Abstract
Purpose
We assessed the cardiovascular safety profile of degarelix, a new gonadotropin-releasing hormone antagonist.
Materials and Methods
This is the first report to our knowledge on cardiovascular safety data from a completed 1-year randomized controlled trial of leuprolide acetate vs degarelix. Outcomes considered in these analyses included the QT interval by central reading and analysis, and cardiovascular adverse events. On multivariate analyses relationships between selected baseline factors and cardiovascular events were evaluated.
Results
There were no significant differences between treatment groups for mean change in Fridericia’s correction of QT during the trial. Markedly abnormal Fridericia’s correction of QT values (500 milliseconds or greater) were observed in only a small number of subjects by treatment group, that is 2 (less than 1%) in the pooled degarelix group and 2 (1%) in the leuprolide group. Supraventricular arrhythmias were the most common type of arrhythmias, affecting 2% of subjects in the pooled degarelix group and 4% in the leuprolide group. Other arrhythmias occurred in 1% or less of subjects by treatment group. The most frequently reported cardiac disorder was ischemic heart disease, which occurred in 4% of subjects treated with degarelix and 10% of those on leuprolide. Cox proportional hazard ratio estimates for selected baseline covariates showed a significantly increased risk of cardiovascular events by age (p = 0.0459) and systolic blood pressure (p = 0.0061).
Conclusions
In men with prostate cancer degarelix and leuprolide have similar cardiovascular safety profiles. These observations suggest that the cardiovascular events associated with both agents result from hypogonadism rather than a direct drug effect.
Keywords: adenocarcinoma, gonadotropin-releasing hormone, drug monitoring
Gonadotropin-releasing hormone agonists are associated with a greater risk of myocardial infarction and sudden cardiac death.1–3 Several mechanisms may contribute to greater CV disease risk following treatment with GnRH agonists including obesity, insulin resistance and increased cholesterol.2 In addition, GnRH agonists are associated with prolongation of the QT interval.4 Hypogonadism, the intended therapeutic effect of treatment with a GnRH agonist, appears sufficient to explain the observed treatment related metabolic alterations and the association with greater CV morbidity.5,6
Degarelix is a new GnRH antagonist approved for the treatment of prostate cancer.7 It binds to GnRH receptors in the anterior pituitary gland, and decreases the secretion of luteinizing hormone and follicle-stimulating hormone, which leads directly to a rapid decrease in testosterone production.8,9 Degarelix achieves serum testosterone levels in the castrate range (0.5 ng/ml or less) within 1 to 3 days after administration.10 In a randomized controlled trial degarelix and leuprolide caused similar maximum suppression of serum testosterone levels.7 There is limited information about the CV safety of degarelix and other GnRH antagonists, although the observed effects on serum testosterone levels suggest a CV safety profile similar to that of other GnRH agonists. In this study we evaluate data from a comparative phase III trial to compare the CV related safety of degarelix (a GnRH antagonist) with leuprolide (a GnRH agonist) for the treatment of prostate cancer in subjects needing androgen ablation therapy.7
MATERIALS AND METHODS
The trial design and subject population have been described for the original study that evaluated the efficacy and safety of degarelix 240/80 mg, degarelix 240/160 mg and leuprolide 7.5 mg.7 The present analysis was performed on data relating to CV events and QT/QTc prolongation.
A 12-lead ECG was performed using unified standardized validated equipment at all included sites at screening on days 0, 3, 84 (±7 days), 168 (±7 days) and every 84 days (±7) thereafter until the end of trial visit (day 364 [±7 days] or at early withdrawal for all subjects). At day 0, 3 separate ECG recordings were made. Recordings were made before dosing if a dose visit was scheduled. Acquisitions of ECGs were made digitally and measurements were performed centrally by Covance®. The ECG was transmitted for central reading and evaluation to a selected group of board certified cardiologists.
ECG recordings were analyzed to assess any effect of the trial drugs on cardiac repolarization in terms of QT prolongation according to current guidelines.11 The overall clinical significance of ECG abnormalities was evaluated by the investigators and by cardiologists at Covance.
