Abstract
Thermal ablation procedures including radiofrequency ablation and cryoablation have been increasingly utilized for treatment for small renal cell carcinoma. Currently, CT and MR imaging are usually used to assess residual or recurrent disease after thermal ablation of renal tumor. After thermal ablation, the zone of ablation is usually seen as an area of hypoattenuation on computed tomography (CT) and is generally hypointense at T2-weighted magnetic resonance (MR) imaging and iso- to hyperintense at T1-weighted imaging relative to renal parenchyma. The ablation zone frequently involutes over time. Residual tumor after thermal ablation is most common at the margin of the ablation zone and often seen as nodular or crescent-shaped areas of contrast enhancement. Accurate assessment of ablated tumors at postprocedural imaging is essential for evaluating the adequacy of treatment and guiding further management. Complications are uncommon and usually minor, but should be detected on post-procedural imaging studies.
INTRODUCTION
The incidence of renal cell carcinoma (RCC) has steadily increased during the past decades in the United Sates [1, 2]. Increase in diagnosis of renal cell carcinoma has been attributed to increased tumor incidence and increased detection of incidental lesions with the widespread use of cross-sectional imaging [3, 4]. Nephron-sparing procedures for the management of small renal tumors have become increasingly accepted during the last decade including partial nephrectomy, wedge resection [5], and more recently thermal ablation procedures. Image-guided percutaneous thermal ablation techniques have potential advantages over surgical resection, including a decreased convalescence with reduced morbidity [6–9].
Currently, the most widely used energy modalities for thermal ablative techniques are radiofrequency (RF) ablation and cryoablation [10]. Thermal ablative techniques have been used to destroy renal tumor tissue through heating (RF ablation) or freezing (cryoablation). Thermal ablation procedures can be performed with image-guided percutaneous approaches, intraoperative ultrasonography, or direct visualization during laparoscopic or open surgery. RF ablation and cryoablation techniques rely on controlled energy delivery to minimize collateral damage to normal renal parenchyma and other surrounding structure [10].
Patients who can particularly benefit from thermal ablation procedures are those who are poor operative candidates as a result of inadequate renal function and/or comorbid disease, and who are genetic predisposition to multiple renal tumors, such as patients with von Hippel-Lindau syndrome, who usually undergo multiple partial nephrectomies for recurrent RCCs [10, 11].
Initial results of RF ablation and cryoablation in the treatment of renal tumors suggest satisfactory outcomes by imaging when careful patient selection and rigorous attention to technical details are utilized [12]. Matin et al. reviewed treatment and follow-up information of 616 patients who underwent RF ablation or cryoablation for renal masses in a multi-institutional study at 7 institutions, and reported that after salvage therapy for incomplete treatment after primary RF ablation or cryoablation, thermal ablative therapy failed in only 4.2% [13]. Treatment failure rates for laparoscopic cryoablation ranged from 3% to 7% for small peripheral exophytic renal lesions [14–16].
Unlike with surgical resection, in which the entire pathological specimen can be examined to confirm treatment success, renal tumors treated with thermal ablation have relied on radiographic imaging to determine treatment success. Postoperative pathological tumor staging is not available by thermal ablation procedures [17]. The follow-up imaging studies are often interpreted by abdominal radiologists, and not by interventional radiologists. Findings to indicate residual tumor and local tumor progression, and post procedure complications should be recognized on follow-up imaging studies. The purpose of this article is to review normal spectrum of post ablation CT and MR imaging findings, and findings to indicate residual tumor and local tumor progression, and potential complications after thermal ablation procedures for treatment of renal tumors.
BRIEF TECHNICAL CONSIDERATION
It is generally accepted that the success rate of RF ablation in treating RCC depends on tumor's size and location [7, 8, 11, 18]. Although no established threshold or tumor diameter is associated with RF ablation treatment success or failure, it is generally considered that RCC with a greatest diameter of 4 cm or less (T1a) has a higher probability of complete ablation than does RCC larger than 4 cm [10].
Tumor location can be classified based on the classification of Gervais et al [7]. Renal tumors can be classified as exophytic (lesions protruding into the perirenal fat), parenchymal (confirmed in the renal parenchyma), central (protruding into the renal sinus), or mixed with central and exophytic components (protruding into both perirenal fat and renal sinus) [7].
Some investigators reported that tumors located centrally or hilar regions are more difficult to treat and have an increased risk of incomplete treatment or radiographic recurrence [9, 19, 20]. For thermal ablation, a major renal vessel proximity to renal tumor distributes heat away from the tumor (heat-sink phenomenon) and may result in incomplete local treatment [21]. Exophytic RCC may be more likely to be completely ablated than parenchymal or central RCC as perirenal fat can produce a thermally insulating effect, resulting in more efficient thermal ablation [10].
