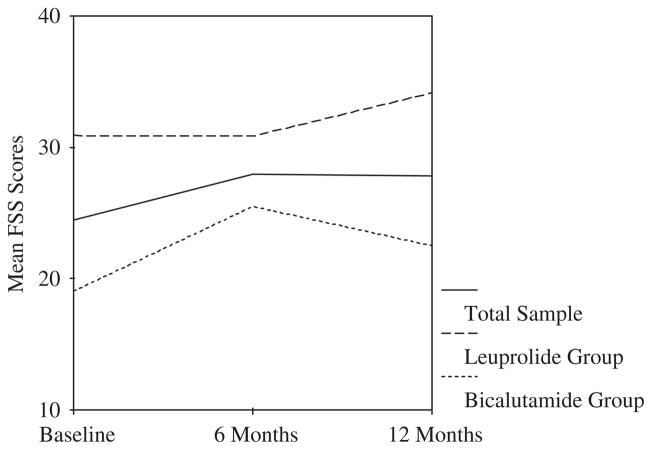
Overall, fatigue scores (i.e. measured with the FSS) increased significantly from baseline (M = 24.43, SD = 11.75) to six months (mean difference = 3.50, SE = 1.68, p = 0.04) and remained steady at 12 months (mean difference = 3.36, SE = 1.59, p = 0.04) (see Figure 2). In the bicalutamide condition, fatigue scores increased significantly from baseline (M = 19.04, SD = 9.08) to 6 months (mean difference = 6.46, SE = 2.14, p = 0.01) and then decreased somewhat at 12 months, though still higher than baseline (mean difference = 3.46, SE = 1.39, p = 0.02). There were no statistically significant changes in fatigue for the leuprolide condition across the three time points. Comparing between the two types of hormone therapy, the leuprolide group reported greater fatigue than the bicalutamide group at baseline (M = 30.86 versus 19.44, t(44) = 3.82, SE = 2.99, p<0.001) and 12 months (M = 34.86 versus 22.50, t(43) = 3.09, SE = 4.00, p = 0.003) but not at 6 months.
Figure 2.
Fatigue Severity Scale (FSS) scores across time for total sample and by treatment group. Effect size estimates (Cohen’s d) for 6- and 12-month change in FSS scores compared to pretreatment baseline: total sample = 0.28 at 6 months and 0.26 at 12 months; leuprolide group = −0.004 at 6 months and 0.25 at 12 months; bicalutamide group = 0.57 at 6 months and 0.31 at 12 months
Depression and fatigue
Fatigue and depression in the overall sample were significantly correlated at each time point: baseline (r = 0.34, p = 0.02), 6 months (r = 0.69, p<0.001), and 12 months, (r = 0.58, p<0.001).
To address potential confounding of depression by fatigue, repeated-measures ANCOVA were conducted, which showed that BDI scores did not vary significantly across the three study time points when controlling for baseline FSS scores alone or when controlling for change in FSS scores from baseline to 6 or 12 months. In addition, the observed differences in BDI scores between the two types of hormone therapy at 6 and 12 months were no longer significant after controlling for patients’ FSS scores using linear regression analyses, (p = 0.23 and p = 0.49, respectively).
Discussion
Although case reports of men receiving hormone therapy for prostate cancer and data from studies of hypogonadal men who are not being treated for prostate cancer would suggest that hormone therapy for prostate cancer would be associated with the development of depressive symptoms, this was not supported by this prospective study. Depressive symptoms measured by changes in BDI scores did not significantly increase at 6 and 12 months after starting hormone therapy. Rates of clinically significant depressive symptoms also did not differ significantly at each of the three time points and, cross-sectionally, appear consistent with previous studies of depressive symptoms in men with prostate cancer [17–19]. There were no significant differences in depression between the two types of hormone therapy when controlling for patient fatigue levels.
Fatigue, in contrast, was found to significantly increase over the course of treatment with hormone therapy. One possible reason for this may be the observed development of anemia, which is described elsewhere. Men in the leuprolide group, which experienced more fatigue, also had a significantly greater decrease in mean hemoglobin concentration compared to the bicalutamide group [10].
Noting the different courses of depression and fatigue in men with prostate cancer is clinically important because fatigue and depression can be difficult to distinguish from each other [21]. People with cancer-related fatigue can often be misdiagnosed as having depression because of the presence of low energy, sleep disturbances, poor concentration, and discouragement over functional limitations. In this study that prospectively assessed both depression and fatigue, it appears that fatigue increased significantly after starting hormone therapy for prostate cancer, while depression did not. Additionally, when fatigue was controlled in analyzing the difference in depressive symptoms between the two types of hormone therapy treatment, the small difference was no longer observed.
Although the power of this small sample may be questioned because the primary study was powered to detect a difference in bone density between treatment groups, the small effect sizes that were observed for depression cast doubt on the clinical significance of an increase in BDI scores if it exists. A change of one or two points on the BDI is not clinically significant and could be explained by an increase in fatigue, which is included as an item in the BDI.
These findings, however, do not negate the presence of major depressive disorder in this population. Although few men followed through on psychiatric referral for diagnosis, the 12-month incidence of major depressive disorder in all of the participants was at least (2/43) 4.7% and could have been as high as (7/43) 16.3% if all men who met BDI criteria for mild depression were evaluated and met DSM IV criteria for a diagnosis of major depressive disorder. This rate of major depressive disorder falls within the range of ambulatory patients with other types of cancer [32].