The QT intervals were corrected for heart rate using the method of Fridericia12 rather than Bazett, which is not considered optimal.13 Subjects with a marked baseline prolongation of the QT/QTcF interval, eg repeated demonstration of a QTcF interval greater than 450 milliseconds, were excluded from the trial. Subjects were also excluded from study if they had a history of additional risk factors for torsades de pointes ventricular arrhythmias, eg heart failure, hypokalemia or a family history of long QT syndrome. The use of concomitant medication associated with prolongation of the QT/QTcF interval (www.torsades.org) was also prohibited at screening. However, during the trial these drugs were allowed at the investigator’s discretion based on the safety evaluation.
AEs were assessed throughout the study according to the National Cancer Institute Common Terminology Criteria for Adverse Events. The CV safety assessment focused on AEs related to QT/QTc prolongation (greater than 500 milliseconds) and serious CV events suggesting an arrhythmia. AEs of interest, especially if an effect on QT/QTc interval was evident, included torsades de pointes, sudden death, ventricular tachycardia, ventricular fibrillation and flutter, syncope and seizures. In addition, baseline risk factors were analyzed including treatment arm, medical history of cardiac or vascular disorders, diastolic and systolic blood pressure, and pretrial medications for CV disorders, diabetes or cholesterol.
All safety analyses were performed for the intent to treat analysis set. A baseline QRS greater than 120 milliseconds was noted in 46 subjects who were excluded from the analysis of QT prolongation. A QTcF of 500 milliseconds or greater was considered markedly abnormal. The changes from baseline in QTcF by greater than 30 milliseconds and greater than 60 milliseconds are summarized by treatment using box plots. Hazard ratio estimates for treatment and selected baseline covariates (age, body mass index, smoking status, alcohol consumption, hyperlipidemia, hypertension, diastolic blood pressure, systolic blood pressure, heart rate, medical history of manifest atherosclerotic CV disease at baseline [including coronary, cerebrovascular and peripheral vascular disease] and treatment regimens) were analyzed using a Cox proportional hazard model, with separate models executed for each baseline variable.
RESULTS
In the original study subjects were randomized to 1 of 3 groups of degarelix 240/80 mg (202), degarelix 240/160 mg (207) or 7.5 mg leuprolide (201). A total of 504 subjects completed the study (in groups of 169, 163 and 172, respectively). Baseline demographic and clinical characteristics were similar among the 3 treatment groups (table 1). The use of concomitant medications was consistent with many subjects having a history of cardiac disease or hypertension. The proportion of patients using concomitant drugs capable of prolonging the QT interval was similar among the groups (data not shown). The primary end point of the original study was suppression of testosterone to 0.5 ng/ml or less from day 28 to 364, which was achieved by 97.2%, 98.3% and 96.4% of patients in the degarelix 240/80 mg, degarelix 240/160 mg and leuprolide groups, respectively.7
Table 1.
Patient demographics and clinical characteristics
| Degarelix 240/80 mg | Degarelix 240/160 mg | Leuprolide 7.5 mg | |
|---|---|---|---|
| Median pt age (range) | 72 (51–89) | 72 (50–88) | 74 (52–98) |
| Median kg/m2 body mass index (range) | 25.8 (17.3–42.2) | 26.4 (16.1–38.9) | 26.4 (19.2–42.7) |
| Median mm Hg blood pressure (range): | |||
| Systolic | 133 (90–190) | 135 (100–184) | 134 (97–196) |
| Diastolic | 80 (56–110) | 80 (53–110) | 80 (50–110) |
| Median beats/min heart rate (range) | 70 (45–107) | 70 (44–100) | 71 (46–112) |
| No. hypertension (%) | 111 (54) | 108 (53) | 104 (52) |
| No. alcohol consumption (%) | 118 (57) | 106 (52) | 106 (53) |
| No. atherosclerotic disease (%) | 66 (32) | 66 (33) | 64 (32) |
| No. ischemic heart disease (%) | 54 (26) | 54 (27) | 57 (28) |
| No. hyperlipidemia (%) | 43 (21) | 33 (16) | 40 (20) |
| No. smoking (%) | 27 (13) | 25 (12) | 27 (13) |
Other patient baseline demographics and baseline characteristics for the original comparative phase III trial have been previously published.7
Results for QTcF at baseline, day 3 and the end of the study are summarized in table 2. There were no significant differences among treatment groups for mean change in QTcF to day 3 or until trial end. Mean percentage changes in QTcF from baseline were approximately 0.01% to 1.0% at day 3 and 2.9% to 3.5% at the end of study visits. Mean increases in QTcF from baseline were less than 15 milliseconds.