FOLLOWUP IMAGING PROTOCOLS
Follow-up interval and duration of CT and MR imaging may vary depending on institutions. Definition of the appropriate length of follow-up and the time points for technical success are currently not well established [22]. The natural history of small RCCs (< 3.5 cm) on average is less than 4 mm of growth per year [23]. Therefore, most investigators perform imaging at 3–6 months intervals after an early scan after ablation to document the absence of viable enhancing tumor and lack of tumor growth [10, 24]
The timing of initial scan varies among institutions from as early as immediately after [8, 11, 24] to 1 week [25] after thermal ablation procedure, to as late as 1 months in order to assess treatment adequacy and baseline size of the ablated tumor and ablation zone.
A multi-institutional study by Matin et al reviewed 616 patients who underwent RF ablation or cryoablation for renal masses reported that most incomplete treatments (70%) were detected within the first 3 months [13]. They suggested that a minimum of 3 to 4 imaging studies in the first year after thermal ablation treatment, at 1, 3, possibly 6, and 12 months [13]. Subsequent follow-up is often performed at 6 months interval [11, 25] or yearly thereafter [26].
CT protocol at our institution typically includes noncontrast scan of the ablated tumor, and arterial phase, and portal venous phase imaging of the ablated tumor, and excretory phase CT of the abdomen and pelvis after administration of iodinated contrast media at a rate of 3 to 4 ml/s. Contrast enhanced CT of the chest may also be included as requested by urologists.
Our MR imaging protocol is as follows: Axial unenhanced T1-weighted images are obtained using two-dimensional (2D) spoiled gradient-recalled echo, or three-dimensional (3D) fast spoiled gradient-recalled echo methods, and T2-weighted images are obtained using fast spin-echo and single-shot fast spin-echo methods. Axial gadolinium-enhanced T1-weighted images are obtained at approximately 30 and 70 seconds after the injection of 0.1 mmol/kg of gadolinium chelate injected at a rate of 2 ml/sec with either a 2D or a 3D technique. A breath-hold fat-suppression MR imaging technique should be used. A coronal T1-weighted image is then obtained immediately after the axial images. A phased-array torso or body coil should be used for all acquisitions.
CT AND MR IMAGING FINDINGS AFTER RF ABLATION AND CRYOABLATION PROCEDURES
Currently, tumor enhancement characteristics and tumor size measurements on follow-up CT or MR imaging are used to assess residual or recurrent disease [7, 16, 27, 28]. Farrell et al. defined successful ablation as being when the lesion showed less than 10 HU of contrast medium enhancement on CT or no qualitative evidence of enhancement after intravenous gadolinium contrast-enhanced MR imaging [28].
In most cases, the operator aims to treat a volume slightly larger than the tumor to ensure complete ablation with an adequate safety margin [25]. A circumferential ablative margin of 5–10 mm beyond the margin of the tumor is often used for thermal ablation [10, 11, 17, 29]. Therefore, the zone of ablation seen as an area of non-enhancement on early post ablation CT or MR imaging is generally larger than the original tumor [24] (Fig 1). On MR imaging, the zone of ablation appears generally hypointense at T2-weighted MR imaging and iso- to hyperintense at T1-weighted imaging relative to renal parenchyma [30–32] (Fig 2).
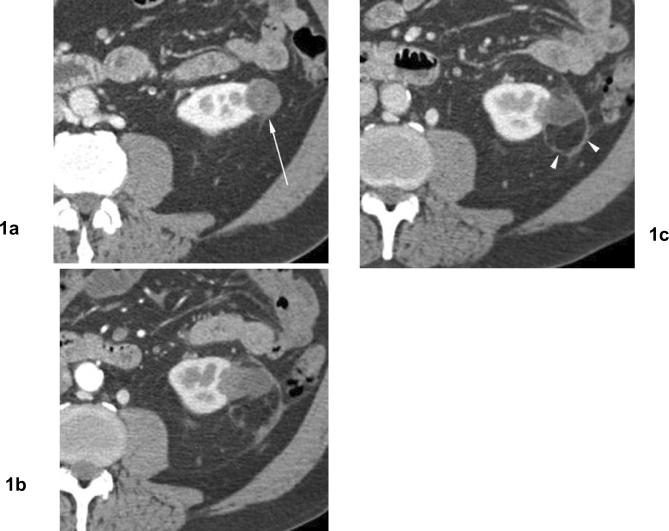
Fig 1. Involution of treated tumor after cryoablation. Papillary type renal cell carcinoma.
(a) Contrast enhanced axial CT before cryoablation shows exophytic hypodense mass in the left kidney (arrow). Biopsy showed papillary type renal cell carcinoma. (b) Venous phase axial CT 4 months after cryoablation shows heterogeneous hypodense area in the ablation zone with a curvilinear hyperattenuation area, or “halo”. (c) Venous phase axial CT 16 months after cryoablation shows decreasing in size of ablation zone without evidence of contrast enhancement. Persistent curvilinear hyperattenuation area in the perinephric fat surrounding the ablated tumor (arrowheads).
