Abstract
Bone health and maintenance of bone integrity are important components of comprehensive cancer care in both early and late stages of disease. Risk factors for osteoporosis are increased in patients with cancer, including women with chemotherapy-induced ovarian failure, those treated with aromatase inhibitors for breast cancer, men receiving androgen-deprivation therapy for prostate cancer, and patients undergoing glucocorticoid therapy. The skeleton is a common site of metastatic cancer recurrence, and skeletal-related events are the cause of significant morbidity. The National Comprehensive Cancer Network (NCCN) convened a multidisciplinary task force on Bone Health in Cancer Care to discuss the progress made in identifying effective screening and therapeutic options for management of treatment-related bone loss; understanding the factors that result in bone metastases; managing skeletal metastases; and evolving strategies to reduce bone recurrences. This report summarizes presentations made at the meeting.
Keywords: NCCN Clinical Practice Guidelines, bone health, breast cancer, prostate cancer, dual x-ray absorptiometry, bone mineral density, FRAX™ analysis, osteopenia, osteoporosis, bisphosphonates, aromatase inhibitors, chemotherapy, imaging, bone metastases
Background
Bone health is emerging as an important issue among clinicians who care for cancer patients. The most commonly diagnosed cancers among women and men in the United States are breast and prostate. The American Cancer Society estimated that 184,450 new cases of breast cancer and 186,320 new cases of prostate cancer were diagnosed in 2008.1 Although most patients will not experience bone metastases, those who do develop metastatic disease have a high likelihood of the tumor involving bone or bone marrow. The incidence of bone metastases is 73% in patients with metastatic breast cancer, 68% in those with prostate cancer, and in nearly all patients with myeloma.2
Complications of bone metastases include pain, hypercalcemia, nerve compression, and pathologic fractures, and significant morbidity and mortality are associated with bone metastases. In addition, bone health can be significantly impacted by cancer treatments in patients with early stage cancer. Treatment–related bone loss may lead to osteoporosis and its complications, including fractures, pain, and diminished quality of life.
Managing and maintaining bone health in patients with cancer requires understanding normal bone metabolism and how it is affected by both bone metastasis and the drugs used to treat cancer, including the effect of chemotherapy-induced menopause and anti-estrogen therapies on bone loss; the role of bone markers and imaging techniques to assess bone loss, bone metastases, and therapeutic strategies to maintain bone health; treatment of bone metastases, including surgery and radiation therapy for pathologic fractures; and emerging data in preventing bone metastases. Since publication of a previous NCCN Task Force Report in 2006, new data on bone health, treatment, and the role of bisphosphonates to prevent bone metastases in cancer patients have emerged, prompting an update. This task force focuses on bone health and bone metastases in solid tumors.
Ten expert task force members were chosen, representing endocrinology, medical oncology, imaging, and orthopedic surgery. All task force members are affiliated with NCCN member institutions and were identified and invited solely by NCCN. During a day-long meeting in December 2008, panel members provided didactic presentations integrating expert judgment with key literature review on screening, detection, and treatment options for osteoporosis; cancer therapy–induced bone loss; reducing risk of recurrences; pathophysiology of bone metastases; and imaging, management, and treatment of bone metastases, particularly in breast and prostate cancer patients. This report summarizes the NCCN Bone Health in Cancer Care Task Force meeting.
Screening for and Detecting Osteoporosis
Osteoporosis and its associated increase in fracture risk is a major health issue for cancer patients. Much of the morbidity and mortality associated with bone loss can be prevented with appropriate screening, lifestyle interventions, and therapy. The hormone deprivation state resulting from certain cancer therapies enhances osteoclastic bone resorption, promoting bone loss. Glucocorticoids are commonly used for supportive therapy (e.g., premedications for taxanes or antiemetics) in the treatment of solid tumors and are often used in hematologic malignancies as well. These therapy-related affects can combine with other important clinical factors such as age, prior fracture history, and family history of fracture, further increasing fracture risk.3,4 Screening and modifying risk factors for development of osteoporosis is a critical issue for all cancer survivors and their health care providers.
Bone is a dynamic tissue that undergoes formation and resorption throughout the life of an individual to maintain skeletal integrity. This homeo-static process involves a continuous cycle of bone matrix and mineral resorption (osteoclastic activity) and bone formation (osteoblastic activity). In the most common form of osteoporosis, resorption exceeds formation, leading to low bone mass, deterioration of bone tissue, and disruption of bone architecture. This leads to compromised bone strength and an increased risk of fractures. Advancing age and the onset of menopause further increase the rate of bone resorption, magnifying the impact of the remodeling imbalance.
Many non-oncologic factors are associated with an increased risk of osteoporosis-related fracture. These include lifestyle factors such as smoking, excess alcohol intake, inadequate exercise, low calcium intake, and vitamin D deficiency; genetic factors such as parental history of hip-fractures; and the use of specific pharmacologic agents such as glucocorticoids, proton pump inhibitors, anticoagulants, certain antidepressants, and agents that lower sex steroids or block their effects. In general, the more risk factors present, the greater the risk of fracture.
According to the National Osteoporosis Foundation (NOF) guidelines for preventing and treating osteoporosis, “all postmenopausal women and men age 50 and older should be evaluated clinically for osteoporosis risk to determine the need for bone mineral density (BMD) testing.” The NCCN Clinical Practice Guidelines in Oncology: Breast Cancer and Prostate Cancer (to view the most recent version of these guidelines, visit the NCCN Web site at www.nccn.org) recommend that patients for whom planned therapy includes medications that lower sex steroids should be evaluated at baseline and with periodic follow-up dual-energy x-ray absorptiometry (DEXA) scans to evaluate risk of fracture.5,6 Osteoporosis risk factors unique to or commonly found in cancer patients are chemotherapy-induced menopause, gonadotropin-releasing hormone (GnRH) suppression of gonadal function, anti-estrogen and anti-androgen therapies, glucocorticoids (used predominantly in treatment of hematologic malignancies or as supportive agents in solid tumors), inadequate calcium intake, vitamin D deficiency, and inadequate exercise.
Bone health is currently assessed using BMD levels. Bone strength is defined by BMD and bone quality. The U.S. Preventive Service Task Force clinical guidelines recommend BMD screening for all women 65 years and older and for women aged 60 to 64 who are at high risk for bone loss.7 ASCO guidelines agree with those and further suggest BMD screening for women with breast cancer who have high risk factors such as family history of fractures, body weight less than 70 kg, and prior non-traumatic fracture, for postmenopausal women of any age receiving aroma-tase inhibitor (AI) therapy, and for premenopausal women with therapy-induced ovarian failure.8 For men, the NOF recommends BMD testing for men 70 years and older. The NCCN Clinical Practice Guidelines recommend screening for osteoporosis in men on androgen-deprivation therapy (ADT) as outlined in NOF guidelines.5
The WHO defines osteoporosis by BMD. Technology widely used to confirm the diagnosis of osteoporosis is DEXA measurement of the hip and spine. DEXA is generally considered the “gold standard” method of measuring BMD for diagnosing osteoporosis and monitoring the effects of osteoporosis therapy. BMD may be expressed in absolute terms, in grams per square centimeter (g/cm2), and in relative terms as the difference in standard deviation (SD) from expected BMD for the patient's age and sex (Z-score) or from that of “young normal” adults of the same sex (T-score). In 1994, WHO established diagnostic criteria for osteoporosis, based on T scores.9 Under the WHO criteria, BMD within 1 SD of a “young normal” adult (T-score of ≥ −1.0) is considered normal, 1.0 to 2.5 SD below (T-score of −1.0 to −2.5) constitutes low bone mass or osteopenia, and 2.5 SD or more below (T-score ≤ −2.5) constitutes osteoporosis.
Although DEXA measurement is considered the gold standard, its limitations must also be recognized. For example, results can vary with the machine used, different underlying dual-energy methods used, differences in calibration, different detectors used, different reference standards, and also by anatomic site (e.g., hip vs. vertebrae). These factors support the recommendation that serial BMD monitoring should be performed on the same piece of equipment using the same reference standards. In addition, osteoarthritis or calcification of the aorta, if present, may lead to falsely high BMD. DEXA scan exposes patients to low levels of radiation, equal to one-tenth of a chest x-ray.
WHO Fracture Risk Algorithm
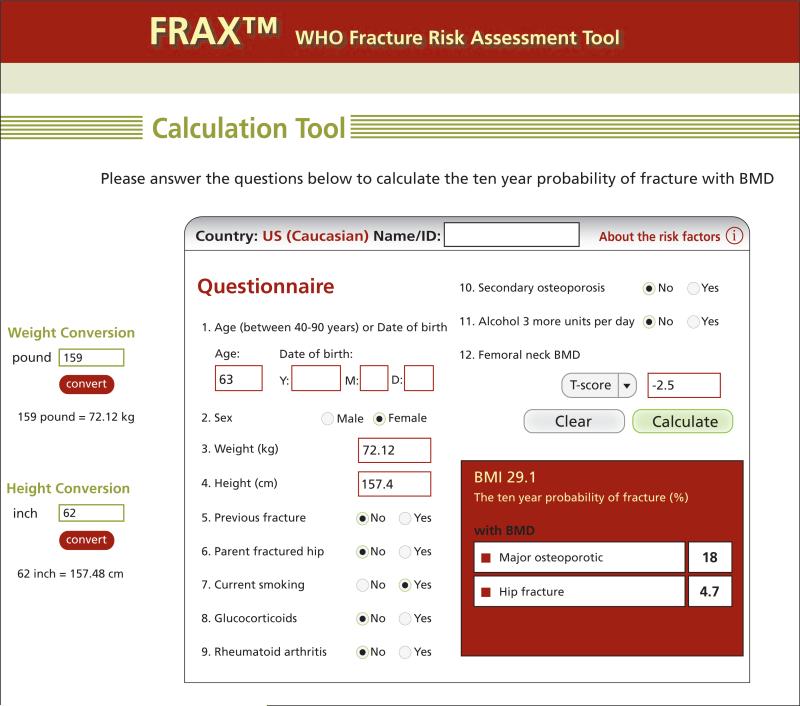
Recently, WHO developed a fracture risk algorithm (FRAX), a risk assessment tool that combines both bone density measurements and clinical factors in assessing fracture risk (available at www.shef.ac.uk/FRAX/).10 This tool provides an estimate of the 10-year probability of hip fracture and major osteoporotic fracture based on age, sex, clinical risk factors, femoral neck BMD (T-score), and other information. FRAX models were developed from studying population-based cohorts in Europe, North America, Asia, and Australia. It has separate calculation tools for U.S. white, black, Asian, and Hispanic populations.
FRAX analysis is optimized for postmenopausal women and men aged 50 and older and is intended to predict risk for patients previously untreated for bone loss. It includes a “secondary osteoporosis” risk modifier that can be used to factor hypogonadism and premature menopause into the fracture risk. The WHO FRAX tool provides an individualized 10-year fracture risk estimate that can be used to guide intervention and therapy. An example of the analysis results is shown in Figure 1. Current Medicare guidelines recommend therapeutic intervention for patients with a 10-year FRAX risk of 3% for hip fractures and more than 20% for all major fractures. The NCCN Bone Health in Cancer Care Task Force recommends using the FRAX algorithm in the baseline assessment of all cancer patients at increased risk for bone loss and fracture because of cancer or -related therapy. FRAX calculations can be performed with or without BMD data, making it useful in situations in which bone density is unavailable.
Figure 1.
Example (reproduction) fracture risk analysis results for a white woman in the United States using the FRAX™ online tool. This algorithm incorporates bone density and other risk factors into a comprehensive estimate of fracture risk. The tool is available at www.shef.ac.uk/FRAX/.
Abbreviations: BMD, bone mineral density; FRAX™, Fracture Risk Assessment Algorithm.
Other Techniques for Assessing Bone Health
Bone Turnover Markers
Biochemical markers of bone remodeling can be broadly subdivided into markers of bone formation and bone resorption. Bone formation markers include osteocalcin, bone-specific alkaline phosphatase (BAP), and N-terminal and C-terminal pro-peptides of type I procollagen (P1NP, P1CP).11 Bone resorption markers include N-terminal and C-terminal cross-linking telopeptides of type I collagen (NTX and CTX); both can be detected in serum or urine using enzyme-linked immunosorbent assay or chemiluminescence based techniques.
Bone biomarkers can be used to assess risk of fracture independent of age, BMD, and prior fracture. Several cohort studies have shown that bone markers such as CTX and BAP are predictive of vertebral and hip fractures,12–14 and bone turnover markers may improve the diagnosis of women at high risk of fracture. However, bone metabolism markers cannot be translated into a patient-specific estimate of fracture risk. Therefore, bone markers are not widely used clinically for assessing osteoporosis. In addition, many physiologic factors affect bone marker levels. For example, bone markers can vary with bed rest, seasonal changes, menstrual cycle, and time of day, and are affected by comorbid conditions such as kidney or liver disease, leading to marked variability ranging from 15% to 40%. Studies have shown that overnight fasting significantly reduces variation for CTX, and, for the urinary NTX marker, obtaining a second morning-void urine sample reduces variability caused by diurnal changes in bone resorption.15,16 Additionally, studies have shown lower physiologic variability for serum markers compared with urine markers.17,18
Vertebral Fractures
Vertebral fractures are the most common osteoporotic fractures.19 Independent of BMD and other clinical risk factors, existing vertebral fractures are a strong predictor of future vertebral and other fractures. Studies by Black et al.20 and Melton et al.21 show that women with vertebral fractures have a 5-fold increased risk of a new vertebral fracture and a 2-fold increased risk of hip fracture.20,21 This risk of sustaining subsequent fractures is grossly under-recognized.
Current DEXA technology from several manufacturers incorporates software that permits lateral vertebral assessment, a technique that provides crisp lateral images of the thoracic and lumbar spine with relatively low radiation exposure.22 Patients with evidence of an existing vertebral fracture should be carefully assessed for all factors affecting future fracture risk and risk intervention strategies, including possible therapeutic intervention, should be undertaken.
In summary, all patients who begin cancer therapy that induces early menopause, reduces sex steroids or interferes with their action, or includes glucocorticoids should undergo assessment of bone loss risk and subsequent risk for osteoporosis and fracture. Risk for osteoporotic fractures can be considered a potential toxicity for a wide array of cancer therapies. Obtaining bone-related history and physical and using the FRAX calculator to assess overall fracture risk are recommended to estimate fracture risk. Cancer patients with elevated fracture risk should be evaluated every 24 months to monitor the impact of cancer treatment on bone mass. In selected circumstances, such as when bone loss risks have changed significantly or a major therapeutic intervention has been undertaken, 12-month follow-up DEXA is reasonable. Counseling regarding modifiable risk factors for osteoporosis, including increasing calcium and vitamin D intake and physical activity and reducing smoking and alcohol use, should be provided to all patients. Therapeutic intervention should be strongly considered in patients with a BMD below a T-score of −2.0, particularly in those with additional risk factors for fragility fracture.