Table 2.
Summary of QTcF results in subjects with QRS 120 msec or less at baseline
| Degarelix 240/80 mg | Degarelix 240/160 mg | Pooled Degarelix | Leuprolide 7.5 mg | |
|---|---|---|---|---|
| Baseline: | ||||
| No. | 191 | 183 | 374 | 184 |
| Mean (SD) | 405 (19.8) | 401 (18.5) | 403 (19.2) | 403 (18.8) |
| Day 3: | ||||
| No. | 188 | 181 | 369 | 181 |
| Mean (SD) | 409 (22.8) | 402 (18.4) | 405 (21.0) | 403 (21.8) |
| Change (SD) | 4.43 (15.4) | 0.576 (16.6) | 2.54 (16.1) | 0.198 (17.5) |
| Mean % change | 1.06 | 0.148 | 0.612 | 0.0132 |
| End of trial: | ||||
| No. | 191 | 185 | 376 | 184 |
| Mean (SD) | 419 (21.9) | 413 (20.6) | 416 (21.4) | 417 (22.3) |
| Change (SD) | 14.1 (18.4) | 11.9 (15.9) | 13.0 (17.2) | 14.0 (20.4) |
| Mean % change | 3.46 | 2.94 | 3.21 | 3.45 |
Patients with baseline QRS greater than 120 msec were excluded from the QTc analysis.
A total of 62 (17%) subjects treated with degarelix and 28 (15%) treated with leuprolide had a post-baseline QTcF of 450 milliseconds or greater (table 3). Post-baseline QTcF values of 480 milliseconds or greater were recorded for 7 (2%) subjects treated with degarelix and 4 (2%) treated with leuprolide. Markedly abnormal QTcF values (500 milliseconds or greater) were noted in 2 (less than 1%) subjects treated with degarelix and 2 (1%) on leuprolide.
Table 3.
Incidence of markedly abnormal changes in QTcF from baseline in subjects with QRS 120 msec or less at baseline
| Degarelix 240/80 mg | Degarelix 240/160 mg | Pooled Degarelix | Leuprolide 7.5 mg | |
|---|---|---|---|---|
| No. with normal baseline value + post-baseline value recorded | 189 | 184 | 373 | 181 |
| No. msec post-baseline QTcF (%): | ||||
| 450 or Greater | 35 (19) | 27 (15) | 62 (17) | 28 (15) |
| 480 or Greater | 4 (2) | 3 (2) | 7 (2) | 4 (2) |
| 500 or Greater | 2 (1) | 0 | 2 (less than 1) | 2 (1) |
Subjects with QTcF 480 or more and 500 or more msec are included in the total for 450 or more msec.
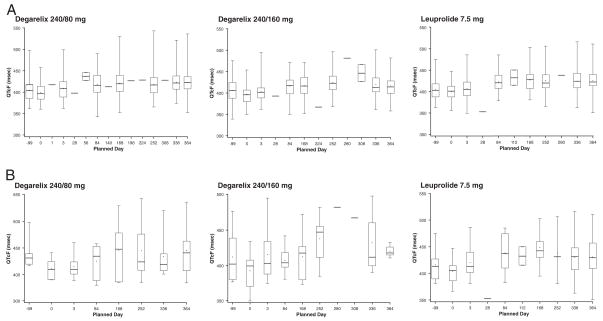
Box plots of change from baseline in QTcF values are depicted for each treatment group in parts A (greater than 30 milliseconds) and B (greater than 60 milliseconds) of the figure. The plots display the range (minimum to maximum), IQR (25th and 75th percentiles), median and mean. It should be noted that baseline values are lower because subjects with marked baseline prolongation were excluded from study and, therefore, any subsequent prolongation is probably related to the study drug. Nevertheless, the study suggests an upward trend in all 3 treatment arms.