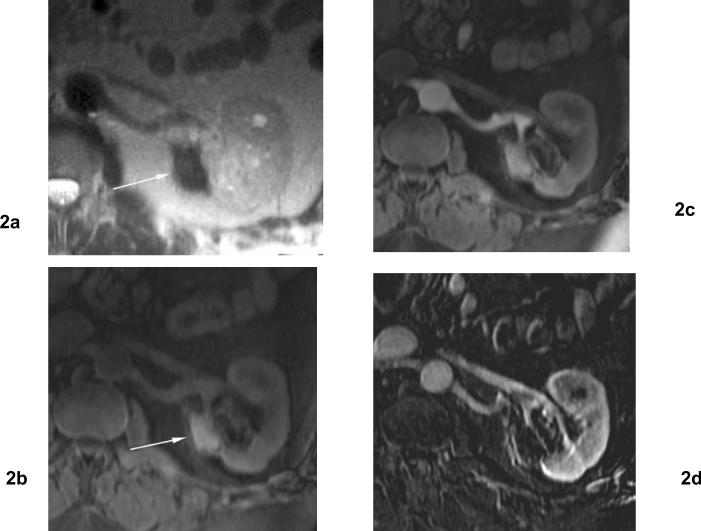
Fig 2. 4 months after cryoablation for clear cell type renal cell carcinoma of the left kidney.
(a) Axial T2 weighted MR image shows a hypointense area with focal contour deformity (arrow) in the ablation zone. (b) Axial fat-suppressed T1 weighted MR image shows an area of hyperintensity in the ablation zone (arrow). (c) Axial contrast enhanced T1 weighted MR images obtained 4 months after cryoablation shows noncontrast enhancement in the ablation zone. (d) Subtraction image. The ablation zone demonstrates no evidence of enhancement.
Ablated renal tumor itself usually decreases in size immediately after RF ablation. Ganguli et al. evaluated the maximal diameter of 72 solid renal tumors on noncontrast CT immediately before and after treatment with RF ablation, and reported mean tumor diameter decreased 5.4 mm immediately after RF ablation (27.5 mm before RF ablation, and 22.1 mm after RF ablation) [24]. This measurement only included ablated tumor, and not including ablative margin which may extend the expected margins of the treated tumor [24].
Shortly after thermal ablation, thin rim enhancement has been occasionally demonstrated peripheral to the zone of ablated renal parenchyma on CT [25] and MR imaging [16, 30] and this finding resolves gradually over time and is barely detectable after 3 months [30]. This is considered benign periablational enhancement, and typically suggests a benign physiologic response to thermal injury (initially reactive hyperemia; subsequently, fibrosis and giant cell reaction) [33]. It is a relatively concentric, symmetric, and uniform process with smooth inner margins, and it needs to be differentiated from residual unablated tumor at the treatment margin which shows irregular peripheral enhancement [22].
Long term imaging of thermal ablation zones has shown involution of zone of ablation over time (Figs 1, 3). Merkele et al. prospectively evaluated 18 patients who underwent galolinium-enhanced MR imaging after RF ablation for solid renal tumors and found that the size of RF ablation zone initially increases approximately 10% within the first 2 weeks after ablation, and involution of these zones was observed by decreasing in size of ablation zone of an average of approximately 30% during the following 6 months [30]. In a study of 56 patients with renal tumors who underwent laparoscopic cryoablation, Gill et al [16] observed a gradual involution in the size of the ablation zone by an average of 75% 3 years after ablation, and 38% of ablation zones were undetectable on MR imaging. These studies assessed changes in ablation zone size often including renal parenchyma that was treated to create an ablation margin, and not only tumor size changes [24]. However, it is important to note that no or minimal involution does not imply treatment failure [22].
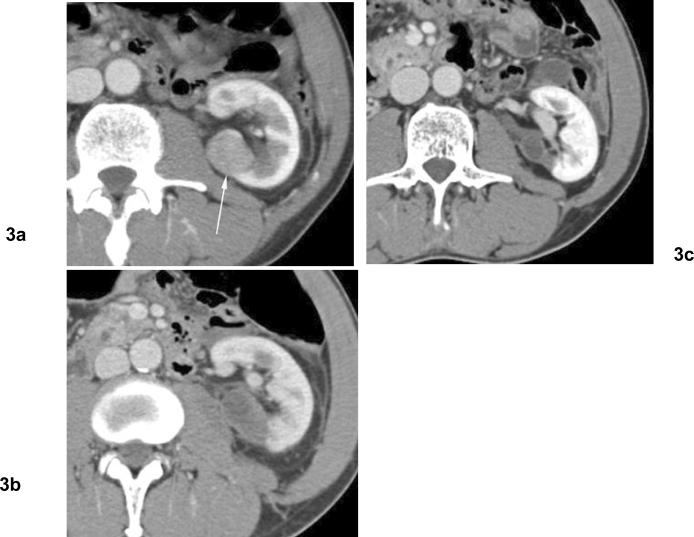
Fig 3. Involution of treated tumor after cryoablation. Papillary type renal cell carcinoma.