Treatment Options for Osteoporosis
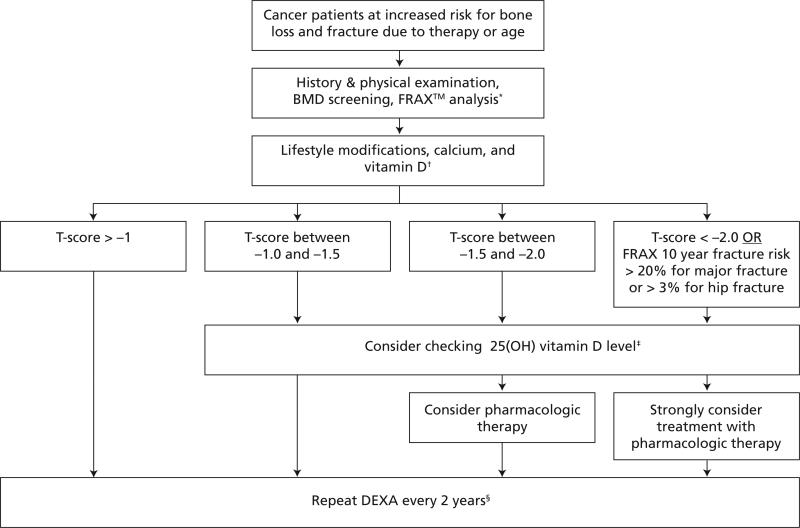
Initial strategies for preventing bone loss and osteoporosis include lifestyle behaviors such as performing regular weight-bearing, muscle-strengthening, and balance exercises, avoiding tobacco use, and limiting alcohol intake. Ensuring an adequate intake of calcium and vitamin D is important. In addition to lifestyle and nutrition interventions, pharmacologic options should be considered for patients at high risk for bone loss or fracture. Available agents fall into 2 categories: antiresorptive agents (bisphosphonates, selective estrogen receptor modulators [SERMs], estrogen, calcitonin) and anabolic agents (parathyroid hormone). An algorithm for the screening and management of cancer patients at increased risk for bone loss and fracture is shown in Figure 2.
Figure 2.
Algorithm for management of bone health of cancer patients in the United States.
Abbreviations: 25(OH), serum hydroxy; BMD, bone mineral density; DEXA, dual-energy x-ray absorptiometry; FRAX™, Fracture Risk Assessment Algorithm.
*See section on “Screening and Detection of Osteoporosis” for FRAX™ algorithm.
†See section on “Update on Treatment Options for Osteoporosis” for lifestyle modifications and calcium and vitamin D repletion.
‡See section on “Update on Treatment Options for Osteoporosis” to correct vitamin D deficiency.
§In selected cases, longer or shorter intervals may be considered. If a major change in patient risk factors or a major intervention occurs, repeating DEXA scan at one year is reasonable.
Note: In addition to monitoring changes in BMD, the oncologist should obtain a lateral thoracic and lumbar x-ray of the spine to determine if vertebral compression deformities are present if there is: 1) a historical height loss > 4 cm (1.6 in) or a prospective height loss > 2 cm (0.8 in), or 2) complaint of acute back pain. Consider referral to a bone health specialist if loss of vertebral height > 20% is present.
Lifestyle Modifications
An excellent patient resource for bone health and lifestyle behavior is the NOF Web site.23 Physical activity can improve muscle mass and strength, balance, and bone strength. Weight-bearing exercise has been associated with a decreased risk of hip fractures, probably due to a reduction in fall risk and also through modest effects on preservation of bone density.24–27 Tai chi, physical therapy, and dancing are considered good options to improve balance and prevent falls. Adults should aim for at least 30 minutes of moderate physical activity daily (either in 1 continuous session or in a number of shorter bursts). This activity can include a mix of weight-bearing, strength training, and balance training exercises. Fracture risk reduction should also include strategies to reduce falls, such as checking for and correcting vision and hearing problems, evaluating for neurologic problems, reviewing prescription medications for side effects that may affect balance, and improving at-home safety. A home safety checklist can be found on the NOF website.28 Wearing hip protectors may prevent hip fracture in the event of a fall29,30 and can be considered for patients who have a high risk for falling.
Behaviors such as tobacco abuse and excessive alcohol consumption are associated with a variety of ill health outcomes, including increased risk for osteoporosis and fracture. Counseling patients on these topics is important on many levels and should not be overlooked. Recommended interventions after counseling should be individualized.
Calcium and Vitamin D Supplementation
Adequate intake of calcium and vitamin D is critical to bone mineralization. Some, but not all, randomized studies have shown calcium and vitamin D supplementation to decrease fracture risk.31,32 However, many of the negative studies have been hampered by poor compliance with supplements or suboptimal supplementation.
The NOF,23 National Institutes of Health Office of Dietary Supplements,33,34 and the Surgeon General's report on osteoporosis35 recommend a total daily calcium intake (from food and supplements) of at least 1000 mg per day for individuals under 50 years of age without major osteoporosis risk factors, and at least 1200 mg per day for those older than 50 years. Calcium supplements are available as calcium carbonate or calcium citrate. Calcium carbonate requires gastric acid for optimal absorption and should be taken with food. Calcium citrate, which does not require gastric acid for absorption, can be taken between meals and is the preferred option for patients receiving proton pump inhibitors. For optimal absorption, calcium supplements should be taken in divided doses of no more than 600 mg at one time.
The safe upper limit of calcium set by the National Academy of Sciences is 2500 mg per day.36 For patients at risk for nephrolithiasis, increasing dietary calcium in food has been associated with lower risk for nephrolithiasis compared with calcium supplements. Measurement of urinary calcium excretion and other markers of lithogenic risk is prudent in patients with a history of calcium nephrolithiasis.37
Vitamin D plays a major role in gastrointestinal calcium absorption and is essential for maintaining normal bone mineralization. It is naturally present in very few foods, but is added as a supplement to some food products and is available as a dietary supplement. It is also produced endogenously when ultraviolet rays strike the skin, triggering vitamin D synthesis. Use of sun block, recommended to reduce the risk of skin cancer, leads to substantial reduction of cutaneous vitamin D synthesis. Vitamin D supplementation increases BMD38 and reduces the risk of falls (possibly by impacting balance).39 The NOF recommends 800 to 1,000 international units (IU) of vitamin D per day for adults aged 50 and older.
Serum 25 hydroxy vitamin D [25(OH) D] levels are the best indicator of vitamin D status, but considerable discussion surrounds the serum concentrations of 25(OH) D associated with deficiency, adequacy for bone health, and optimal overall health. For bone health, vitamin D should ideally be supplemented in amounts sufficient to bring serum 25(OH) D levels to 30 ng/ml (75 nmol/L) or higher.40 In supplements, vitamin D is available in 2 forms: D2 (ergocalciferol) and D3 (cholecalciferol). These 2 forms are metabolized differently, and vitamin D3 could be more effective in raising 25(OH) D concentrations and maintaining those levels for a longer time when longer dosing intervals are employed.41,42 No difference in maintaining 25(OH) D levels was found when daily dosing was studied.43
One common regimen for patients with serum 25(OH) D levels below 30 ng/mL is prescription vitamin D (ergocalciferol) 50,000 IU weekly for 8 weeks, followed by a recheck of the serum 25(OH) D level, with subsequent dosing based on the results.40 For patients with 25(OH) D levels between 20 and 30, an alternative suggested by the panel is adding 1000 IU over the counter vitamin D2 or D3 per day to the patient's current intake and rechecking the level in 3 months. Vitamin D toxicity (hypercalcemia, hyperphosphatemia, and activation of bone resorption) is uncommon but may occur with daily doses of more than 50,000 IU per day that produce 25(OH) D levels larger than 150 ng/mL.40
Therefore, current expert opinion on supplementation for adults older than age 50 is 1200 mg of calcium (from all sources) and 800 to 1000 IU of vitamin D daily. The NCCN Bone Health in Cancer Care Task Force also recommends these ranges for younger patients at risk for cancer treatment–associated bone loss. A caveat to the vitamin D intake recommendation is that many patients need more than the recommended amount and should be repleted based on serum 25(OH) D level.
Pharmacologic Agents
The United States FDA–approved pharmacologic options for preventing or treating osteoporosis include bisphosphonates, SERMs, estrogen, calcitonin, and teriparatide (Table 1). For FDA approval, a drug must show that it reduces the risk of vertebral fractures; non-vertebral anti-fracture efficacy is not a requirement.44
Table 1.
FDA Approved Medications for Osteoporosis Prevention and Treatment
| Dosing |
Mechanism; Class |
|
|---|---|---|
| Estrogen | See Kalantaridou et al.221 | Antiresorptive; steroid hormone |
| Calcitonin | 200 IU intranasal daily | Antiresorptive; peptide hormone |
| Alendronate | 35 mg orally weekly | Antiresorptive; bisphosphonate |
| 70 mg orally weekly; with or without vitamin D: 2800, 5600 IU | ||
| Risedronate | 35 mg orally weekly | Antiresorptive; bisphosphonate |
| 150 mg orally monthly | ||
| Ibandronate | 150 mg orally monthly, or 3 mg intravenous push every 3 months | Antiresorptive; bisphosphonate |
| Zoledronic acid | 5 mg intravenous yearly (over 15 minutes) | Antiresorptive; bisphosphonate |
| Raloxifene | 60 mg orally daily | Antiresorptive; selective estrogen receptor modulator |
| Teriparatide (recombinant parathyroid hormone) | 20 mg subcutaneous daily for a maximum of 2 years | Anabolic; peptide hormone fragment |
Bisphosphonates
Bisphosphonates decrease bone resorption and increase mineralization by inhibiting osteoclast activity.45 Bisphosphonates approved by the FDA for postmenopausal osteoporosis are alendronate, ibandronate, risedronate, and zoledronic acid. All except ibandronate are approved in both men as well as women. Because compliance is a significant problem with daily oral bisphospho-nate dosing, a trend toward less frequent oral dosing and intravenous options have emerged (Table 1). Generally, oral formulations (alendronate, ibandronate, and risedronate) are considered first line. Use of intravenous bisphosphonates (ibandronate or zoledronic acid) may be considered, particularly for patients who cannot tolerate the oral formulations.
Several bisphosphonates were studied in the context of cancer treatment–induced bone loss. Some settings in which bisphosphonates have shown efficacy at preserving BMD changes during anti-cancer treatment include breast cancer patients receiving AIs or those with chemotherapy-induced menopause or other forms of ovarian suppression, prostate cancer patients undergoing ADT, and hematologic malignancy patients undergoing stem cell transplantation.46–49 Details of some of these trials are presented in subsequent sections of this report.
Because of potential gastrointestinal toxicities, oral bisphosphonates should be avoided in patients with esophageal emptying disorders and those who cannot sit upright; these patients are at high risk for pill esophagitis.50 Intravenous bisphosphonates are generally not recommended in patients with creatinine clearance less than 30 mL/min because they can increase serum creatinine and may, rarely, cause acute renal failure.51 The risk for renal insufficiency appears related to dose, infusion rate, and hydration. Oral bisphosphonates appear to have better renal safety in patients with lower creatinine clearance.52 Calcium intake and vitamin D status should be optimized when starting any bisphosphonate. Vitamin D deficiency should be corrected before treating with intravenous bisphosphonates because hypocalcemia has been reported in patients with unrecognized vitamin D deficiency.
Osteonecrosis of the jaw (ONJ) has emerged as a complication of bisphosphonate treatment. The etiology is unknown, and it occurs in 1% to 10% of patients with intravenous bisphosphonate used at the higher doses for treating metastatic bone disease.53,54 The incidence of ONJ with bisphosphonate to treat osteoporosis (or prevent cancer treatment–induced bone loss) appears to be low (< 10,000–100,000),53 accounting for roughly 4% of reported ONJ cases, compared with 95% of cases reported with intravenous bisphosphonate in patients with bone metastases.53,54 In cancer therapy–induced bone loss prevention trials with zoledronic acid (4 mg every 6 months), no cases of ONJ have been reported to date. However, the fact that no previous trial of oral bisphosphonates reported ONJ cases is important to recognize. This highlights the difficulty of identifying low frequency but serious effects in clinical trials.55,56 Risk factors associated with ONJ include dental extractions. Therefore, dental examination and prophylactic measures should be considered before starting bisphosphonate therapy. Patients should also be advised against unnecessary invasive oral surgery while on bisphosphonate therapy.57 The ongoing SWOG S0702 trial, involving 7000 patients with metastatic bone disease treated with zoledronic acid, is designed to prospectively investigate risk factors, incidence, outcome, and mechanisms associated with ONJ.
In a phase III fracture prevention trial of yearly zoledronic acid in women with postmenopausal osteoporosis, Black et al.20 reported a higher risk of serious atrial fibrillation for patients receiving zoledronic acid 5 mg yearly when compared with placebo (1.3% vs. 0.4%). This serious adverse event was not more common in other studies of osteoporotic patients in which zoledronic acid was dosed 5 mg yearly.58 Additionally, in studies in which 4 mg of zoledronic acid was administered every 3 to 4 weeks for preventing skeletal-related events in patients with skeletal malignant involvement, no increase in atrial fibrillation was seen. In response to concerns regarding atrial fibrillation, the FDA released an early communication letter stating that evidence was not strong enough to associate atrial fibrillation with bisphosphonate use and that further investigation is warranted before making any conclusions.59
During the past 2 years, a small but concerning number of cases of subtrochanteric hip fractures have developed in patients on long-term bisphosphonate therapy. Although a causal relationship has not been established with certainty, the unusual nature of the horizontal fractures that occur at angles perpendicular to the long axis of the femur have raised concerns about long-term use of bisphosphonates.60,61
Patients treated with zoledronic acid may also experience acute phase reactions. This physiologic reaction is associated with fever and flu-like symptoms including myalgia, arthralgias, fever, fatigue, and nausea. It occurs in roughly 30% of patients after the initial dose and may persist for a few days. Acute phase reaction is not common with subsequent dosing.62
Estrogen/Hormonal Therapy
Estrogen is an antiresorptive agent with proven anti-fracture efficacy as shown in the Women's Health Initiative study. Estrogen therapy alone or combined estrogen and progesterone were associated with a 33% to 34% reduction in hip fracture.49 The same study reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis in postmenopausal women.49 Because of these risks, the FDA recommends that “estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.”59 Estrogen replacement therapy is highly controversial in women with a history of breast cancer, including those who had hormone receptor negative disease, due to the increased risk of breast cancer recurrence.63 In young patients with cancers other than breast cancer who experience chemotherapy-induced premature menopause, estrogen may be a treatment option both for menopausal symptoms and bone health. Data in young women with spontaneous premature ovarian failure argues against an increased risk of breast cancer or other adverse events with full replacement doses.64 Therefore, in women with chemotherapy-induced menopause who are not at increased risk for breast cancer, replacement of estrogen/progesterone until the normal age of menopause is not likely to produce a higher risk for adverse events seen in the WHI study and may be beneficial for bone health.