Figure.
Box plot for change from baseline in QTcF greater than 30 milliseconds (A) and greater than 60 milliseconds (B) by treatment group
The most common type of arrhythmia was supraventricular arrhythmia, occurring in 2% (10 of 409) of subjects in the pooled degarelix group and 4% (9 of 201) of those in the leuprolide group (table 4). Other arrhythmias occurred in 1 to 4 subjects (1% or less) by treatment group, and included AV conduction disturbances, bundle branch block, bradycardia, cardiac arrest and ventricular arrhythmias. Both instances of cardiac arrest in subjects treated with degarelix were considered serious AEs and led to treatment discontinuation. QT intervals were not prolonged in these subjects. Bradycardia was reported as a serious AE in 1 patient treated with degarelix (240/80 dose group).
Table 4.
Incidence of arrhythmias
| No. Degarelix 240/80 mg (%) | No. Degarelix 240/160 mg (%) | No. Pooled Degarelix (%) | No. Leuprolide 7.5 mg (%) | |
|---|---|---|---|---|
| Supraventricular* | 6 (3) | 4 (2) | 10 (2) | 9 (4) |
| AV conduction disturbances† | 1 (less than 1) | 3 (1) | 4 (less than 1) | 3 (1) |
| Bundle branch block (lt + rt) | 0 | 2 (less than 1) | 2 (less than 1) | 3 (1) |
| Bradycardia | 0 | 1 (less than 1) | 1 (less than 1) | 2 (less than 1) |
| Ventricular‡ | 0 | 2 (less than 1) | 2 (less than 1) | 2 (less than 1) |
| Cardiac arrest | 2 (less than 1) | 0 | 2 (less than 1) | 0 |
Includes supraventricular extrasystoles, atrial fibrillation, supraventricular tachycardia, tachycardia, arrhythmia and palpitations.
Includes AV block, first and second degree.
Includes ventricular extrasystoles and ventricular arrhythmia.
Weight gain was common in both groups, reported in 10% (40 of 409) of subjects in the pooled degarelix group and 12% (24 of 201) of those in the leuprolide group (table 5). Hypercholesterolemia and hypertension occurred in 2% to 6% of subjects with similar incidences in those treated with degarelix and leuprolide. Other cardiac risk factors generally occurred in 1% or less of subjects. Diabetes mellitus was reported for 2% (7 of 409) of subjects on degarelix and 1% (3 of 201) of those on leuprolide. Increases in blood glucose, glucose in urine and glucose intolerance were reported for less than 1% of subjects.
Table 5.