(a) Axial contrast enhanced CT before cryoablation shows solid mass in the left kidney posteriorly (arrow). Biopsy showed papillary type renal cell carcinoma. (b) Venous phase contrast enhanced CT 2 months after cryoablation shows heterogeneous hypodensity in ablation zone without contrast enhancement. (c) Venous phase contrast enhanced CT 10 months after cryoablation shows involution of treated tumor without contrast enhancement.
A curvilinear hyperattenuation area or halo, is a relatively common finding, lying roughly parallel to the tumor but extends beyond the borders of the original renal tumor [18] (Figs 1, 4). This halo may be seen to evolve with time, months or even years after treatment [25] (Fig 6). A cortical wedge-shaped infarct may be seen Following RF ablation as a result of segmental arterial thrombosis in the treatment zone [25, 34].
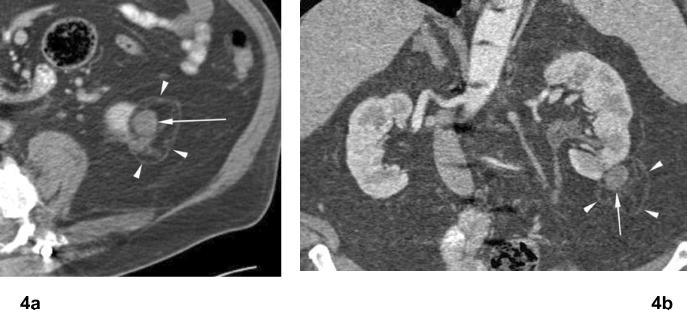
Fig 4. Treated tumor after RF ablation. Papillary type renal cell carcinoma.
(a) Axial and (b) coronal contrast enhanced CT images 4 years after RF ablation shows persistent exophytic mass in lower pole of the left kidney, unchanged compared to early post ablation CT images (not shown). There is no contrast enhancement in ablated tumor (arrow). There is a curvilinear hyperattenuation area, or “halo” in perinephric fat surrounding ablated tumor (arrowheads).
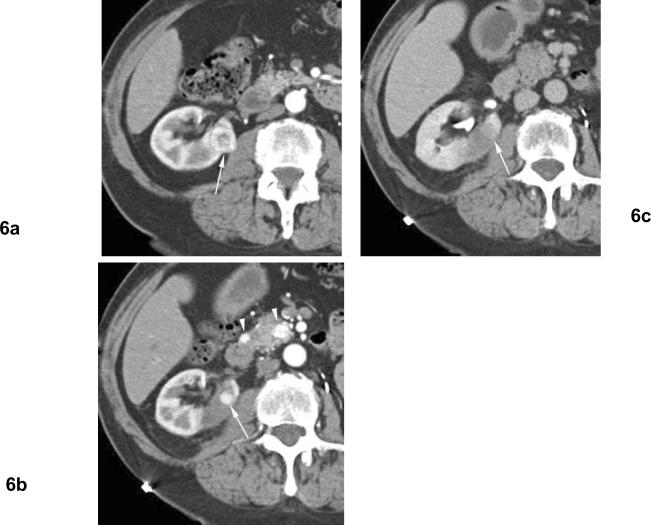
Fig 6. Residual unablated tumor after cryoablation. Clear cell type renal cell carcinoma. The patient is status post left nephrectomy for renal cell carcinoma.
(a) Arterial phase contrast enhanced CT before cryoablation shows heterogeneously enhancing mass (arrow) in the right kidney medially. (b) Arterial phase contrast enhanced CT 1 month after cryoablation shows nodular contrast enhancement at the periphery of ablation zone (arrow) indicating residual unablated tumor. Note enhancing small masses in the pancreas (arrowheads) indicating metastatic foci. (c) Excretory phase contrast enhanced CT 1 months after cryoablation shows washout of contrast material from the nodular enhancement, and unablated tumor is seen as subtle hypodense area relative to normal renal parenchyma (arrow).
LOCAL TUMOR PROGRESSION AFTER ABLATION
Any tumor after thermal ablation treatment that enhances more than 10 HU on CT imaging or increase in the signal intensity on MR imaging after contrast material administration [7], or a serial increase in tumor size when compared with that on images from the immediate after ablation [29] is considered to be untreated tumor and re-treatment is required [25].
Characteristic findings on CT or MR imaging of residual unablated tumor have been described as nodular (Fig 5, 6) or crescent-shaped areas (Fig 7) of contrast enhancement [27]. Thermal ablation occurs in a spherical distribution, and residual tumor is usually seen as an enhancing tissue at the periphery of the ablated tumor [25].