SERMs
Although tamoxifen has shown favorable impact on bone density in postmenopausal breast cancer patients, raloxifene is currently the only SERM that is FDA approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene is a less potent antiresorptive agent than bisphosphonates. Raloxifene has been shown to decrease the incidence of vertebral fracture; however, randomized studies have failed to document any benefit against non-vertebral or hip fractures.65
Raloxifene, unlike estrogen, is not associated with an increase in myocardial infarction. Raloxifene has been associated with an increased risk of fatal stroke (hazard ratio [HR], 1.49; absolute risk increase, 0.7/1000), and venous thromboembolism (HR, 1.44; absolute risk increase, 1.3/1000) in the RUTH (Raloxifene Use for The Heart) trial of postmenopausal women with a history of coronary artery disease or cardiovascular risk factors.66 A decreased risk of invasive breast cancer was shown in the RUTH trial, confirming previous findings from an osteoporosis treatment trial67 and also from a trial of postmenopausal women at high risk for breast cancer.68 Hot flushes, leg cramps, peripheral edema, and gall-bladder disease are more common with raloxifene than with placebo.66,69–71 The hot flushes induced by raloxifene may be accentuated in early menopause. Raloxifene use is not indicated in premenopausal women at high risk for breast cancer; it has resulted in decreased BMD in clinical trials.72
The efficacy of raloxifene in combination with an AI for breast cancer remains unknown. In the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial, the concurrent use of tamoxifen (a SERM) and anastrozole (an AI) had less anti-tumor efficacy than anastrozole alone.73 Thus, combined AI and SERM should not be used outside a clinical trial. Newer SERMs may have potential for use concomitant with AIs, although the effects on bone are unknown.74 For women with a history of breast cancer, bisphosphonates are probably the best choice for preventing bone loss or treating established osteoporosis.
Parathyroid Hormone(1-34, Teriparatide)
Recombinant parathyroid hormone (PTH) 1-34 or teriparatide is the first anabolic agent approved for treatment of postmenopausal osteoporosis. It has been shown to reduce the incidence of vertebral and non-vertebral fractures. It is administered daily by subcutaneous injection for 2 years. Because of the potential increased risk for osteosarcoma, it is contraindicated in patients with increased baseline risk of osteosarcoma such as those with Paget's disease of bone, open epiphyses, or prior radiation therapy involving the skeleton (which includes many patients with cancer). Furthermore, teriparatide is not indicated in patients with bone metastases, including those who may have micrometastatic or occult disease. A recent study involving 200,000 patients shows no significant difference in incidence of osteosarcoma between the treated group and the general population.75 Although no data in patients with cancer exist, teriparatide is best avoided in patients with a history of malignancy prone to metastasize to bone.
The drug works to sequentially increase bone resorption followed by bone formation. This marked increase in bone turnover may be favorable to propagation of microscopic bone metastases76 through liberation of bone-derived growth factors and cytokines, and potentially through direct anabolic effects on tumor cells. However, in cases of severe osteoporosis with fractures occurring on bisphosphonate therapy, the benefits may outweigh these theoretical risks. In such patients with a remote history of cancer, teriparatide could be cautiously considered.77
Receptor Activator of Nuclear Factor kB Ligand Inhibition
Receptor activator of nuclear factor kB ligand (RANK-L) is an essential cytokine expressed on the surface of preosteoblastic and osteoblastic cells. RANK-L activates its receptor RANK, which is expressed on osteoclasts and their precursors, ultimately promoting osteoclast formation and activation. Denosumab is a human monoclonal antibody to RANK-L that blocks osteoclast differentiation, proliferation, and function. A 3-year, phase III trial of 7868 postmenopausal women with osteoporosis randomized participants to receive either 60 mg subcutaneous denosumab or placebo every 6 months.78 At the end of 36 months, treatment with denosumab showed a statistically significant reduction in the incidence of new vertebral fractures, new non-vertebral fractures, and hip fractures compared with placebo treatment. No serious adverse events reported with denosumab were significantly increased relative to placebo. A randomized phase III non-inferiority trial compared denosumab with alendronate in 1189 postmenopausal women.79 At 12 months, denosumab produced a significantly greater increase in BMD at the hip (3.5% vs. 2.6% for alendronate; P < .0001) and greater suppression of bone turnover markers (CTX-I and P1NP). Denosumab appears to be a promising new agent that may receive FDA approval for managing postmenopausal osteoporosis. Denosumab is currently being evaluated in patients previously treated with bisphosphonates80 and in patients with cancer (both for prevention of cancer therapy–induced bone loss and skeletal-related events).
Role of Bone Biomarkers
Bone biomarkers are useful for monitoring patient response and effectiveness of antiresorptive therapies. Changes in bone turnover markers can reflect response to antiresorptive therapy in weeks to months rather than the months to years required for changes in BMD. This may be helpful in avoiding the time and expense of a potentially ineffective therapy or detecting of nonadherence with therapy, permitting the earlier start of potentially more-effective therapy.
Chestnut et al.81 compared changes in urinary NTX levels and bone mass in 109 postmenopausal women undergoing hormone replacement therapy. Patients with the highest quartile for baseline levels of NTX and those with decreasing levels over 6 months also had the largest percentage gain in BMD. Ravn et al.82 compared short-term changes (3 to 12 months) in urine CTX and other biomarkers to changes in BMD measured after 2 years in postmenopausal patients receiving alendronate. A 50% decrease in CTX showed an 87% positive predictive value for prevention of bone loss.
Treatment Duration
No published guidelines are available on duration of antiresorptive therapy and whether to institute drug holidays. Treatment with alendronate for 10 years was shown to be well tolerated and with a positive impact on bone density versus placebo.83 The results of the Fracture Intervention Trial Long-term Extension (FLEX) study suggest that postmenopausal women with a history of alendronate use for 5 years who discontinued it for 5 subsequent years had a modest absolute increase in clinical vertebral fractures (5.3% vs 2.4%) but no difference in morphometric vertebral fractures or non-vertebral fractures.84 Patients at very high fracture risk may benefit by continuing beyond 5 years.84 In the setting of continued risk for cancer treatment–induced bone loss, such as AI use for more than 5 years, no data on duration are available. Factors to consider for duration of anti-osteoporosis therapy include BMD, response to therapy, and risk factors for continued bone loss or fracture.
Impact of Therapy-Induced Ovarian Failure on Bone Health
Nearly all premenopausal women with breast cancer receiving standard chemotherapy experience at least temporary amenorrhea, and 50% to more than 70% will have permanent ovarian failure or early meno-pause.85–87 Development of chemotherapy-induced ovarian failure is considered a high risk factor for bone loss.8 The most important factor for predicting premature menopause or ovarian failure is age at time of chemotherapy treatment; greater risk is seen with increasing age.88 Additional factors include cumulative dose and duration of alkylating agents, such as cyclophosphamide.86,87 One of the challenges in studying this issue is the lack of standard definition for chemotherapy-induced ovarian failure in the literature. For example, some studies define it as at least 3 to 6 months of amenorrhea. However, distinguishing between temporary amenorrhea that will reverse and permanent ovarian failure is important, because bone loss is of greatest magnitude with ovarian failure.88,89
Several small studies have identified additional risk factors for ovarian failure independent of age: baseline BMD before adjuvant chemotherapy might predict individual risk of developing ovarian failure. In a multivariate analysis of 49 premenopausal patients undergoing adjuvant chemotherapy, a higher baseline BMD increased the risk of ovarian failure.90
Studies have also shown accelerated bone loss as a consequence of ovarian failure after adjuvant chemotherapy.90–96 In a prospective study by Shapiro et al.90 involving 49 young women with breast cancer receiving adjuvant chemotherapy, 35 women experienced chemotherapy-induced ovarian failure. In patients with ovarian failure, a highly significant bone loss was seen in the lumbar spine by 6 months, but no significant change was seen in patients who retained ovarian function. Bone loss associated with chemotherapy-induced menopause is several-fold higher than that seen with natural menopause or AI therapy-induced bone loss in postmenopausal women.91,97–99
Several studies have reported that bisphosphonates, including clodronate and risedronate, attenuate the bone loss associated with chemotherapy-related ovarian failure.53,93–95 Zoledronic acid was tested in the CALGB 79809 study, in which premenopausal patients beginning adjuvant chemotherapy were randomized between early zoledronic acid (4 mg, every 3 months) or delayed zoledronic acid (given 1 year after adjuvant chemotherapy). The primary end point was change in lumbar spine BMD. Density was preserved in patients treated with early zoledronic acid at 12 months, compared with a 6.6% loss reported for the control group (delayed group). Similarly, in a randomized placebo-controlled trial of 4 mg of zoledronic every 3 months for 1 year, BMD was preserved in the lumbar spine and hip.100
Bisphosphonates are also effective for minimizing loss of BMD in women receiving ovarian suppression with GnRH.101,102 In the Austrian Breast and Colorectal Cancer Study Group (ABCSG)-12 trial, premenopausal breast cancer patients receiving endocrine treatment including a GnRH agonist were randomized to 4 mg of zoledronic acid treatment or not every 6 months for 3 years. The addition of the bisphosphonate prevented bone loss in both the lumbar spine and hip. Additionally, a recent report noted fewer breast cancer recurrences with the addition of zoledronic acid.102
Although studies showing the ability of bisphosphonates to preserve BMD in young women with cancer treatment–related ovarian failure are encouraging, no study to date has shown an impact on the clinically relevant endpoint of fractures.
AI-Induced Bone Loss
As reviewed in the NCCN Breast Cancer Guidelines (to view the most recent version of these guidelines, visit the NCCN Web site at www.nccn.org),6 AIs play an important role in the treatment of estrogen or progesterone receptor (PR)–positive breast cancers in postmenopausal women in both the adjuvant and metastatic settings. Randomized studies of AIs compared with or after tamoxifen therapy have led to the widespread use of AIs as adjuvant therapy in postmenopausal, estrogen receptor (ER)–positive breast cancer.102 AIs act by inhibiting aromatase enzyme involved in conversion of the androgen precursors to estrogen. Lower estrogen levels are associated with increased bone resorption and fracture risk. AIs cause a rapid decline of circulating estrogen levels, leading to bone loss,103,104 and are divided into steroidal (exemestane) and non-steroidal (letrozole or anastrazole). Exemestane binds irreversibly to the catalytic site of aromatase, whereas letrozole and anastrozole bind reversibly to the heme group of the enzyme. The NCCN Breast Cancer Guidelines Panel considers the 3 selective AIs (anastrozole, letrozole, exemestane) to be similar in antitumor activity and toxicity profiles.
Several reviews of AIs and their impact on bone health were published recently.98,105–108 In the ATAC trial,109 the annual incidence of fractures was higher in women receiving anastrozole (2.93%) compared with tamoxifen (1.9%) throughout 5 years of treatment. After treatment, the fracture rates of both groups were similar, suggesting that AI-related fracture rates decrease after treatment.
The Breast International Group (BIG) 1-98 trial compared adjuvant therapy with tamoxifen to letrozole.110 As with the ATAC trial, increased incidence of bone fracture was seen in patients on AI (8.6% vs. 5.8% at 51 months). The Intergroup Exemestane Study (IES) compared adjuvant tamoxifen for 5 years with initial adjuvant tamoxifen followed by exemestane.111 Because exemestane is a steroidal AI with androgenic properties, researchers hypothesized that it might have less impact on bone loss and fractures than anastrozole and letrozole. However, the incidence of fracture at 58 months was significantly higher (7%) in the exemestane group than in the tamoxifen group (5%).112
The recently closed MA-27 trial randomizing postmenopausal breast cancer patients to either adjuvant exemestane or anastrozole will hopefully clarify whether the androgenic nature of exemestane results in less impact on bone density.113 The MA-17 trial compared an additional 5 years of letrozole versus placebo after an initial 5 years of adjuvant tamoxifen.114 The design of this trial allowed for a more direct look at the effect of AIs on bone without the confounding factor of tamoxifen present in the comparator arm. The incidence of a new diagnosis of osteoporosis was 5.8% in the letrozole group compared with 4.5% in the placebo group (P = .07), with similar fracture rates in both groups. These results suggest that the difference in bone loss and fracture rates in the adjuvant studies may be primarily due to a bone protective effect of tamoxifen as opposed to a bone destructive effect of the AIs.
Several of the large adjuvant trials have evaluated bone loss and fractures in more detailed breakout studies of women receiving AI therapy.98,106,115 The ATAC study evaluated risk factors for fractures in patients on AI.109 Older age was associated with higher risk. Additionally, a prospective study of the ERs trial assessed BMD changes in postmenopausal women. Among anastrozole-treated patients, median BMD decreased from baseline to 5 years in lumbar spine (−6.08%) and total hip (−7.24%) compared with the tamoxifen group (lumbar spine, +2.77%; total hip, +0.74%). Importantly, no patients with normal BMD at baseline became osteoporotic at 5 years.
Role of Bisphosphonates
Several studies have analyzed the impact of bisphosphonate therapy on maintaining bone density in patients on AI treatment. Two trials examined the effects of oral bisphosphonates in patients receiving anastrozole therapy. The SABRE (Study of Anastrozole with the Biophosphonate Risedronate) trial116 was an open-label intervention study in which all patients received anastrozole and were assigned to a bisphosphonate treatment group based on T-score. Patients with a low-risk T score (> −1) received no intervention; patients with a T score greater than −2 received risedronate; and patients with a T score between −1 and −2 were randomized to risedronate or placebo. For patients at low risk, bone loss during short-term follow-up was minimal. For other patients, risedronate therapy at doses established for preventing and treating osteoporosis resulted in favorable effects on BMD over 24 months.117
The ARIBON study evaluated the impact of ibandronate on BMD in postmenopausal, early stage breast cancer patients receiving anastrozole.118 Patients with a T score greater than −1 received no intervention; patients with a T score of −1.0 to −2.5 were randomized to ibandronate or placebo; patients with a T score less than −2.5 received ibandronate treatment. The addition of ibandronate to anastrozole led to a significant increase in BMD at the spine and hip after 1 year, which was maintained for 2 years.