While this study is the first prospective study of both depressive symptoms and fatigue in men with prostate cancer receiving hormone therapy, our conclusions are limited by the small sample size, the lack of a control group, and the low rate of psychiatric evaluation of the men with elevated BDI scores. Larger, controlled studies that are adequately powered for depression and fatigue as the primary outcomes are required.
Acknowledgments
This study was funded by a grant from the Dana Foundation.
Footnotes
None of the authors have conflicts of interests or disclosures.
References
- 1.Ryan CJ, Small EJ. Early versus delayed androgen deprivation for prostate cancer: new fuel for an old debate. J Clin Oncol. 2005;23:8225–8231. doi: 10.1200/JCO.2005.03.5311. [DOI] [PubMed] [Google Scholar]
- 2.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 3.Berthelet E, Pickles T, Lee KW, et al. Long-term androgen deprivation therapy improves survival in prostate cancer patients presenting with prostate-specific antigen levels >20 ng/mL. Int J Radiat Oncol Biol Phys. 2005;63:781–787. doi: 10.1016/j.ijrobp.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Moyad MA. Promoting general health during androgen deprivation therapy (ADT): a rapid 10-step review for your patients. Urol Oncol. 2005;23:56–64. doi: 10.1016/j.urolonc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54:85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 6.Konski A, Watkins-Bruner D, Bereton H, et al. Long-term hormone therapy and radiation is cost-effective for patients with locally advanced prostate carcinoma. Cancer. 2006;106:51–57. doi: 10.1002/cncr.21575. [DOI] [PubMed] [Google Scholar]
- 7.Herr HW, O’Sullivan M. Quality of life in asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163:1743–1746. [PubMed] [Google Scholar]
- 8.Stone P, Hardy J, Huddart R, et al. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36:1134–1141. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen AC, Petrylak DP. Complications of androgen-deprivation therapy in men with prostate cancer. Curr Urol Rep. 2005;6:210–216. doi: 10.1007/s11934-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, Goode M, Zeitman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and bosy composition. J Clin Oncol. 2004;22:2546–2553. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 12.Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord. 1998;48:157–161. doi: 10.1016/s0165-0327(97)00168-7. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt DE, Mellow A. Depression during hormonal treatment of prostate cancer. J Am Board Fam Practice. 1995;8:317–320. [PubMed] [Google Scholar]
- 15.Freeman MP, Freeman SA. Treatment of leuprolide induced depression with intramuscular testosterone: a case report. J Clin Psychiatry. 2003;64:341–343. doi: 10.4088/jcp.v64n0317h. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt PJ, Berlin KL, Danaceau MA, et al. The effects of pharmacologically induced hypogonadism on mood in healthy men. Arch Gen Psychiatry. 2004;61:997–1004. doi: 10.1001/archpsyc.61.10.997. [DOI] [PubMed] [Google Scholar]
- 17.Pirl WF, Siegel G, Goode M, et al. Depression in men with prostate cancer receiving androgen deprivation therapy: a pilot study. Psycho-Oncology. 2002;11:518–523. doi: 10.1002/pon.592. [DOI] [PubMed] [Google Scholar]
- 18.Cliff AM, MacDononagh RP. Psychosocial morbidity in prostate cancer II: a comparison of patients and partners. BJU Int. 2000;86:834–839. doi: 10.1046/j.1464-410x.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- 19.Roth AJ, Kornblith AB, Batel-Copel L, et al. Rapid screening for psychological distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.van Andel, Kurth KH. The impact of androgen deprivation therapy on health related quality of life in asymptomatic men with lymph node positive prostate cancer. Eur Urol. 2003;44:209–214. doi: 10.1016/s0302-2838(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson PB, Donovan KA, Weitzner MA. Distinguishing fatigue and depression in patients with cancer. Semin Clin Neuropsychiatry. 2003;8:229–240. [PubMed] [Google Scholar]
- 22.Curt CA, Breitbart W, Cella D, et al. Impact of cancerrelated fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 23.Richardson J, Sheldon D, Krailo M, et al. The effect of compliance with treatment on survival among patients with hematologic malignancies. J Clin Oncol. 1990;8:356–364. doi: 10.1200/JCO.1990.8.2.356. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel D, Kato PM. Psychosocial influences on cancer incidence and progression. Harv Rev Psychiatry. 1996;4:10–26. doi: 10.3109/10673229609030518. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann C, Brand-Driehorst S, Kaminsky B, et al. Diagnostic groups and depressed mood as predictors of 22-month mortality in medical inpatients. Psychosom Med. 1998;60:570–577. doi: 10.1097/00006842-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284:2907–2911. doi: 10.1001/jama.284.22.2907. [DOI] [PubMed] [Google Scholar]
- 27.First M, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York State Psychiatric Institute, Biometrics Research Department; New York: 1999. [Google Scholar]
- 28.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 29.Kathol RG, Mutgi A, Williams J, et al. Diagnosis of major depression in cancer patients according to four sets of criteria. Am J Psychiatry. 1990;147:1021–1024. doi: 10.1176/ajp.147.8.1021. [DOI] [PubMed] [Google Scholar]
- 30.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 32.Pirl WF. Evidence-based report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monog. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]