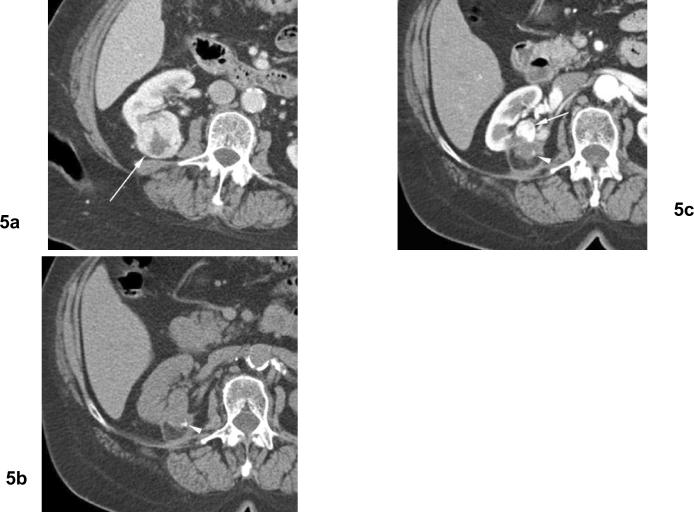
Fig 5. Residual unablated tumor. Calcifications in ablation zone after cryoablation twice for clear cell type renal cell carcinoma.
(a) Arterial phase contrast enhanced CT before cryoablation shows heterogeneously enhancing mass (arrow) in the right kidney posteriorly. (b) Noncontrast CT (15 months after 2nd cryoablation) shows small calcification (arrowhead) in the ablation zone. (c) Arterial phase contrast enhanced CT (15 months after 2nd cryoablation) shows nodular contrast enhancement at the periphery of ablation zone (arrow) indicating residual unablated tumor.
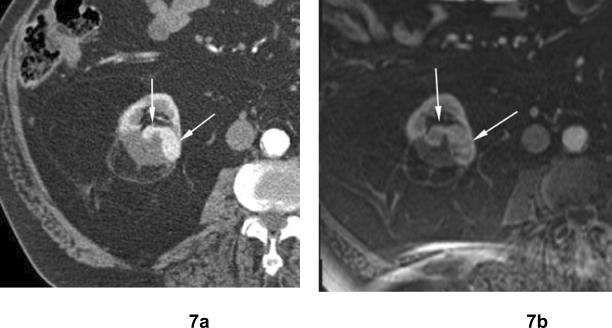
Fig 7. Residual unablated tumor after cryoablation. Clear cell type renal cell carcinoma.
(a) Arterial phase contrast enhanced CT 6 months after cryoablation shows crescent-shaped areas of contrast enhancement (arrows) at the periphery of ablated zone in the right kidney indicating residual unablated tumor. (b) Axial contrast enhanced fat-suppressed T1-weighted MR image 12 months after cryoablation shows crescent-shaped areas of contrast enhancement in the right kidney which has slightly increased in size compared to (a).
Both noncontrast and contrast-enhanced scans are necessary to assess degree of contrast enhancement. On noncontrast CT, relatively high attenuation within ablation zone may be seen due to hemorrhage or calcifications (Fig 5) [25] and noncontrast CT is necessary to determine presence or absence of contrast enhancement. For MR imaging, subtraction or quantitative assessment is used to evaluated contrast enhancement, since ablated renal tumor often demonstrate high signal intensity on T1-weighted images (Fig 2).
Late arterial phase images are useful to evaluate residual hypervascular RCC which may exhibit avid differential enhancement in this phase [25] (Figs 5, 6, 7). In contrast, delayed contrast enhancement is seen in many hypovasuclar tumors, and residual tumor is often best appreciated in a comparison of portal venous or delayed images with baseline images [22].
Javadi et al. reported three cases of atypical findings on CT after RF ablation for small RCC. CT of two patients showed enlarged area of soft-tissue density in and around the ablation zone, and in one of these two patients CT showed evidence of enhancement after contrast material administration. However, percutaneous biopsy showed no viable tumor in these two patients. CT of one patient showed increasing perinephric soft tissue density with no contrast enhancement, and percutaneous biopsy showed fragments of viable RCC along with fragments of fibrous tissue with a foreign body-type giant cell reaction and fragments of renal parenchyma [35]. They suggested that when atypical findings are noted on follow-up CT or MR imaging, close follow-up with more frequent surveillance imaging and percutaneous biopsy should be performed [35].
It should be noted that postprocedural imaging findings are only a rough guide to the success of ablation therapy, since microscopic foci of residual disease, by definition, cannot be expected to be identified [22]. Correlation with postablation radiographic success and pathological specimen has not been rigorously validated after thermal ablation of renal tumors [36]. Recently, Weight et al. evaluated 109 renal lesions in 88 patients treated with percutaneous radio frequency ablation, and 192 lesions in 176 patients treated with laparoscopic cryoablation. Thirty four patients after RF ablation and 95 patients after cryoablation underwent biopsy immediately after 6 months abdominal CT or MR imaging. Six of 13 patients who had positive biopsy at 6 months after RF ablation demonstrated no contrast enhancement on CT or MR imaging. In patients treated with cryoablation, all 6 patients who had positive biopsies revealed enhancement on CT or MR imaging just before biopsy. They recommend post RF ablation follow-up biopsy due to the significant risk of residual renal cell cancer without radiographic evidence, although the clinical significance of these viable cells remains to be determined [36].