The Zometa-Femara Adjuvant Synergy Trials (Z-Fast and ZO-Fast) were designed to compare effects of upfront versus “delayed” initiation of an intravenous bisphosphonate, zoledronic acid (4 mg intravenously every 6 months), in preventing AI-associated bone loss.119,120 All patients received adjuvant letrozole. The “delayed therapy” group received zoledronic acid only when bone loss became clinically significant or a fragility fracture occurred (10% of patients in the study). A pooled analysis of approximately 1600 patients was performed and showed that upfront use of zoledronic acid was associated with preservation of BMD.55 These studies suggest that both oral and intravenous bisphosphonates can mitigate the bone loss effects of AIs, although none of these trials have shown a reduction in fractures. No clinical trials have directly compared oral versus intravenous bisphosphonates in this setting. Importantly, health care professionals should recognize that AIs do cause bone loss. However, bone density monitoring and intervention strategies should be individualized for patients on AIs, with drug therapy reserved for those at greatest risk.
Role of RANK-L Inhibition
Ellis et al.121 conducted a randomized, double-blind, placebo-controlled phase III trial evaluating the effect of denosumab in patients receiving adjuvant AI therapy. The primary end point was the percentage change from baseline in lumbar spine BMD. Patients with early stage (nonmetastatic), hormone receptor–positive breast cancer were randomized to either denosumab, 60 mg, or placebo every 6 months for a total of 4 doses while receiving AI therapy. At 12 and 24 months, lumbar spine BMD increased by 5.5% and 7.6%, respectively, in the denosumab group compared with placebo (P < .0001). After 24 months, the increase in BMD in the total hip, femoral neck, trochanter, and radius was 4.7%, 3.5%, 5.9%, and 6.1%, respectively.
Management of Bone Health in Prostate Cancer
Prostate cancer is the most commonly diagnosed malignancy in American men. Because prostate cancer growth is driven by androgen hormones, ADT, either by orchiectomy or using GnRH agonists, is commonly used for treatment. According to the NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer (available at www.nccn.org), long-term ADT is used for locally advanced, recurrent, and metastatic prostate cancer.5 Osteoporosis and greater fracture risk have emerged as important long-term adverse events in ADT.
The term ADT is used because the intended therapeutic effect is lower testosterone levels. Because estradiol is produced from testosterone by aromatase activity, ADT also reduces estradiol levels.122,123 A compelling body of data suggest that estradiol has important effects in men.124 Selective deficiency of estradiol, caused by genetic deficiency of aromatase or inactivation of the ER, produces profound osteoporosis in the presence of normal testosterone levels. Estrogen receptors are expressed in osteoclasts and osteoblasts. In population-based studies of older men, low estradiol levels are associated with low bone mass and greater fracture incidence.125 In these studies, low estradiol levels are more closely associated with fracture incidence than low testosterone levels.
A number of studies have associated ADT with increased fracture risk. A Medicare claims-based study characterized the relationship between GnRH agonists and risk for clinical fractures.126 Men (n = 10,617) with nonmetastatic prostate cancer were matched for age, race, geographic location, and comorbidity; 3887 men were treated with GnRH agonist and 7774 men were not.126 GnRH agonist use was associated with a faster time to fracture and a significantly increased risk for any clinical fracture, hip/femur fractures, and vertebral fractures. Short-term treatment did not confer any greater fracture risk, suggesting reversal of the hypogonadal effects on the bone.
Another study used both SEER and Medicare databases to evaluate the risk of fracture after ADT for prostate cancer.127 Records of more than 50,000 men with prostate cancer revealed that the frequency of any fracture was significantly higher in those receiving ADT. The relative risk of the occurrence of any fracture or one resulting in hospitalization increased with increasing doses of GnRH agonist received during the first year after diagnosis. Many studies have shown that GnRH agonist treatment is associated with accelerated bone loss. In one prospective analysis, for example, Mittan et al.128 examined the effects of GnRH analogue treatment on bone loss and bone resorption in men with prostate cancer compared with age-matched control subjects. After 12 months of GnRH therapy, a significant decrease was seen in BMD of the total hip and ultra-distal radius in men receiving GnRH compared with the control group. Similar data on BMD loss in ADT have come from several other clinical trials.129–132
Randomized studies have focused on bisphosphonate therapy in hypogonadal men with prostate cancer using BMD end points. Intravenous pamidronate and zoledronic acid given once every 3 months prevented ADT-induced bone loss in the spine and hip compared with control groups.133,134 In contrast to pamidronate, zoledronic acid increased BMD. Mean lumbar spine BMD was increased by 5.6% in men receiving zoledronic acid (n = 42) but decreased by 2.2% in the placebo group (n = 37).133
A second randomized controlled trial of zoledronic acid evaluated the efficacy of a single annual dose.135 Mean BMD of the lumbar spine and hip increased by 4.0% and 0.7%, respectively, in men receiving zoledronic acid. In contrast, mean BMD decreased 3.1% and 1.9% in the spine and hip, respectively, with placebo.
Yearly dosing of zoledronic acid is effective in general populations with osteoporosis and is FDA approved. Whether more frequent dosing is indicated in cancer patients with accelerated bone absorption remains to be defined. Greenspan et al.136 have shown the efficacy of alendronate in preventing BMD loss in patients with nonmetastatic prostate cancer undergoing ADT. In a randomized, double-blind, placebo-controlled trial in men treated with weekly oral doses of alendronate, BMD increased over 1 year by 3.7%. Although long-term data on the impact of bisphosphonates on fracture prevention is not available, these studies provide evidence that bisphosphonates effectively reduce bone loss in men receiving ADT. The NCCN Prostate Cancer Guidelines (available at www.nccn.org) recommend calcium and vitamin D supplementation for all patients, and consideration of bisphosphonate therapy with zoledronic acid (4 mg, annually) or alendronate (70 mg orally, weekly) as options in men receiving ADT who are at substantial risk for fracture based on standard risk assessment tools.5
Consistent with the important role of estradiol/ER signaling in bone metabolism in men, several small randomized, controlled trials have shown that SERMs increase BMD in men undergoing ADT for prostate cancer. In one study, raloxifene increased BMD of the hip and tended to increase BMD of the lumbar spine.137 Toremifene, a SERM approved for the treatment of advanced breast cancer, increased BMD of the hip and spine in men receiving ADT for prostate cancer in another trial.138 Two large randomized, placebo-controlled trials to prevent fractures during ADT were recently completed. In one multicenter study with a primary end point of new vertebral fractures, 1389 men in the United States and Mexico receiving ADT for prostate cancer were randomly assigned to either toremifene or placebo. Secondary end points included BMD, serum lipids, vasomotor flushing, and breast symptoms. Interim analyses reported that toremifene significantly increased BMD of the lumbar spine, total hip, and fem-oral neck.138 Toremifene also significantly improved lipid profiles in men on ADT.139
In a recently completed global study, 1468 men receiving ADT for prostate cancer were randomly assigned to either the RANK-L inhibitor, denosumab, subcutaneously every 6 months or placebo. The primary study end points were BMD and new fractures. Complete results are expected this year. Two other large studies to evaluate the role of denosumab for preventing bone metastases and disease-related skeletal complications in men with prostate cancer are ongoing.
In conclusion, ADT is associated with significant morbidity, including osteoporosis and increased incidence of clinical fractures. Strategies to reduce morbidity include educating patients about risks, encouraging healthy lifestyle modifications, supplementation with calcium and vitamin D, screening for osteoporosis, and drug therapy in appropriate individuals. The results of recently completed large randomized controlled trials will help establish evidence-based guidelines for fracture prevention in prostate cancer survivors.
Role of Adjuvant Bisphosphonates in Reducing Recurrence
Breast Cancer
Preclinical in vitro studies have shown that bisphosphonates inhibit the adhesion of breast cancer cells to extracellular bone matrix, inhibit tumor cell invasion,140–143 and induce apoptosis via the ras pathway in human breast cancer cells.144 In an animal study, pretreatment of nude mice with bisphosphonates before inoculation of tumor cells reduced the development of osteolytic lesions.145 Taken together, these studies suggest that bisphosphonates may inhibit critical steps in the development of bone metastases in addition to inhibiting resorption.
Three randomized trials in early stage breast cancer investigated whether oral clodronate can prevent bone metastases and improve survival; 2 of the 3 showed a survival benefit. In a large (n = 1069) placebo-controlled trial, breast cancer patients receiving standard systemic therapies were randomized to receive oral clodronate (1600 mg, daily) or placebo for 2 years as adjuvant treatment.146 This trial reported a reduced risk of bone metastases with clodronate of 51 versus 73 events (HR, 0.69; P = .04) at 5 years, and 19 versus 35 events (HR, 0.55; P = .048) during the 2 years on treatment. Survival at 5 years, the preplanned study end point, favored the clodronate group with a hazard rate of uncertain significance because of multiple analyses (for all patients: HR, = 0.77; P = 0.048). The most recent report includes survival data with long-term follow-up that showed a continued separation of the survival curves between years 5 and 10.146
In a second smaller, randomized, open-label study, 302 women with breast cancer and micrometastases detected in a bone marrow aspirate at diagnosis were randomized to receive either clodronate (1,600 mg, daily) or no bisphosphonate for 2 years. Additionally, patients received standard adjuvant systemic therapy. Patients who received clodronate had a 50% reduction in the incidence of bone metastases (P = .003), and a significantly longer bone metastasis-free survival (P < .001). Distant metastases were detected in 21 of 157 patients (13%) who received clodronate compared with 42 of 145 patients (29%) in the control group (P < .001).147 A later analysis at 8.5 years’ follow-up continued to confirm a significant improvement in overall survival for clodronate patients, although the significance in disease-free survival no longer persisted.148
In a third small randomized, open-label study investigating 3 years of adjuvant clodronate therapy in 299 patients with lymph node-positive breast cancer, the results showed no reduction in bone metastases in the clodronate-treated arm, although bone as a first site of relapse was less frequent in the clodronate group than in the control arm (14% vs. 30%). However, a worrisome increase in visceral metastases and reduction in overall survival at 5 years was seen for patients receiving clodronate.149 A possible explanation for these adverse outcomes is an imbalance in hormone-negative cases between the arms of the study, with significantly more PR-negative (45% vs. 31%; P = .03) and a trend towards more ER-negative (35% vs. 23%) tumors in the clodronate group. This difference was potentially exacerbated by the practice in this trial of assigning endocrine therapy alone to all postmenopausal women and chemotherapy alone to all premenopausal women, regardless of ER/PR status. The negative impact of clodronate on overall survival appears to be neutralized when the imbalance in hormone receptor negativity is corrected. Even without correction, the survival detriment no longer showed significance at 10 years.
A meta-analysis using the 5-year data from these 3 adjuvant clodronate trials did not show a statistically significant difference in overall or bone metastasis-free survival when the data were pooled.150 A marked heterogeneity among the trials in part explains the wide confidence interval around the HR (HR, 0.75; 95% CI, 0.31–1.82).
A recent study investigating the adjuvant use of zoledronic acid reported an improvement in disease-free survival, in addition to favorable effects on BMD.102 The ABCSG-12 trial enrolled 1,800 premenopausal women with ER-positive breast cancer. All patients received ovarian suppression for 3 years with a luteinizing hormone-releasing hormone analogue, goserelin. Patients were randomized in a 2 by 2 design to receive tamoxifen versus anastrozole, and zoledronic acid (4 mg, every 6 months) or not. At the first efficacy analysis, reported after 137 events (70 distant relapses) with approximately 60 months follow-up, no difference in outcome was seen with respect to the endocrine therapy randomization. However, the authors found a statistically significant improvement in disease-free survival for patients who received zoledronic acid (HR, 0.64; P = .01), with a similar trend toward improved overall survival (HR, 0.60; P = .10). The absolute benefit in disease-free survival was 3.2%.
Although ABCSG-12 clearly provides additional support for the metastasis-suppressing potential of adjuvant bisphosphonates, this study enrolled only a narrow subset of breast cancer patients: premenopausal women with ER-positive tumors who did not receive adjuvant chemotherapy. Although the results are promising, caution must be taken not to over-extrapolate these findings, or this dose schedule, to all breast cancer patients.
The Z-FAST and ZO-FAST trials enrolled ER-positive postmenopausal women receiving letrozole and randomized them to “upfront” versus “delayed” zoledronic acid therapy (4 mg, every 6 months for 5 years) in an attempt to reduce bone loss–related morbidity.55,119,120 Time to recurrence was a secondary end point. A recent combined analysis of these trials showed lower recurrence rates in the group receiving upfront zoledronic acid therapy (1.1% vs. 2.3%; P = .04).55
Two additional large trials of adjuvant bisphosphonates have met their targeted accrual goals, with efficacy analysis pending. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-34 trial (closed to accrual in 2004) enrolled women with stage I or II breast cancer and compared 3 years of daily oral clodronate (1600 mg, daily) to placebo. The primary end point was disease-free survival. Another clinical trial asking “Does Adjuvant Zoledronic Acid Reduce Recurrence in Patients with High-Risk Localized Breast Cancer?” (AZURE) enrolled stage II and III breast cancer patients, comparing standard adjuvant cancer therapy alone or given with “intensive” zoledronic acid for 5 years (4 mg monthly for 6 months, followed by once every 3 months for 2 years, then once every 6 months through year 5). This trial closed to accrual in 2006.
The North American Breast Cancer Intergroup, in combination with the NSABP, is conducting SWOG S0307, a comparison of 3 different bisphosphonates in the adjuvant breast cancer setting. This trial is randomizing 4500 women with stage I or III breast cancer receiving standard adjuvant therapy to oral clodronate (1600 mg, daily) versus oral ibandronate (50 mg, daily) versus zoledronic acid (4 mg intravenously, monthly for 6 months, then every 3 months), all for 3 years duration.
These unreported trials include all patient and tumor subsets, including both pre- and postmenopausal women, ER-positive and -negative tumors, and patients who received a range of standard systemic therapy, including chemotherapy. The results of these trials will be critical in determining how broadly applicable bisphosphonates are across the spectrum of breast cancer patients. Several additional ongoing early stage bisphosphonate trials are evaluating various agents, doses, schedules, and adjuvant settings, including residual disease after preoperative chemotherapy and in elderly populations.