Incidence of cardiac risk factors
| No. Degarelix 240/80 mg (%) | No. Degarelix 240/160 mg (%) | No. Pooled Degarelix (%) | No. Leuprolide 7.5 mg (%) | |
|---|---|---|---|---|
| Wt increased | 18 (9) | 22 (11) | 40 (10) | 24 (12) |
| Blood pressure increased | 1 (less than 1) | 2 (1) | 3 (less than 1) | 1 (less than 1) |
| Cholesterol increased | 1 (less than 1) | 0 | 1 (less than 1) | 1 (less than 1) |
| Blood glucose increased | 0 | 1 (less than 1) | 1 (less than 1) | 0 |
| Glucose urine | 0 | 2 (1) | 2 (less than 1) | 1 (less than 1) |
| Hypercholesterolemia | 7 (3) | 12 (6) | 19 (5) | 5 (2) |
| Diabetes mellitus | 1 (less than 1) | 6 (3) | 7 (2) | 3 (1) |
| Noninsulin dependent diabetes mellitus | 1 (less than 1) | 3 (1) | 4 (1) | 1 (less than 1) |
| Hyperlipidemia | 2 (1) | 2 (1) | 4 (1) | 2 (1) |
| Dyslipidemia | 1 (less than 1) | 0 | 1 (less than 1) | 0 |
| Hypertriglyceridemia | 0 | 0 | 0 | 1 (less than 1) |
| Glucose intolerance | 0 | 1 (less than 1) | 1 (less than 1) | 0 |
| Hypertension | 12 (6) | 14 (7) | 26 (6) | 8 (4) |
| Hypotension | 3 (1) | 1 (less than 1) | 4 (1) | 1 (less than 1) |
| Accelerated hypertension | 2 (1) | 0 | 2 (less than 1) | 0 |
| Labile blood pressure | 0 | 0 | 0 | 1 (less than 1) |
The most frequently reported cardiac disorder was ischemic heart disease, occurring in 4% (18 of 409) of subjects in the pooled degarelix group and 10% (21 of 201) of those in the leuprolide group (table 6). The most common events in this class were myocardial ischemia and myocardial infarction, which each occurred in 2% of subjects treated with leuprolide but in less than 1% of those on degarelix. Cardiac failure occurred in less than 1% of subjects in the pooled degarelix group and in 2% of those on leuprolide.
Table 6.
Incidence of other cardiovascular related treatment emergent AEs
| No. Degarelix 240/80 mg (%) | No. Degarelix 240/160 mg (%) | No. Pooled Degarelix (%) | No. Leuprolide 7.5 mg (%) | |
|---|---|---|---|---|
| Ischemic heart disease | 8 (4) | 10 (5) | 18 (4) | 21 (10) |
| Peripheral vascular atherosclerosis | 3 (1) | 2 (1) | 5 (1) | 1 (less than 1) |
| Cerebrovascular disease | 5 (2) | 2 (1) | 7 (2) | 1 (less than 1) |
| Cardiac failure | 2 (1) | 3 (1) | 5 (1) | 5 (2) |
Peripheral vascular atherosclerosis occurred in 1% (5 of 409) of subjects in the pooled degarelix group and 1% or less (1 of 201) in the leuprolide group. Of subjects in the pooled degarelix group 2% (7 of 409) experienced a stroke compared with less than 1% (1 of 201) in the leuprolide group. Other atherosclerotic events affected 1 or 2 subjects only.
Serious arrhythmias occurred in 2% (10 of 409) of subjects in the pooled degarelix group and 5% (10 of 201) of those in the leuprolide group. Two subjects died of cardiac arrest (both in the 80 mg degarelix group) and 3 died of myocardial infarction (1 in the 80 mg degarelix group and 2 in the leuprolide group). Cardiac failure resulted in the death of 2 subjects, 1 treated with degarelix and 1 with leuprolide. Cardiac disorder resulted in the death of 1 subject treated with leuprolide. Based on inspection of the last ECG recordings made before death, neither death was considered related to the trial drugs.
Cardiac disorders led to discontinuation in 1% (6 of 409) of subjects in the pooled degarelix group and 2% (5 of 201) of those in the leuprolide group. Two subjects, each in the pooled degarelix group, discontinued the study because of cardiac arrest or cardiopulmonary failure. All other CV related AEs leading to discontinuation occurred in only 1 (less than 1%) subject.
Cox proportional hazard ratio estimates for selected baseline covariates showed a significantly increased risk for CV events by age (p = 0.0459) and systolic blood pressure (p = 0.0061). There were no statistically significant differences by treatment arm, diastolic blood pressure and pretrial medication for CV disorders, diabetes or cholesterol. No significant association was found between baseline factors and cardiac arrhythmias using the high level group term cardiac arrhythmias.