POTENTIAL COMPLICATIONS
Ablative techniques may cause complications previously not associated with renal tumor treatment [37]. Generally, the complication rate with renal cryoablation and RF ablation is low and minor [8] and require observation only [37], and the complication rate is similar to that of other laparoscopic and percutaneous renal procedures [37]. Minimal asymptomatic perirenal fluid or blood collection is commonly seen early after thermal ablation and is considered a side effect. Minor complications require no therapy, or require overnight admission for observation only with no consequence. Major complications require therapy, with or without permanent adverse sequelae [10]. A multi-institutional review defining the complications associated with percutaneous and laparoscopic cryoablation (139 cases) and RF ablation (133 cases) reported a total of 30 complications (11.1%) including 25 minor (9.2%) and 5 major (1.8%) complications. Calyceal damage with stricture or urine leakage may occur particularly with more centrally located tumors (Fig 8). Ureteral stricture with hydronephrosis has been reported when renal tumors adjacent to the ureter or ureteropelvic junction was targeted [27, 37, 38] (Fig 9). The technique of “hydrodissection” with instillation of sterile water or solution of 5% dextrose has been used to decrease the risk of bowel injury [39], but thermal injury to adjacent bowel such as colon (Fig 10) [40] and duodenum [25] may occur. Thermal injury to the pancreas has also been reported [41].
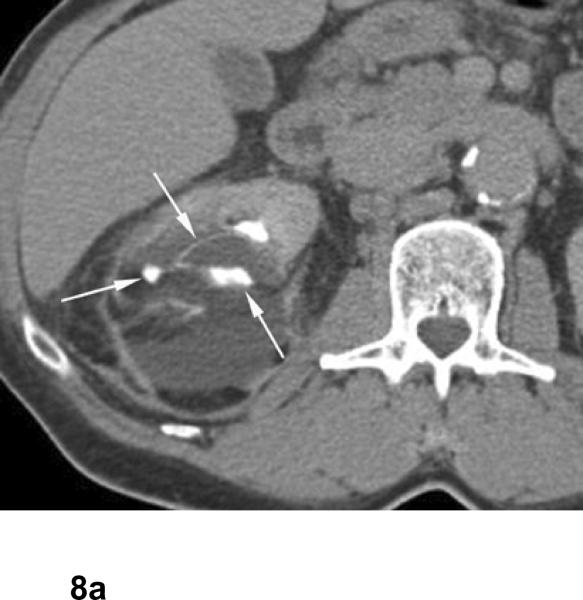
Fig 8. Urinary leak from upper pole calyx after cryoablation for papillary renal cell carcinoma. The patient is 1 month after percutaneous cryoablation.
Excretory phase axial and anterior volume rendered images show fluid collection around the ablated zone with contrast extravasation from upper pole calyx (arrows).
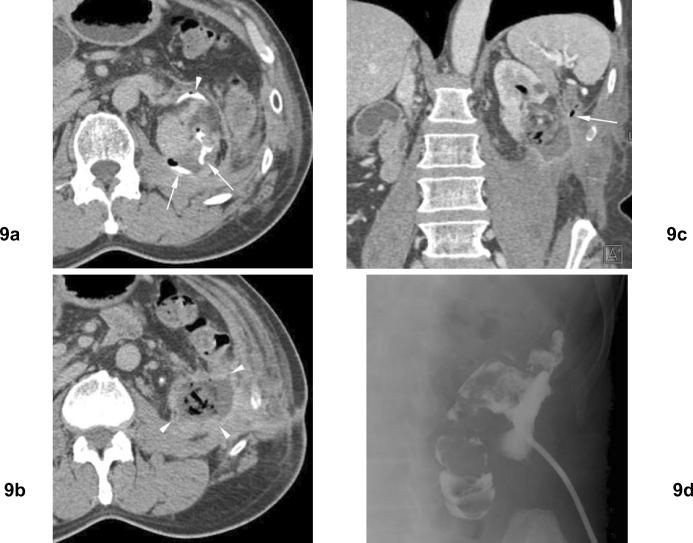
Fig 9. Left perinephric abscess and pyelocolonic fistula following open cryoablation of left renal cell carcinoma. Remote history of left partial nephrectomy for renal cell carcinomas.
(a) Excretory phase axial CT 1 month after open cryoablation shows extravasation of contrast (arrows) in ablation zone. Ureter is displaced anteriorly (arrowhead) due to prior partial nephrectomy. (b) Excretory phase axial CT slightly more inferior to (a) shows an abscess inferior to the left kidney. (c) Venous phase coronal CT shows the abscess in ablation zone with air within the renal collecting system. The descending colon is closely related to this fluid collection (arrow). (d) Percutaneous drain was placed to the abscess, and urine leak was found in the draining fluid. Injection of contrast material shows communication of abscess to colon, indicating pyelocolonic fistula.