Prostate Cancer
In prostate cancer, trials have yet to show any reduction in recurrence or death from the adjuvant use of bisphosphonates. A randomized controlled trial to evaluate the effects of zoledronic acid on time to first bone metastasis in men with prostate cancer, no bone metastases, and rising prostate-specific antigen despite ADT was terminated approximately halfway into accrual when interim analysis showed a lower-than-expected event rate.151 A randomized, double-blind, placebo-controlled trial of oral clodronate versus placebo in patients with nonmetastatic prostate cancer found no difference in bone metastases–free survival or overall survival after nearly 10 years of follow-up.152
Several trials evaluating the adjuvant use of osteoclast-targeted therapy in prostate cancer are ongoing. One of the objectives of the Randomized Androgen Deprivation and Radiotherapy (RADAR) trial is to determine whether 18 months of zoledronic acid will reduce relapse risk by impeding the development of bony metastases. The ZEUS trial will assess the efficacy of zoledronic acid every 3 months versus best supportive care in preventing skeletal metastases in patients with high-risk prostate cancer. Additionally, the RANK-L inhibitor denosumab is being tested in an ongoing large (1500 patients) international randomized, phase III, placebo-controlled trial in men with hormone-refractory prostate cancer with the end point of bone metastasis–free survival.
Conclusions
The adjuvant bisphosphonate trials in breast cancer reported to date support the potential role of these drugs in impacting recurrence and survival in early stage breast cancer. The data do not yet support the addition of adjuvant bisphosphonates as standard of care for all patients. The promising, yet somewhat contradictory, results of the 3 reported breast cancer adjuvant clodronate studies suggest that bisphosphonates can impact disease recurrence, but highlight the need for further investigation. Extrapolation of the ABCSG-12 findings to postmenopausal women, ER-negative tumors, and women receiving chemotherapy will require data from ongoing clinical trials. These studies will aid in defining the optimal patient and tumor populations for the addition of adjuvant bisphosphonates, as well as optimal doses and schedules of administration, and long-term toxicities. Whether doses used in metastatic disease are required for prevention or whether lower doses will suffice is unknown. It is unclear whether adjuvant bisphosphonates should be given continuously and orally, whether intravenous therapy is preferable, and whether less intensive intravenous regimens will be as effective as more intensive regimens. The optimal duration of adjuvant bisphosphonate therapy is also unknown.
Bone Metastases
Pathophysiology
Normal bone homeostasis involves constant remodeling by the coordinated actions of osteoclasts and osteoblasts. In metastatic bone disease, as in osteoporosis, this balance is tipped to favor osteoclast-mediated resorption. The interactions between the tumor and the bone microenvironment shape the characteristics of the bone metastases. Metastatic bone disease associated with breast cancer is often predominantly osteolytic, whereas lesions from prostate cancer are predominantly osteoblastic on imaging. However, bone metastases are frequently heterogeneous, and histological examination often shows evidence of both osteolytic and osteoblastic features.153,154
At the cellular level, evidence of reciprocal signaling between the tumor and bone microenvironment can be seen. Cancer cells interact with osteoclasts, osteoblasts, and the bone matrix through multiple pathways.155 Tumor cells in the bone microenvironment may produce factors such as interleukins, prostaglandins, and PTH–related protein (PTHrP) which directly and indirectly stimulates bone resorption. Osteolysis releases cytokines and growth factors that were stored within the bone matrix, such as transforming growth factor (TGF)-beta, insulin-like growth factor (IGF)-1, and platelet-derived growth factor (PDGF). These factors may induce tumor cell proliferation, thereby generating a vicious cycle of tumor growth and bone destruction.155 Understanding the biology of this process has provided useful insights for developing therapy to prevent or reduce the morbidity of bone metastases. In addition, therapies that specifically target the bone microenvironment are being incorporated into clinical practice.
Frequency
The frequency of bone metastases varies by cancer type and duration of advanced disease. The incidence of bone metastases in patients with advanced disease is 73% in breast, 68% in prostate, 42% in thyroid, 35% in kidney, and 36% in lung cancers. Bone metastases can cause significant morbidity.156
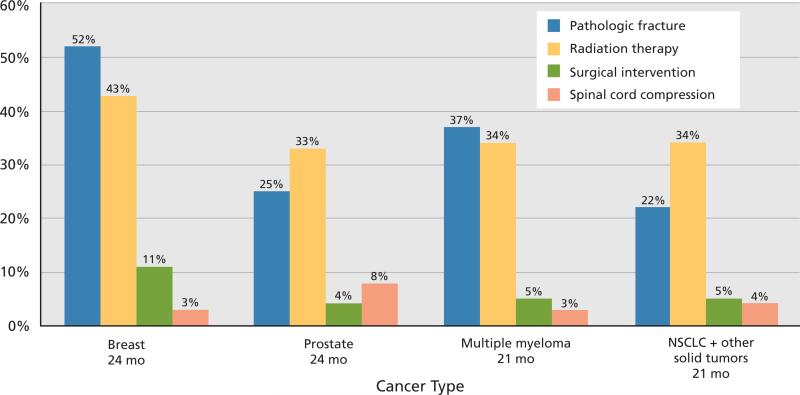
Patients with bone metastases are at risk for skeletal-related events, including fracture, need for radiation to bone, spinal cord compromise, and hypercalcemia or need for surgery (Figure 3). The risk of experiencing an additional skeletal-related event within 1 year of a first event is substantial. In an analysis performed in the United Kingdom, skeletal-related events accounted for 63% of hospital costs for patients with metastatic breast cancer.157 Cancer patients with bone involvement are at risk for pain, particularly with motion, and the effects of bone metastases can negatively impact a patient's quality of life.
Figure 3.
Prevalence of skeletal-related events in patients with metastatic bone disease not treated with a bisphosphonate. The data are obtained from 4 major trials of placebo versus an intravenous bisphosphonate in different tumor types.165,166,222,223 Abbreviation: NSCLC, non–small cell lung cancer.
Therapies
In addressing bone metastases, the mainstay of oncology care is attempting to control the tumor burden. This is typically addressed through antineoplastic therapies. Bone-specific interventions are often incorporated into the treatment plan and may be localized or systemic. Surgery and radiation therapy can be used to address local control of specific lesions. Bone-directed systemic interventions include bisphosphonates and radiopharmaceutical therapy. Throughout the management of the patient, attention must be paid to pain control and minimizing the risk of potentially catastrophic events, such as fracture or spinal cord compromise.
Bisphosphonates to Reduce Skeletal Morbidity
Large randomized clinical trials have shown that the addition of bisphosphonate therapy to standard anti-cancer therapy can decrease the frequency of skeletal-related events by approximately a third.158–162 Studies have also shown that bisphosphonate therapy can reduce pain and improve quality of life in patients with bone metastases.161,163–167 Bisphosphonates have been incorporated into the routine clinical management of metastatic bone disease in a number of tumor types.8,161,168,169 In the Unites States, pamidronate and zoledronic acid are FDA approved for treating metastatic bone disease. Clodronate and ibandronate are licensed for use in bone metastases outside the United States.
Treatment of Bone Metastases in Breast Cancer
In clinical trials, bisphosphonates have shown treatment benefits for breast cancer patients with bone metastases. Pamidronate reduced the frequency of skeletal morbidity in placebo-controlled trials involving breast cancer patients with bone lesions who were receiving hormone or chemotherapy.166 The skeletal morbidity rate was 2.4 events per year in the pamidronate arm and 3.7 in the placebo arm (P < .001). The median time to skeletal complication was 12.7 months in the pamidronate group and 7 months in the placebo group (P < .001). In the pamidronate arm, 51% had skeletal complications at up to 24 months on treatment, compared with 64% in the placebo arm (P < .001).
In preclinical testing, zoledronic acid appeared to be a more potent bisphosphonate than pamidro-nate, and clinically it showed superiority over pamidronate in treating hypercalcemia of malignancy.170 A randomized, phase III, multicenter trial was conducted to compare zoledronic acid to pamidronate in patients with bone lesions secondary to breast cancer or multiple myeloma, with the objective of determining the safety and efficacy of long-term therapy with these agents.171 The 13-month core phase of the trial showed that zoledronic acid had an efficacy and safety profile comparable with pamidronate. In a 25-month extension phase, the overall incidence of skeletal-related events other than hypercalcemia of malignancy was similar between the zoledronic acid and pamidronate groups. The percentage of patients who required radiotherapy to bone was 19% for zoledronic acid compared with 24% for pamidronate (P = .037). A comparable median time to first event was seen in both groups (376 days with zoledronic acid, 356 with pamidronate; P = .151). Zoledronic acid reduced the mean annual incidence of skeletal complications, or skeletal morbidity rate, by 25% compared with pamidronate, with 1.04 events per year for zoledronic acid and 1.39 for pamidronate (P = .084). In the overall patient population, zoledronic acid reduced the risk of developing a skeletal complication by an additional 16% compared with pamidronate, with a risk ratio derived from the multiple-event analysis of 0.841 (P = .030).
A randomized trial in Japan compared zoledronate, 4 mg, to placebo every 4 weeks for 1 year in 228 women with breast cancer with at least 1 osteolytic bone metastasis.172 The placebo control was used because no intravenous bisphosphonate is approved for this indication in Japan. In this trial, zoledronate reduced the rate of skeletal-related events by 39% (P = .027). The absolute reduction in the number of patients having an event was 20% (number needed to treat = 5). In addition, bone pain scores were significantly improved within 4 weeks of treatment and remained modestly reduced for 52 weeks. No serious (grade 3 or 4) toxicities or substantial declines in renal function were seen. This study corroborates the benefit of zoledronate in reducing skeletal-related events seen in previous studies.
The ASCO clinical practice guidelines for breast cancer suggest that patients in whom radiographic evidence of bone metastases exist should receive therapy with either zoledronic acid, 4 mg over 15 minutes, or pamidronate, 90 mg delivered over 2 hours, both every 3 to 4 weeks.8 Currently evidence is insufficient to support superiority of one over the other, and, in countries where they are approved, clodronate or ibandronate are also therapeutic options. Due to increasing concerns over potential bisphosphonate-associated toxicities, all women should undergo dental examination with appropriate preventive dental care before starting bisphosphonate therapy. Serum creatinine also should be monitored before each dose of pamidronate or zoledronate.
The optimal duration of bisphosphonate therapy has not been well defined. The longest phase III clinical trials in patients with bone metastases examined a 3 to 4 week dosing of bisphosphonates for only 2 years. Little data is available beyond 2 years of treatment. The unknown potential additional benefit from continuing bisphosphonates must be weighed against the potential toxicities of long-term administration. The ASCO bisphosphonate guidelines for breast cancer recommend continuing bisphospho-nate therapy indefinitely or until the patient's performance status declines substantially. The consensus of the NCCN Task Force is that continuation of bisphosphonate therapy should be reconsidered at 2 years. Continued bisphosphonate treatment should be considered in patients with active cancer or an existing focus of bone metastasis. Discontinuation should be considered for patients with no active disease or who have experienced significant deterioration of renal function. The presence of bisphospho-nate-associated ONJ or sub-trochanteric hip fracture is not necessarily an indication for discontinuation. If a clear indication was seen for starting therapy (bone metastasis or active cancer known to metastasize to bone) and those indications continue to exist, continued therapy may be appropriate. The development of low-frequency significant side effects should not detract from the overall usefulness of the agents. If bisphosphonates are discontinued, an active bone surveillance program should be initiated.
The optimal dosing interval of bisphosphonate therapy in bone metastases is also unknown. No data are available to support lengthening intervals between treatments, although this is the subject of important ongoing investigations. The ongoing OPTIMIZE 2 trial is studying breast cancer patients with bone metastases who received approximately 1 year of monthly zoledronic acid. Patients are randomized in a double-blind fashion to continue monthly dosing for an additional year versus changing dosing intervals to every 3 months. The ongoing BISMARK trial is a randomization between standard dosing—once every 4 weeks—of zoledronic acid and intervals based on markers of bone turnover, which are evaluated at 15- to 16-week intervals. For patients with low bone turnover at the time of the marker evaluation, 1 dose of zoledronic acid is given every 15 or 16 weeks. For patients with intermediate markers of bone turnover, 2 doses are given in this time frame. For patients with high levels of bone turnover, one dose every 4 weeks is given. This protocol therefore allows for changes in the dosing interval of zoledronic acid based on current bone activity. The CALGB 70604 trial randomizes patients with meta-static breast or prostate cancer or multiple myeloma involving the bone to receive zoledronic acid, 4 mg every 4 or 12 weeks, and will investigate the rate of SREs between the groups over 2 years.
Pamidronate and zoledronic acid are FDA approved for use in patients with metastatic bone disease and have been shown to be efficacious in reducing or delaying the onset of skeletal-related events in patients with breast and solid tumors with documented bone metastases. Bisphosphonates are effective in reducing bone pain and improving quality of life. Currently, questions remain on how to optimally use bisphosphonates. Ongoing clinical trials will help identify optimal dosing schedules, duration, and the role of other novel agents in the treatment of bone metastases.
Treatment Bone Metastases in Prostate Cancer
An estimated 65% to 75% of patients diagnosed with advanced prostate cancer experience a skeletal-related event. Their median 5-year survival is 25%, and almost all patients who die of prostate cancer have skeletal involvement.157
Almost all men diagnosed with metastatic disease are initially offered hormonal therapy, and almost all show response to the treatment. Significant improvement in pain relief, a decline in prostate-specific antigen levels, and improvement in quality of life are seen. Unfortunately, in most cases, the disease relapses after a median response of about 2 years. Unlike in breast cancer, only 3 chemotherapy drugs have been approved for patients with prostate cancer. The NCCN Prostate Cancer Guidelines (available at www.nccn.org) recommend docetaxel administered every 3 weeks in combination with prednisone as preferred first-line treatment for castration-recurrent disseminated prostate cancer.5 Alternate regimens included in these NCCN Guidelines include 3-week docetaxel with estramustine, weekly docetaxel with prednisone, or mitoxantrone with prednisone.
Bisphosphonates have been shown to be effective in reducing bone complications in patients with osteolytic bone metastases caused by a variety of solid tumors. Because prostate cancer is primarily osteoblastic, researchers initially thought that bisphosphonates may not be as effective in this disease. However, studies have revealed that bone resorption in metastatic prostate cancer is high, reflecting substantial osteoclastic activity. Therefore, biologic rationale exists for the use of bisphosphonates in prostate cancer.