DISCUSSION
This study compared the CV safety profile of leuprolide acetate and degarelix, a GnRH antagonist, using data from a recently reported randomized controlled trial. Mean changes in the QTcF interval were similar for subjects treated with degarelix and leuprolide. There were no significant differences in rates of new ischemic heart disease, arrhythmias or marked prolongation of QTcF values.
The medical history was as expected for the group of elderly subjects with histologically proven adenocarcinomas of the prostate enrolled in the trial. Approximately a quarter of the subjects (25% to 28%) had a history of ischemic heart disease. Treatment of men with prostate cancer using ADT has been associated with an increased risk of CV disease within months of commencing therapy.14 ADT is most commonly achieved by GnRH agonists such as leuprolide and acute coronary syndromes are 1 of the reported AEs of these agents, resulting from decreased serum testosterone concentrations.14 Consequences of decreased circulating testosterone associated with ADT include changes in body composition, alterations in lipid profiles and decreased insulin sensitivity.15 It is plausible that this pattern of metabolic changes during ADT may increase CV disease risk, although the association between ADT use and CV mortality remains controversial. Given the metabolic effects of ADT, this highlights the importance of monitoring and quantifying the incidence of CV events in the short-term and long-term treatment of prostate cancer with ADT.
QTc prolongation has been reported with GnRH agonists, combined androgen blockade or a GnRH antagonist in the treatment of prostate cancer, with 9 to 21 milliseconds increases in QTc.4 Hypogonadism rather than a direct drug effect appears to account for the observed QTc prolongation as repolarization was noted to be slower and longer in castrated compared with normal cases.16 Interestingly women with virilization exhibited a shorter and faster repolarization than normal women and castrated men. Our observation that mean changes in the QTcF interval were similar for subjects treated with degarelix and leuprolide provides further support of hypogonadism as the mechanism responsible for prolongation of the QTc interval.
In the present study we evaluated these potential effects in a head-to-head comparison of degarelix and leuprolide. There were no major changes from baseline to the end of study visits. The figure suggests an upward trend in QTcF values from baseline in all 3 treatment arms, although the effect appears to level off after day 168. Many drugs that prolong the QT interval block the cardiac hERG channel.17 However, degarelix had no effect on hERG channels in HEK239 cells,18 nor did degarelix have a direct effect on QRS or QT intervals in dog isolated cardiac Purkinje fibers.19 It is likely that the small QT prolonging potency relates to the decreased testosterone level itself, suggested by the fact that both drugs (degarelix and leuprolide) have a similar effect on QT interval.
The incidence of CV events during GnRH agonist therapy has been thoroughly documented by several investigators. The incidence of events varied between 11.9/1,000 and 69.4/1,000 subject-years.1,20 In the present study we compared the incidence rates in subjects treated with leuprolide and degarelix monitored for 1 year. Although the incidence of events was relatively low, the numerical data suggest a comparable safety profile for the 2 drugs. This finding emphasizes that the main driving force of complications is ADT.
Arrhythmias are a cardiac complication of particular concern. The incidence of such events was comparable between the degarelix and leuprolide groups, and the most frequent AE was supraventricular arrhythmia, which occurred in 2% of the degarelix group and 4% of the leuprolide group. Other arrhythmias noted were AV conduction disturbances (respectively less than 1% and 1%), bundle branch block (less than 1% and 1%), bradycardia (less than 1% in both groups), ventricular arrhythmias (less than 1% in both groups) and cardiac arrest (less than 1% of degarelix subjects, no leuprolide subjects). Similar incidences of atherosclerotic events that manifested as peripheral vascular atherosclerosis were noted in the pooled degarelix and leuprolide groups (1% or less). The long-term safety of degarelix and leuprolide in terms of these types of events is yet to be established.
Hazard ratio estimates for selected baseline covariates showed a significantly increased risk with higher age (p = 0.0459) and systolic blood pressure (p = 0.0061). There were no statistically significant differences by treatment arm, diastolic blood pressure and pretrial medication for CV disorders, diabetes or cholesterol. The lack of association between obvious CV risk factors (eg type 2 diabetes and CV events) could be due to the relatively low prevalence of these diseases in the treatment arms. In summary, these analyses indicate that degarelix has an overall CV safety profile similar to that of leuprolide.