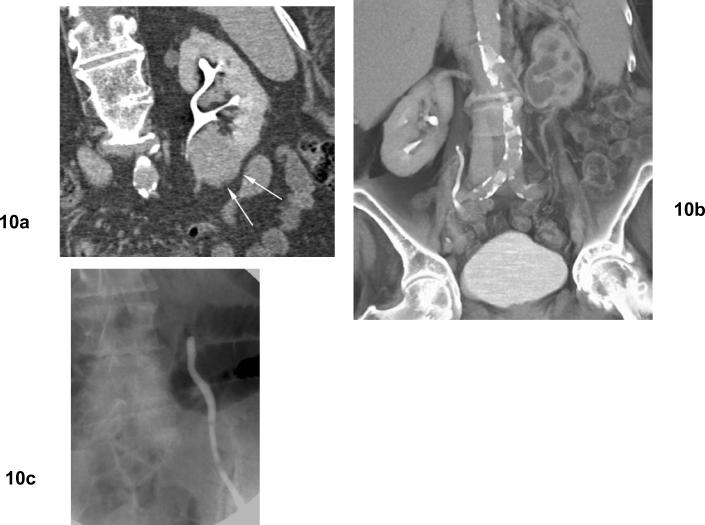
Fig 10. Ureteral stricture and hydronephrosis after cryoablation for clear cell type renal cell carcinoma.
(a) Excretory phase coronal CT shows mass in the lower pole of the left kidney adjacent to the ureteropelvic junction. (b) Excretory phase coronal CT shows left hydronephrosis developed after cryoablation. (c) Retrograde ureterography shows obstruction of the left proximal ureter at the level of the ureteropelvic junction.
CONCLUSION
Small renal tumors are increasing being treated by RF ablation and cryoablation. Residual tumor after thermal ablation is most common at the margin of the ablation zone and seen as nodular or crescent-shaped areas of contrast enhancement on CT and MR imaging. Accurate assessment of ablated tumors at postprocedural imaging is essential for evaluating the adequacy of treatment and guiding further management. Complications are uncommon and usually minor, but should be detected on post-procedural imaging studies.
REFERENCES
- 1.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166(5):1611–1623. [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr. Rising incidence of renal cell cancer in the United States. Jama. 1999;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 4.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997) Urology. 2000;56(1):58–62. doi: 10.1016/s0090-4295(00)00534-3. [DOI] [PubMed] [Google Scholar]
- 5.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163(2):442–445. [PubMed] [Google Scholar]
- 6.Pavlovich CP, Walther MM, Choyke PL, et al. Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol. 2002;167(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- 7.Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217(3):665–672. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- 8.Zagoria RJ, Hawkins AD, Clark PE, et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol. 2004;183(1):201–207. doi: 10.2214/ajr.183.1.1830201. [DOI] [PubMed] [Google Scholar]
- 9.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 10.Clark TW, Millward SF, Gervais DA, et al. Reporting standards for percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2006;17(10):1563–1570. doi: 10.1097/01.RVI.0000236718.12560.47. [DOI] [PubMed] [Google Scholar]
- 11.Mylona S, Kokkinaki A, Pomoni M, Galani P, Ntai S, Thanos L. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with solitary kidney: 6 years experience. Eur J Radiol. 2008 doi: 10.1016/j.ejrad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Matin SF, Ahrar K. Nephron-sparing probe ablative therapy: long-term outcomes. Curr Opin Urol. 2008;18(2):150–156. doi: 10.1097/MOU.0b013e3282f4a869. [DOI] [PubMed] [Google Scholar]
- 13.Matin SF, Ahrar K, Cadeddu JA, et al. Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol. 2006;176(5):1973–1977. doi: 10.1016/j.juro.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Lawatsch EJ, Langenstroer P, Byrd GF, See WA, Quiroz FA, Begun FP. Intermediate results of laparoscopic cryoablation in 59 patients at the Medical College of Wisconsin. J Urol. 2006;175(4):1225–1229. doi: 10.1016/S0022-5347(05)00682-8. discussion 1229. [DOI] [PubMed] [Google Scholar]
- 15.Nadler RB, Kim SC, Rubenstein JN, Yap RL, Campbell SC, User HM. Laparoscopic renal cryosurgery: the Northwestern experience. J Urol. 2003;170(4 Pt 1):1121–1125. doi: 10.1097/01.ju.0000088020.00147.8d. [DOI] [PubMed] [Google Scholar]
- 16.Gill IS, Remer EM, Hasan WA, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173(6):1903–1907. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- 17.Wright AD, Turk TM, Nagar MS, Phelan MW, Perry KT. Endophytic lesions: a predictor of failure in laparoscopic renal cryoablation. J Endourol. 2007;21(12):1493–1496. doi: 10.1089/end.2007.9850. [DOI] [PubMed] [Google Scholar]
- 18.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226(2):417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 19.Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology. 2006;240(1):82–89. doi: 10.1148/radiol.2401050788. [DOI] [PubMed] [Google Scholar]