Effectiveness of clodronate treatment was evaluated in symptomatic bone progression-free survival in 2 studies.173,174 In the first, 311 men with hormone-sensitive metastatic prostate cancer were randomly assigned to receive oral clodronate or placebo for 3 years.173 The second trial used clodronate in conjunction with mitoxantrone and prednisone versus mitoxantrone and prednisone alone.174 Clodronate treatments resulted in no pain relief or improvement in quality of life in both studies.
Similarly, a randomized placebo-controlled study of pamidronate (90 mg every 3 weeks) versus placebo in 378 metastatic prostate cancer patients found no differences in pain control or reduction of skeletal-related events.175 Zoledronic acid is the only bisphosphonate with proven clinical benefit in reducing skeletal complications in patients with hormone-refractory prostate cancer. In a double-blind phase III trial, patients with hormone-refractory disease (rising prostate-specific antigen despite medical or surgical castration) and a history of bone metastases were randomized to zoledronic acid, 4 mg (n = 214), zoledronic acid, 8 mg (n = 221), or placebo (n = 208) every 3 weeks for 15 months.176 The primary end point was time to occurrence of skeletal-related events. Risk of renal impairment was elevated with the 8-mg dose, and medication for this arm was therefore reduced to 4 mg. Patients taking placebo had significantly more events than those on zoledronic acid (44.2% vs. 33.2%; P = .021). Subsequently, of 122 patients who completed 24 months on the study, fewer patients treated with zoledronic acid developed skeletal complications (38% vs. 49% for the placebo group; P = .028).167 The delay in onset to the first skeletal-related event was about 31% longer for those on zoledronic acid. Overall, the authors noted a decrease in fracture, spinal cord compression, need for antineoplastic therapy, and need for radiation and surgery in patients receiving zoledronic acid compared with placebo.
In conclusion, the main risk factor for skeletal complications is the presence of bone metastases in patients with hormone-refractory prostate cancer. These patients should be considered for bisphosphonate therapy. As with other tumors, insufficient data are available to guide choice, dose, and route of administration of bisphosphonates in prostate cancer patients. Bisphosphonates have no proven role in hormone-naïve patients with advanced prostate cancer; currently, hormone therapy adequately controls underlying disease in this setting. Research into the earlier use of bisphosphonates in these patients is ongoing.177 A phase III trial is currently recruiting patients with hormone-refractory prostate cancer to compare time to development of a skeletal event on zoledronic acid versus denosumab.178 Better understanding of the role of bisphosphonates and other novel bone-targeting agents in treatment-related bone loss, prevention and treatment of metastases, and antitumor effects, will probably expand the role they play in managing advanced prostate cancer.
Investigational Targets for Treatment of Bone Complications
New classes of agents with anti-osteoclastic activity are in various stages of investigation for treating and preventing bone metastases and osteoporosis. These include the RANK-L inhibitor, denosumab, the Src kinase inhibitor, dasatinib, and several versions of cathepsin k inhibitors. The relative efficacy and toxicity profiles of these agents compared with bisphosphonates will be of great interest. Whether these agents will be optimally used as an alternative for or in combination or sequence with bisphosphonates remains to be studied.
RANK-L
RANK-L mediates the formation, function, and survival of osteoclasts. Denosumab, the monoclonal antibody that binds and neutralizes RANK-L, has been evaluated in phase II studies in patients with bone metastases naïve to intravenous bisphosphonate therapy and those with elevated levels of the bone resorption marker urinary-N-telo-peptide despite ongoing intravenous bisphosphonate treatment.179,180 Denosumab was well tolerated, with strong suppression of bone-resorption markers. Phase III clinical trials are ongoing comparing denosumab to zoledronic acid in the treatment of bone metastases in patients who have not previously received intravenous bisphosphonates.
Src
Src is involved in signaling cascades important for receptor-mediated osteoclast formation and function, including RANK. Preclinical data show that Src plays a role promoting bone metastases. Therefore, inhibition of Src could have negative effects not only on osteoclast activity, but also on tumor cells that invade bone and promote osteoclast activity.Src inhibitors represent potential novel therapeutics for bone metastases and are currently under investigation in patients with metastatic bone disease. Dasatinib is approved by the FDA for treatment of imatinib-resistant or intolerant chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia. It is currently being evaluated in clinical trials for patients with metastatic bone disease from solid tumors.181–183
Cathepsin K
Cathepsin K is a cysteine protease produced by osteoclasts. It mediates bone resorption and degradation.184 Cathepsin K is also produced by cancer cells and helps promote cancer cell invasion. Preclinical studies have shown that inhibitors of cathepsin K can reduce osteolysis and skeletal tumor burden by breast cancer cells.185 Odonacatib, a cathepsin K inhibitor, is currently in clinical trial.186
Imaging of Bone Metastases
Numerous imaging techniques are available to evaluate bone metastases, including plain film radiography, CT, MRI, 99mTc bone scanning (radionuclide bone scan), and PET, both with fluorodeoxyglucose (FDG) and 18F sodium fluoride. Imaging has an important role in detection, diagnosis, treatment planning, and follow-up of bone metastases. The results of imaging studies must be interpreted within clinical context. If bone metastases are present or suspected, imaging or biopsy may be required to confirm the diagnosis and establish the extent of disease. Response to therapy can be evaluated using radiographs (plain films) and by correlating the radiographic changes with bone scan, clinical, and laboratory findings.
Plain film radiography, the oldest imaging technique for evaluating bone metastases, recognizes alterations in bone density, such as osteolytic and osteoblastic changes. In case of indeterminate bone scan findings, plain film may be helpful in further characterizing a suspicious lesion. Plain films may also be helpful in detecting the cause of bone pain. Plain films can assess cortical destruction by the cancer, providing valuable information regarding fracture risk. Unfortunately, plain films are relatively insensitive in the detection of early or small metastatic lesions. A 30% to 50% loss in bone density must occur to recognize an osteolytic lesion on plain film.
CT, like plain films, is a map of bone density, with tomographic capability. Compared with plain films, CT has an improved target-to-background ratio and improved sensitivity. Muindi et al.187 and Durning et al.188 found that CT is more sensitive than plain film radiography in detecting metastatic lesions. CT is used to assess lesion size and cortical reaction and can also identify alterations in adjacent soft tissue. As with plain films, CT is useful for characterizing suspicious lesions on bone scans. The usefulness of CT in detecting early involvement of the bone marrow, however, is limited. In addition, skeletal coverage is limited with CT because of its relatively high radiation dose, making it unsuitable as a screening tool.
MRI is associated with a high sensitivity (82%–100%) and specificity (73%–100%) for bone marrow metastases. Unlike CT and plain film, MRI does not assess bone density, but is helpful in assessing tissue alterations. Therefore, MRI can detect metastases that have infiltrated bone marrow189 before they provoke an osseous bone response. MRI is more sensitive for detecting early lesions and marrow-based metastases than plain films, CT, or radionuclide bone scans. Although MRI is a good choice for detecting marrow infiltration, its role in bone metastases is generally limited because it is more expensive and not as readily available as CT.
Skeletal scintigraphy (bone scan) is an effective method for screening the whole body for bone metastases.190 99mTc-methylene diphosphonate (MDP), is the most frequently used radiotracer. Because technetium-labeled MDP is taken up by active osteoblasts, 99mTc-planar bone scans detect metastatic tumor deposits in bone by the increased osteoblastic activity they induce. Radionuclide bone scans are relatively insensitive for purely osteolytic lesions found commonly in cancers of the kidney and thyroid and multiple myeloma, but they are highly sensitive to osteoblastic and mixed osteolytic-osteoblastic lesions such as from prostate and breast cancer. Bone scans have the disadvantages of poor spatial and contrast resolution.
Sensitivity of 99mTc bone scan is estimated at between 62% and 100%, with the lowest sensitivity seen in patients with predominantly lytic disease. Many benign processes and other entities (i.e., trauma fractures, Paget disease) can produce an area of increased radiotracer uptake that mimics a metastatic deposit. Bone scans are not optimal for monitoring response to treatment, because the osteoblast changes induced by cancer metastases can be long-lived. Osteoblastic activity resulting from healing after therapy (i.e., flare phenomenon) may mislead-ingly suggest advancing disease on bone scans. In many patients, further imaging, such as plain films or CT, is required.
PET can help in identifying bone metastases at an early stage of growth, before host response of the osteoblasts occur. It has higher spatial resolution than bone scan and a quantitative capability. Therefore, PET can better assess response to therapy. Two currently available PET tracers are 18F sodium fluoride and 18F FDG. 18F sodium fluoride is taken up by osteoblasts and therefore is reflective of a reparative response, making fluoride PET scans similar to 99mTc bone scan. 18F fluoride gets incorporated into newly formed bone in increased amounts, reflecting increased turnover. 18F sodium fluoride PET has improved lesion detection over technecium bone scans. However, finding additional lesions may not necessarily alter therapy.
18F FDG-PET can detect early malignant bone-marrow infiltration because of the early increased glucose metabolism in neoplastic cells.191 Unlike 99mTc-MDP bone scans and 18F sodium fluoride PET bone scans, 18F FDG-PET directly assesses the metabolic activity of the metastatic tissue rather than the bony response to the presence of the metastasis. Therefore, FDG-PET can be helpful in detecting purely osteolytic lesions and marrow infiltration, but it may not be helpful for osteoblastic lesions with relatively low metabolic activity. Consequently, lesions present on MRI or PET may not be visible on bone scans, and vice versa. These techniques nicely complement each other when mixed lesions are present. In a comparative study of 3 modalities in detecting bone metastases, Daldrup-Link et al.192 found sensitivities of 90% for FDG-PET, 82% for whole-body MRI, and 71% for 99mTc bone scans. Similar results have emerged from comparative studies of FDG-PET and 99mTc bone scans.193,194 FDG-PET shows a high number of false-positive lesions, which require follow-up imaging with other modalities. In skull metastases, the high rate of glucose metabolism in the normal areas of brain may obscure metastases.
Additional PET tracers are under investigation in cancer imaging. 18F fluoroestradiol can measure ERs. Markers of DNA synthesis such as 11C thymi-dine and 18F fluorothymidine can measure cellular proliferation. 11C acetate can measure lipid biosynthesis, an indication of cellular reproduction.
A model of how a patient might pass through a set of imaging studies is illustrated in Figure 4. Imaging analysis for metastases is focused on the most likely bones of involvement: vertebrae, pelvis, ribs, skull, femur, and humerus. Patients with suspected bone metastases may be assessed initially with skeletal scintigraphy (99mTc-MDP bone scan).195 Given its specificity, if the bone scan is negative and no symptoms are present, clinicians can presume the patient has no metastatic disease. A positive scintigraph is followed by plain film radiography to further localize and characterize the lesion. If radiographs are negative and the patient is symptomatic or has a suspicious lesion, an MRI or CT scan should be considered.
Figure 4.
Algorithm for imaging for cancer patients in the United States.
Modified from Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol 2004;22:2942–2953
In conclusion, understanding the advantagesand disadvantages of different bone imaging techniques will assist the clinician in screening, planning treatment, and assessing response. Multiple imaging modalities may be required to confirm the presence of and optimally evaluate bone metastases. Monitoring bone metastases can be problematic with any imaging technique, because changes in bone often occur very slowly. Marrow regeneration in successfully treated patients may appear to be progressive disease on MRI or PET. For evaluation of impending fracture and the need for surgical intervention, plain films and CT scans provide the best information. Results of imaging studies must be interpreted within the clinical context of the individual patient.
Surgical and Radiation Treatment of Bone Metastases
Complications caused by bone metastases, including pain, fractures, and decreased mobility, can affect quality of life and reduce a patient's performance status. Localized therapies, including radiation and surgery, can be used for preventing impending events, as well as for palliation.
Radiation Treatment
Radiation therapy is commonly used in the management of bone metastases, both for pain relief and prevention of morbidity and disease progression. Radiation therapy has shown responses rates of 60% to 70%. Complete pain relief may occur in 20% to 30% of patients receiving radiotherapy.196,197 However, in many patients, the effects may not be felt for several weeks after treatment starts and relief may last only 3 to 4 months.
External Beam Radiation Therapy
External beam radiation therapy is widely used for cancer patients who present with localized bone pain. The optimal treatment schedule—single versus multiple fraction—is under debate. A systematic review of published trials show no difference between single and multiple fraction in terms of efficacy and toxicity, although a slightly higher re-treatment rate has been seen with single fraction. The Radiation Therapy and Oncology Group (RTOG) conducted one of the largest trials in the United States to study effects of single versus multiple fractions of external beam radiation therapy in treating bone metastases in breast and prostate cancer patients.198 Patients (n = 898) were randomized to a single 8-Gy fraction or 30 Gy given in 10 fractions. No significant difference in response rates were seen between the 2 arms, although a significantly higher re-treatment rate was seen in the single-fraction arm. In the RTOG trial, more acute toxicity (grades 2-4) was seen in the multiple fraction arm compared with the single-fraction arm.198 The higher re-treatment rate with single fraction treatment may be attributed to the bias that giving additional doses of radiation may help patients who not have experienced relief from a single dose. A cost-utility analysis performed in the Netherlands compared 2-year quality-adjusted life expectancies and 12-week societal costs.199 Total societal costs for radiation therapy (including re-treatments, non-medical costs, and non-radiation treatment costs) were estimated at $4700 and $6453 for single fractions and multiple fractions, respectively. Despite multiple studies indicating no difference in response rate, duration of response, use of pain medication, side-effects, or quality of life, radiation oncologists in the United States appear to be reluctant to deliver single-fraction radiation for uncomplicated bone metastases.200
Stereotactic Body Radiation Therapy
Stereotactic body radiation therapy was initially developed for targeting lung lesions and is now being used to treat spine metastases. Although conventional radiation therapy of spinal metastatic tumors is useful for palliation, its effectiveness is limited by spinal cord tolerance. In patients with spinal metastases not causing cord compression, stereotactic radiation can be used to overcome some of the dose limitation associated with conventional radiotherapy. It can also be used for patients who have a good prognosis and for whom more aggressive treatment may be warranted. This technique is characterized by high-dose radiation delivered precisely to an extracranial target in 1 to 5 fractions. Researchers have found response rates are around 90% and duration of 13 months, with little to no long-term toxicity. Re-treatment rates range from 0% to 15%.201 This technique is relatively technically sophisticated and costly but allows better sparing of adjacent critical normal structures.