CONCLUSIONS
In men with prostate cancer degarelix and leuprolide have similar CV safety profiles. Marked prolongation of the QTc interval was uncommon (1% or less) with either agent. The incidence of arrhythmias during a 1-year period was similar for subjects treated with degarelix and leuprolide. These observations suggest that the CV effects of both agents result from hypogonadism rather than a direct drug effect.
Acknowledgments
Supported by Ferring Pharmaceuticals A/S.
IMC Medical Communication, London, United Kingdom, provided editorial support.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- AE
adverse event
- AV
atrioventricular
- CV
cardiovascular
- ECG
electrocardiogram
- GnRH
gonadotropin-releasing hormone
- hERG
human ether-à-go-go-related gene
- QRS
recording of single heartbeat on ECG corresponding to depolarization of right and left ventricles
- QT
measure of time between start of Q wave and end of T wave
- QTc
QT corrected for heartbeat
- QTcF
Fridericia’s correction of QT
References
- 1.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 2.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998. doi: 10.1016/j.juro.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MR. Androgen deprivation therapy and risk for diabetes and cardiovascular disease in prostate cancer survivors. Curr Urol Rep. 2008;9:197. doi: 10.1007/s11934-008-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garnick MB, Pratt CM, Campion M, et al. The effect of hormonal therapy for prostate cancer on the electrocardiographic QT interval: phase 3 results following treatment with leuprolide and goserelin, alone or with bicalutamide, and the GnRH antagonist abarelix. J Clin Oncol. 2004;(suppl):22. abstract 4578. [Google Scholar]
- 5.Hakimian P, Blute MJ, Kashanian J, et al. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU Int. 2008;102:1508. doi: 10.1111/j.1464-410X.2008.07933.x. [DOI] [PubMed] [Google Scholar]
- 6.Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74. doi: 10.1159/000176046. [DOI] [PubMed] [Google Scholar]
- 7.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 8.Gittelman M, Pommerville PJ, Persson BE, et al. A 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America. J Urol. 2008;180:1986. doi: 10.1016/j.juro.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Van Poppel H, Tombal B, de la Rosette JJ, et al. Degarelix: a novel gonadotrophin-releasing hormone (GnRH) receptor blocker–results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur Urol. 2008;54:805. doi: 10.1016/j.eururo.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 10.Frampton JE, Lyseng-Williamson KA. Degarelix. Drugs. 2009;69:1967. doi: 10.2165/10484080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs 2005; [Accessed June 8, 2010]. Available at http://www.ich.org/LOB/media/MEDIA1476.pdf. [Google Scholar]
- 12.Fridericia LS. The duration of systole in the electrocardiogram of normal subjects and of patients with heart disease. Acta Med Scand. 1920;53:469. doi: 10.1046/j.1542-474X.2003.08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol. 1993;72:17B. doi: 10.1016/0002-9149(93)90035-b. [DOI] [PubMed] [Google Scholar]
- 14.Kintzel PE, Chase SL, Schultz LM, et al. Increased risk of metabolic syndrome, diabetes mellitus and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28:1511. doi: 10.1592/phco.28.12.1511. [DOI] [PubMed] [Google Scholar]
- 15.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory forum from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60:194. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidoggia H, Maciel JP, Capalozza N, et al. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 17.Mitcheson JS. hERG potassium channels and the structural basis of drug-induced arrhythmias. Chem Res Toxicol. 2008;21:1005. doi: 10.1021/tx800035b. [DOI] [PubMed] [Google Scholar]
- 18.Ferring Pharmaceuticals A/S. Effect of FE200486 on hERG tail current recorded from stably transfected HEK293 cells. Study Report FE:200486PHA0204. Unpublished data.
- 19.Ferring Pharmaceuticals A/S. Study PHA0203. Unpublished data.
- 20.Keating NL, O’Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]