- 20.Hines-Peralta A, Goldberg SN. Review of radiofrequency ablation for renal cell carcinoma. Clin Cancer Res. 2004;10(18 Pt 2):6328S–6334S. doi: 10.1158/1078-0432.CCR-050004. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(2):323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235(3):728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosniak MA, Birnbaum BA, Krinsky GA, Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995;197(3):589–597. doi: 10.1148/radiology.197.3.7480724. [DOI] [PubMed] [Google Scholar]
- 24.Ganguli S, Brennan DD, Faintuch S, Rayan ME, Goldberg SN. Immediate renal tumor involution after radiofrequency thermal ablation. J Vasc Interv Radiol. 2008;19(3):412–418. doi: 10.1016/j.jvir.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Rutherford EE, Cast JE, Breen DJ. Immediate and long-term CT appearances following radiofrequency ablation of renal tumours. Clin Radiol. 2008;63(2):220–230. doi: 10.1016/j.crad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Wingo MS, Leveillee RJ. Central and deep renal tumors can be effectively ablated: radiofrequency ablation outcomes with fiberoptic peripheral temperature monitoring. J Endourol. 2008;22(6):1261–1267. doi: 10.1089/end.2008.0135. [DOI] [PubMed] [Google Scholar]
- 27.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 2, Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):72–80. doi: 10.2214/ajr.185.1.01850072. [DOI] [PubMed] [Google Scholar]
- 28.Farrell MA, Charboneau WJ, DiMarco DS, et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol. 2003;180(6):1509–1513. doi: 10.2214/ajr.180.6.1801509. [DOI] [PubMed] [Google Scholar]
- 29.Atwell TD, Farrell MA, Callstrom MR, et al. Percutaneous cryoablation of 40 solid renal tumors with US guidance and CT monitoring: initial experience. Radiology. 2007;243(1):276–283. doi: 10.1148/radiol.2431052133. [DOI] [PubMed] [Google Scholar]
- 30.Merkle EM, Nour SG, Lewin JS. MR imaging follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology. 2005;235(3):1065–1071. doi: 10.1148/radiol.2353040871. [DOI] [PubMed] [Google Scholar]
- 31.Boss A, Clasen S, Kuczyk M, et al. Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol. 2005;40(9):583–590. doi: 10.1097/01.rli.0000174473.32130.28. [DOI] [PubMed] [Google Scholar]
- 32.Silverman SG, Tuncali K, Morrison PR. MR Imaging-guided percutaneous tumor ablation. Acad Radiol. 2005;12(9):1100–1109. doi: 10.1016/j.acra.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88(11):2452–2463. [PubMed] [Google Scholar]
- 34.Rendon RA, Gertner MR, Sherar MD, et al. Development of a radiofrequency based thermal therapy technique in an in vivo porcine model for the treatment of small renal masses. J Urol. 2001;166(1):292–298. [PubMed] [Google Scholar]
- 35.Javadi S, Matin SF, Tamboli P, Ahrar K. Unexpected atypical findings on CT after radiofrequency ablation for small renal-cell carcinoma and the role of percutaneous biopsy. J Vasc Interv Radiol. 2007;18(9):1186–1191. doi: 10.1016/j.jvir.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Weight CJ, Kaouk JH, Hegarty NJ, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179(4):1277–1281. doi: 10.1016/j.juro.2007.11.075. discussion 1281–1273. [DOI] [PubMed] [Google Scholar]
- 37.Johnson DB, Solomon SB, Su LM, et al. Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol. 2004;172(3):874–877. doi: 10.1097/01.ju.0000135833.67906.ec. [DOI] [PubMed] [Google Scholar]
- 38.Chen SH, Mouraviev V, Raj GV, Marguet CG, Polascik TJ. Ureteropelvic junction obliteration resulting in nephrectomy after radiofrequency ablation of small renal cell carcinoma. Urology. 2007;69(5):982, e983–985. doi: 10.1016/j.urology.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Farrell MA, Charboneau JW, Callstrom MR, Reading CC, Engen DE, Blute ML. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181(5):1315–1317. doi: 10.2214/ajr.181.5.1811315. [DOI] [PubMed] [Google Scholar]
- 40.Vanderbrink BA, Rastinehad A, Caplin D, Ost MC, Lobko I, Lee BR. Successful conservative management of colorenal fistula after percutaneous cryoablation of renal-cell carcinoma. J Endourol. 2007;21(7):726–729. doi: 10.1089/end.2006.0211. [DOI] [PubMed] [Google Scholar]
- 41.Lee DI, McGinnis DE, Feld R, Strup SE. Retroperitoneal laparoscopic cryoablation of small renal tumors: intermediate results. Urology. 2003;61(1):83–88. doi: 10.1016/s0090-4295(02)02004-6. [DOI] [PubMed] [Google Scholar]