Radioisotopes
When symptomatic bone metastases are widespread and present on both sides of the diaphragm, radionuclides can be useful. Radionuclide therapy using strontium-89 chloride, samarium-153, rhenium-186, and rhenium-188 treatments have resulted in palliation of bone pain and improvement in quality of life in patients with advanced hormone-refractory prostate cancer.202–208 These radionuclides localize to regions of enhanced bone turnover and deliver high local doses of radiation through the emission of beta particles. Patients with diffuse multifocal disease are good candidates for systemic radioisotope therapy.
Surgical Treatment
Surgical management of bone metastasis is performed to relieve pain, provide stabilization, and prevent impending fracture or spinal cord compression. In some situations, surgery provides a greater likelihood of return to ambulatory status than radiation alone.
Although surgical treatment of pathologic fractures is often straightforward, treatment of patients with impending pathologic fractures is preferable. Compared with treatment of fractures of the femur, treatment of impending fractures is associated with a shorter hospital stay, a greater likelihood of discharge to home versus extended care, and a greater likelihood of support-free ambulation.209 The widespread use of bisphosphonate therapy has resulted in a decrease in the incidence of fracture due to bone metastases. Identification of bones at risk remains a “moving target” in the face of better anti-cancer therapies.
Surgeons identify lesions at high-risk for fracture based on general criteria. These include lytic lesions greater than 2.5 cm in diameter, encompassing more than 50% of the bone diameter, or the presence of lesser trochanter avulsion. Other indications for surgery for impending fractures include a lesion in a weight-bearing area and a readily identifiable painful lesion that is refractory to external beam radiation therapy. Verifying that the lesion is the source of pain is important.
These general guidelines must also be interpreted in the specific clinical context. Fracture stabilization must be preceded by an assessment of metastatic disease in other bones, which could compromise rehabilitation. When considering stabilization of a femoral fracture, a long bone survey or a bone scan within 2 to 3 months is recommended to detect other sites of disease that may relate to weight bearing. Differentiating pathologic fractures from traumatic fractures clinically is also important. Preoperative assessment should include estimation of life expectancy, mental status, mobility status, pain level, metabolic status, skin condition, and nutritional status.
From a technical standpoint, one of the easiest bones to stabilize is the proximal femoral shaft; stabilization is more challenging in the pelvis-acetabulum, spine, and periarticular areas. For a periarticular fracture, prosthetic replacement confers fairly predictable pain relief and a return to ambulatory status. Procedures that are applicable to nonmetastatic traumatic fractures often do not apply in the setting of pathologic fractures. For example, a sliding hip screw is commonly used in patients with intertrochanteric osteoporotic fractures. However, these devices are not effective in patients with pathologic fractures, because of the lack of bone healing, particularly with planned subsequent bone radiation.
Fractures within the femoral diaphysis can be stabilized using intramedullary nailing. Some of the interlocking capabilities of plates and nails have increased over the past 3 years with new locking plate technology. Insertion of intramedullary nails is a relatively straightforward procedure that requires general or regional anesthesia and a hospital stay of about 2 days. Humeral shaft metastases are often treated with locked intramedullary nailing or, more recently, an inflatable nail, with excellent pain relief and regained use of the extremity in several days.210,211 Case series of patients treated with intramedullary nailing have reported good outcomes, with complete pain relief and resumption of ambulation in a large proportion of patients. However, these outcomes are may be related to patient selection criteria.212,213
Stabilization of acetabular disease is technically challenging but can generally be done with a variation of hip replacement. Marco et al.214 reported on a case series of 55 patients who were treated with curettage of the tumor, protrusio cup, cement, and pin or screw fixation. Although 76% of patients had a decrease in narcotic usage and half of the nonambulatory patients regained the ability to walk, this procedure has been associated with a 22% complication rate. Saddle prosthesis is another option; a case series of 20 patients showed a similar improvement in analgesia, independence, and ambulation. Again, however, the complication rate was high at 20%.215 This high morbidity underscores the importance of patient selection for extensive surgery.
Additional Minimally Invasive Techniques
A variety of minimally invasive techniques are available, including radiofrequency ablation, percutaneous osteoplasty, also referred to as cementoplasty, percutaneous vertebroplasty, and kyphoplasty.
Radiofrequency ablation uses thermal energy to destroy tumor cells and has been used to treat painful bony metastases. Goetz et al.216 reported on a multicenter prospective study of radiofrequency ablation in which 43 patients with painful bone metastases, the majority of whom had undergone prior radiation therapy, had significant pain relief and reduction in opioid use with minimal side effects.
Percutaneous vertebroplasty and kyphoplasty are the injection of surgical cement, usually polymethylmethacrylate, into fractured vertebral bodies. Kyphoplasty uses a bone tamp that is inflated before the procedure to create a space for injection of polymethylmethacrylate. Kyphoplasty may result in an increase in vertebral height, which may provide a biomechanical advantage over vertebroplasty. That this technique is effective for pain reduction in both metastatic disease and osteoporosis seems clear, although the mechanism of the effect remains unclear.
This technique is growing in popularity; however, published outcomes for the treatment of metastatic disease are still minimal. Fourney et al.217 described a case series of 97 procedures in 56 patients. A total of 84% of patients had marked or complete pain relief. These results appear comparable to the larger volume of literature on kyphoplasty as a treatment of osteoporosis-related vertebral fractures.218
Cementoplasty is the percutaneous injection of polymethylmethacrylate into a metastatic lesion to palliate pain.219 This technique is similar to vertebroplasty. The difference is that it is performed in areas other than the spine and uses 3-dimensional imaging, most commonly CT. This technique is most suited to the pelvis.
Image-guided cryoablation is a relatively new minimally invasive technique. Similar to radiofrquency ablation, the metastatic lesions are accessed percutaneously. Cryoprobes are introduced under anesthesia. As the argon gas released from the probes rapidly expands, it produces rapid cooling with temperatures close to −100°C, leading to intracellular ice ball formation, dehydration, and cell death. Callstrom et al.220 reported on 14 patients with bone metastases treated with image-guided cryoablation. Improvement in pain, decrease in pain interference with activities of daily living, and noted reductions in narcotic use were seen using this therapy.
In conclusion, advances in surgery provide a number of techniques for treating bone pain from metastases. The key to surgical management remains identifying patients with impending fractures and referring them for stabilization before fracture occurs.
Conclusions
Bone health is an increasingly critical issue for all cancer patients and their health care providers. Evaluation and treatment of cancer treatment-related bone loss should be incorporated into comprehensive cancer care. Strategies to reduce morbidity include educating patients about fracture risk, encouraging healthy lifestyle modifications, providing supplementation with calcium and vitamin D, screening for osteoporosis, and starting drug therapy as clinically indicated. BMD combined with clinical risk factors for fracture determined using the FRAX calculator, provides a better estimation of fracture risk than BMD or clinical risk factors alone.
In patients with documented bone metastases, treatment and monitoring should involve a multidisciplinary approach that includes systemic anticancer therapy, osteoclast-targeted therapy, pain control, imaging studies, and surgery and radiation therapy if indicated. In breast cancer, emerging data suggests that bisphosphonates may impact recurrence in early stage breast cancer in addition to impacting bone density.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 5.Mohler J, Amling CL, Bahnson RR, et al. [8 June 2009];NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer, version 2.2009. Available at: http://www.nccn.org.
- 6.Carlson RW, Allred DC, Anderson BO, et al. [8 June 2009];NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, version I.2009. Available at: http://www.nccn.org.
- 7.U.S. Preventive Services Task Force Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526–528. doi: 10.7326/0003-4819-137-6-200209170-00014. [DOI] [PubMed] [Google Scholar]
- 8.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Melton LJ, III, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Collaborating Centre for Metabolic Bone Diseases [May 31, 2009];FRAX WHO fracture risk assessment tool. Available at: http://www.shef.ac.uk/FRAX/.
- 11.Garnero P, Delmas PD. Biochemical markers of bone turnover in osteoporosis. In: Marcus M, Feldman D, Kelsey J, editors. Osteoporosis. Vol. 2. Academic Press; New York: pp. 459–477. [Google Scholar]
- 12.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 13.Ross PD, Kress BC, Parson RE, et al. Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int. 2000;11:76–82. doi: 10.1007/s001980050009. [DOI] [PubMed] [Google Scholar]
- 14.Sornay-Rendu E, Munoz F, Garnero P, et al. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813–1819. doi: 10.1359/JBMR.050609. [DOI] [PubMed] [Google Scholar]
- 15.Christgau S, Bitsch-Jensen O, Hanover Bjarnason N, et al. Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone. 2000;26:505–511. doi: 10.1016/S8756-3282(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 16.Christgau S. Circadian variation in serum CrossLaps concentration is reduced in fasting individuals. Clin Chem. 2000;46:431. [PubMed] [Google Scholar]
- 17.Eastell R, Mallinak N, Weiss S, et al. Biological variability of serum and urinary N-telopeptides of type I collagen in postmenopausal women. J Bone Miner Res. 2000;15:594–598. doi: 10.1359/jbmr.2000.15.3.594. [DOI] [PubMed] [Google Scholar]
- 18.Clowes JA, Hannon RA, Yap TS, et al. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30:886–890. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]
- 19.Cauley JA, Palermo L, Vogt M, et al. Prevalent vertebral fractures in black women and white women. J Bone Miner Res. 2008;23:1458–1467. doi: 10.1359/JBMR.080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black DM, Arden NK, Palermo L, et al. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ, III, Atkinson EJ, Cooper C, et al. Vertebral fractures predict subsequent fractures. Osteoporos Int. 1999;10:214–221. doi: 10.1007/s001980050218. [DOI] [PubMed] [Google Scholar]
- 22.Genant HK, Li J, Wu CY, Shepherd JA. Vertebral fractures in osteoporosis: a new method for clinical assessment. J Clin Densitom. 2000;3:281–290. doi: 10.1385/jcd:3:3:281. [DOI] [PubMed] [Google Scholar]
- 23.National Osteoporosis Foundation guideline. Excerpta Medica, Inc; Belle Mead, NJ: 1999. Physicians guide to prevention and treatment of osteoporosis. [Google Scholar]
- 24.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288:2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Fatalities and injuries from falls among older adults—United States, 1993-2003 and 2001-2005. MMWR Morb Mortal Wkly Rep. 2006;55:1221–1224. [PubMed] [Google Scholar]
- 26.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Eng J Med. 1988;317:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 27.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 1998;53:M112–M119. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- 28.National Osteoporosis Foundation [May 2009];Patient info: fall prevention. Available at: http://www.nof.org/patientinfo/fall_prevention.htm.
- 29.Parker MJ, Gillespie WJ, Gillespie LD. Effectiveness of hip protectors for preventing hip fractures in elderly people: systematic review. BMJ. 2006;332:571–574. doi: 10.1136/bmj.38753.375324.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawka AM, Boulos P, Beattie K, et al. Hip protectors decrease hip fracture risk in elderly nursing home residents: a Bayesian meta-analysis. J Clin Epidemiol. 2007;60:336–344. doi: 10.1016/j.jclinepi.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 32.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health: Office of Dietary Supplements [May 2009];Dietary supplement fact sheet: calcium. Available at: http://ods.od.nih.gov/factsheets/calcium.asp.
- 34.National Institutes of Health: Office of Dietary Supplements [May 2009];Dietary supplement fact sheet: vitamin D. Available at: http://ods.od.nih.gov/factsheets/vitamind.asp.
- 35.Office of the Surgeon General [May 2009];Bone health and osteoporosis: a report of the surgeon general. Issued October 14, 2004. Available at: http://www.surgeongeneral.gov/library/bonehealth/index.html. [PubMed]
- 36.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1997. [Google Scholar]
- 37.Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 38.Adams JS, Kantorovich V, Wu C, et al. Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab. 1999;84:2729–2730. doi: 10.1210/jcem.84.8.5899. [DOI] [PubMed] [Google Scholar]
- 39.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 40.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 41.Cranney C, Horsely T, O'Donnell S, et al. Effectiveness and safety of vitamin D. Evidence Report/Technology Assessment No. 158. Agency for Healthcare Research and Quality; Rockville: 2007. AHRQ Publication No. 07-E013. [Google Scholar]
- 42.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84:694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 43.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 45.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 46.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 47.Smith MR, McGovern FJ, Zeitman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 48.Tauchmanova L, Colao A, Lombardi G, et al. Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2007;92:4536–4545. doi: 10.1210/jc.2006-2870. [DOI] [PubMed] [Google Scholar]
- 49.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 50.Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol. 2008;15:S30–40. doi: 10.3747/co.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003;349:1676–1679. doi: 10.1056/NEJM200310233491721. [DOI] [PubMed] [Google Scholar]
- 52.Van Poznak C, Estilo C. Osteonecrosis of the jaw in cancer patients receiving IV bisphosphonates. Oncology (Williston Park) 2006;20:1053–1062. [PubMed] [Google Scholar]
- 53.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 54.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 55.Brufsky A, Bundred N, Coleman R, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 56.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 57.Weitzman R, Sauter N, Eriksen EF, et al. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients—May 2006. Crit Rev Oncol Hematol. 2007;62:148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. Food and Drug Administration [May 2009];Update of safety review follow-up to the October 1, 2007 early communication about the ongoing safety review of biophosphonates. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm136201.htm.
- 60.Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 61.Kwek EB, Goh SK, Koh JS, et al. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 62.Olson K, Van Poznak C. Significance and impact of bisphosphonate-induced acute phase responses. J Oncol Pharm Pract. 2007;13:223–229. doi: 10.1177/1078155207080806. [DOI] [PubMed] [Google Scholar]
- 63.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363:453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 64.Christin-Maitre S. The role of hormone replacement therapy in the management of premature ovarian failure. Nat Clin Pract Endocrinol Metab. 2008;4:60–61. doi: 10.1038/ncpendmet0699. [DOI] [PubMed] [Google Scholar]
- 65.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 66.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 67.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to EVISTA: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 68.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 69.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65:125–134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 70.O'Regan RM, Gajdos C, Dardes RC, et al. Effects of raloxifene after tamoxifen on breast and endometrial tumor growth in athymic mice. J Natl Cancer Inst. 2002;94:274–283. doi: 10.1093/jnci/94.4.274. [DOI] [PubMed] [Google Scholar]
- 71.Stewart HJ, Forrest AP, Everington D, et al. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. The Scottish Cancer Trials Breast Group. Br J Cancer. 1996;74:297–299. doi: 10.1038/bjc.1996.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eng-Wong J, Reynolds JC, Venzon D, et al. Effect of raloxifene on bone mineral density in premenopausal women at increased risk of breast cancer. J Clin Endocrinol Metab. 2006;91:3941–3946. doi: 10.1210/jc.2005-2827. [DOI] [PubMed] [Google Scholar]
- 73.Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 74.Goss P, Bondarenko IN, Manikhas GN, et al. Phase III, double-blind, controlled trial of atamestane plus toremifene compared with letrozole in postmenopausal women with advanced receptor-positive breast cancer. J Clin Oncol. 2007;25:4961–4966. doi: 10.1200/JCO.2006.09.5455. [DOI] [PubMed] [Google Scholar]
- 75. Refer http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/index.cfm?fuseaction=Search.DrugDetails for latest package insert.
- 76.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 77.Farooki A, Fornier M, Girotra M. Anabolic therapies for osteoporosis. N Engl J Med. 2007;357:2410–2411. doi: 10.1056/NEJMc076405. [DOI] [PubMed] [Google Scholar]
- 78.Cummings SR, McClung MR, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM trial [abstract].. Presented at the American Society of Bone and Mineral Research 30th Annual Meeting; Montreal, Quebec, Canada. September 12–16, 2008; Abstract 1286. [Google Scholar]
- 79.Brown JP, Deal C, de Gregorin LH, et al. Effect of densoumab vs alendronate on bone turnover markers and bone mineral density changes at 12 months based on baseline bone turnover level [abstract].. Presented at the American Society of Bone and Mineral Research 30th Annual Meeting; Montreal, Quebec, Canada. September 12–16, 2008; Abstract 1285. [Google Scholar]
- 80.Kendler DL, Benhamou CL, Brown JP, et al. Effects of denosumab vs. alendronate on bone mineral density (BMD), bone turnover markers (BTM), and safety in women previously treated with alendronate [abstract].. Presented at the American Society of Bone and Mineral Research 30th Annual Meeting; Montreal, Quebec, Canada. September 12–16, 2008; Abstract 138. [Google Scholar]
- 81.Chesnut CH, III, Bell NH, Clark GS, et al. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med. 1997;102:29–37. doi: 10.1016/s0002-9343(96)00387-7. [DOI] [PubMed] [Google Scholar]
- 82.Ravn P, Clemmesen B, Christiansen C. Biochemical markers can predict the response in bone mass during alendronate treatment in early postmenopausal women. Alendronate Osteoporosis Prevention Study Group. Bone. 1999;24:237–244. doi: 10.1016/s8756-3282(98)00183-5. [DOI] [PubMed] [Google Scholar]
- 83.Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85:3109–3115. doi: 10.1210/jcem.85.9.6777. [DOI] [PubMed] [Google Scholar]
- 84.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 85.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol. 2000;18:1570–1593. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 86.Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575–1579. doi: 10.1002/cncr.21385. [DOI] [PubMed] [Google Scholar]
- 87.Burstein HJ, Winer EP. Primary care for survivors of breast cancer. N Engl J Med. 2000;343:1086–1094. doi: 10.1056/NEJM200010123431506. [DOI] [PubMed] [Google Scholar]
- 88.Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 89.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 90.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 91.Headley JA, Theriault RL, LeBlanc AD, et al. Pilot study of bone mineral density in breast cancer patients treated with adjuvant chemotherapy. Cancer Invest. 1998;16:6–11. doi: 10.3109/07357909809039747. [DOI] [PubMed] [Google Scholar]
- 92.Powles TJ, McCloskey E, Paterson AH, et al. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst. 1998;90:704–708. doi: 10.1093/jnci/90.9.704. [DOI] [PubMed] [Google Scholar]
- 93.Saarto T, Blomqvist C, Valimaki M, et al. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol. 1997;15:1341–1347. doi: 10.1200/JCO.1997.15.4.1341. [DOI] [PubMed] [Google Scholar]
- 94.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26:4739–4745. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delmas PD, Balena R, Confravreux E, et al. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol. 1997;15:955–962. doi: 10.1200/JCO.1997.15.3.955. [DOI] [PubMed] [Google Scholar]
- 96.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 97.Eastell R, Hannon RA, Cuzick J, et al. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006;21:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 98.Fogelman I, Blake GM, Blamey R, et al. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos Int. 2003;14:1001–1006. doi: 10.1007/s00198-003-1508-y. [DOI] [PubMed] [Google Scholar]
- 99.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26:4739–4745. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ripps BA, VanGilder K, Minhas B, et al. Alendronate for the prevention of bone mineral loss during gonadotropin-releasing hormone agonist therapy. J Reprod Med. 2003;48:761–766. [PubMed] [Google Scholar]
- 101.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 102.Goss P, Wu M. Application of aromatase inhibitors in endocrine responsive breast cancers. Breast. 2007;16(Suppl 2):S114–119. doi: 10.1016/j.breast.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 103.Geisler J, Lonning PE. Endocrine effects of aromatase inhibitors and inactivators in vivo: review of data and method limitations. J Steroid Biochem Mol Biol. 2005;95:75–81. doi: 10.1016/j.jsbmb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 104.Simpson ER, Dowsett M. Aromatase and its inhibitors: significance for breast cancer therapy. Recent Prog Horm Res. 2002;57:317–338. doi: 10.1210/rp.57.1.317. [DOI] [PubMed] [Google Scholar]
- 105.Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol. 2008;15:S30–40. doi: 10.3747/co.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chowdhury S, Pickering LM, Ellis PA. Adjuvant aromatase inhibitors and bone health. J Br Menopause Soc. 2006;12:97–103. doi: 10.1258/136218006778234020. [DOI] [PubMed] [Google Scholar]
- 107.Body JJ, Bergmann P, Boonen S, et al. Management of cancer treatment-induced bone loss in early breast and prostate cancer—a consensus paper of the Belgian Bone Club. Osteoporos Int. 2007;18:1439–1450. doi: 10.1007/s00198-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 108.Perez EA. Safety of aromatase inhibitors in the adjuvant setting. Breast Cancer Res Treat. 2007;105(Suppl 1):75–89. doi: 10.1007/s10549-007-9704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 110.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 111.Coombes RC, Hall E, Gibson LJ, et al. Intergroup Exemestane Study: a randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 112.Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 113.National Cancer Institute [8 June 2009];Phase III randomized adjuvant study of exemestane versus anastrozole in postmenopausal women receptor-positive primary breast cancer. Available at: http://www.cancer.gov/clinicaltrials/CAN- NCIC-MA27.
- 114.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 115.Coleman RE. Effect of anastrozole on bone mineral density: 5-year results from the ‘Arimidex’, Tamoxifen, Alone or in Combination (ATAC) trial [abstract]. J Clin Oncol. 2006;24(Suppl 1):5s. doi: 10.1200/JCO.2007.11.0726. Abstract 511. [DOI] [PubMed] [Google Scholar]
- 116.Van Poznak C, Hannon RA, Clack G, et al. The SABRE study: effects of risedronate on bone mineral density and bone metabolism in postmenopausal women using anastrozole as adjuvant therapy for hormone receptor-positive early stage breast cancer—first results [abstract]. Breast Cancer Res Treat. 2006;100(Suppl 1) Abstract 4061. [Google Scholar]
- 117.Van Poznak C, Hannon R, Clack G, et al. Managing cancer treatment-induced bone loss: 24-month results from the Study of Anastrozole with the Bisphosphonate RisedronatE (SABRE).. San Antonio Breast Cancer symposium; Abstract 1137. [Google Scholar]
- 118.Lester JE, Gutcher SA, Ellis S, et al. Use of monthly oral ibandronate to prevent anastrozole- induced bone loss during adjuvant treatment for breast cancer: two-year results from the ARIBON study [abstract]. J Clin Oncol. 2008;26(Suppl 1) Abstract 554. [Google Scholar]
- 119.Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving letrozole: ZO-FAST study results. Cancer. 2008;112:1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 120.Schenk N, Llombart A, et al. The E-ZO-FAST trial: zoledronic acid (ZA) effectively inhibits aromatase inhibitor associated bone loss (AIBL) in postmenopausal women (PMW) with early breast cancer (EBC) receiving adjuvant letrozole [abstract].. Presented at the 14th European Cancer Conference; Barcelona, Spain. September 23–27, 2007; Abstract 2008. [Google Scholar]
- 121.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 122.Guise TA, Oefelein MG, Eastham JA, et al. Estrogenic side effects of androgen deprivation therapy. Rev Urol. 2007;9:163–180. [PMC free article] [PubMed] [Google Scholar]
- 123.Basaria S, Lieb J, II, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 124.Khosla S, Melton LJ, III, Atkinson EJ, O'Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 125.Mellstrom D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23:1552–1560. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 126.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 127.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 128.Mittan D, Lee S, Miller E, et al. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab. 2002;87:3656–3661. doi: 10.1210/jcem.87.8.8782. [DOI] [PubMed] [Google Scholar]
- 129.Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 130.Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83:1561–1566. [PubMed] [Google Scholar]
- 131.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 132.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. discussion 2367. [PubMed] [Google Scholar]
- 133.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss in men receiving gonadotropin releasing hormone agonist therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 134.Smith MR, Eastham J, Gleason D, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 135.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 137.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 138.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase III clinical study. J Urol. 2008;179:152–155. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith MR, Malkowicz SB, Chu F, et al. Toremifene improves lipid profiles in men receiving androgen-deprivation therapy for prostate cancer: interim analysis of a multicenter phase III study. J Clin Oncol. 2008;26:1824–1829. doi: 10.1200/JCO.2007.13.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Senaratne SG, Mansi JL, Colston KW. The bisphosphonate zoledronic acid impairs Ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. Br J Cancer. 2002;86:1479–1486. doi: 10.1038/sj.bjc.6600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van der Pluijm G, Vloedgraven H, van Beek E, et al. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Invest. 1996;98:698–705. doi: 10.1172/JCI118841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–2954. [PubMed] [Google Scholar]
- 143.Teronen O, Konttinen YT, Salo T, et al. [Bisphosphonates inhibit matrix metalloproteinases—a new possible mechanism of action]. Duodecim. 1999;115:13–15. [in Finnish] [PubMed] [Google Scholar]
- 144.Boissier S, Magnetto S, Frappart L, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res. 1997;57:3890–3894. [PubMed] [Google Scholar]
- 145.Sasaki A, Boyce BF, Story B, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:3551–3557. [PubMed] [Google Scholar]
- 146.Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. New Engl J Med. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 148.Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19:2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19:10–17. doi: 10.1200/JCO.2001.19.1.10. [DOI] [PubMed] [Google Scholar]
- 150.Ha TC, Li H. Meta-analysis of clodronate and breast cancer survival. Br J Cancer. 2007;96:1796–1801. doi: 10.1038/sj.bjc.6603661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 152.Mason MD, Sydes MR, Glaholm J, et al. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J Natl Cancer Inst. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 153.Roudier MP, Vesselle H, True LD, et al. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin Exp Metastasis. 2003;20:171–180. doi: 10.1023/a:1022627421000. [DOI] [PubMed] [Google Scholar]
- 154.Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 155.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 156.Witham TF, Khavkin YA, Gallia GL, et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2:87–94. doi: 10.1038/ncpneuro0116. quiz 116. [DOI] [PubMed] [Google Scholar]
- 157.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 158.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 159.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 160.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 161.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 162.Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133–1137. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tubiana-Hulin M, Beuzeboc P, Mauriac L, et al. [Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases]. Bull Cancer. 2001;88:701–707. [in French] [PubMed] [Google Scholar]
- 164.Diel IJ, Body JJ, Lichinitser MR, et al. Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer. 2004;40:1704–1712. doi: 10.1016/j.ejca.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 165.Body JJ, Diel IJ, Bell R, et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain. 2004;111:306–312. doi: 10.1016/j.pain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 166.Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 167.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 168.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 169.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 170.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 171.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 172.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 173.Dearnaley DP, Sydes MR, Mason MD, et al. A double-blind, placebo-controlled, randomized trial of sodium clodronate for metastatic prostate cancer (MRC PR05 trial). J Natl Cancer Inst. 2003;95:1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 174.Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–3342. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 175.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 176.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 177.ClinicalTrials.gov [May 2009];Study to evaluate zoledronic acid on quality of life and skeletal-related events as adjuvant treatment in patients with hormone-naïve prostate cancer and bone metastasis who have undergone orchiectomy. Available at: http://www.clinicaltrial.gov/ct2/show/NCT00237146?term=hormone+naive+prostate+cancer&rank=4.
- 178.ClinicalTrials.gov [May 2009];Double-blind study of denosumab compared with zoledronic acid in the treatment of bone metastases in men with hormone-refractory prostate cancer. Available at: http://www.clinicaltrial.gov/ct2/show/NCT00321620?term=denosumab+and+prostate+cancer&rank=2.
- 179.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 180.Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 181.ClinicalTrials.gov [May 2009];Dasatinib in treating patients with stage IV breast cancer that has spread to the bone. Available at: http://clinicaltrials.gov/ct2/show/NCT00410813.
- 182.ClinicalTrials.gov [May 2009];Dasatinib in combination with zoledronic acid for the treatment of breast cancer with bone metastasis. doi: 10.1158/1078-0432.CCR-15-2845. Available at: http://clinicaltrials.gov/ct2/show/NCT00566618?show_desc=Y. [DOI] [PubMed]
- 183.ClinicalTrials.gov [May 2009];Trial of dasatinib (Sprycel®) in subjects with hormone-refractory prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00570700.
- 184.Le Gall C, Bonnelye E, Clezardin P. Cathepsin K inhibitors as treatment of bone metastasis. Curr Opin Support Palliat Care. 2008;2:218–222. doi: 10.1097/SPC.0b013e32830baea9. [DOI] [PubMed] [Google Scholar]
- 185.Le Gall C, Bellahcene A, Bonnelye E, et al. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007;67:9894–9902. doi: 10.1158/0008-5472.CAN-06-3940. [DOI] [PubMed] [Google Scholar]
- 186.ClinicalTrials.gov [May 2009];Archive: view of NCT00692458 on 2008_12_07. Available at: http://clinicaltrials.gov/archive/NCT00692458/2008_12_07.
- 187.Muindi J, Coombes RC, Golding S, et al. The role of computed tomography in the detection of bone metastases in breast cancer patients. Br J Radiol. 1983;56:233–236. doi: 10.1259/0007-1285-56-664-233. [DOI] [PubMed] [Google Scholar]
- 188.Durning P, Best JJ, Sellwood RA. Recognition of metastatic bone disease in cancer of the breast by computed tomography. Clin Oncol. 1983;9:343–346. [PubMed] [Google Scholar]
- 189.Hanna SL, Fletcher BD, Fairclough DL, et al. Magnetic resonance imaging of disseminated bone marrow disease in patients treated for malignancy. Skeletal Radiol. 1991;20:79–84. doi: 10.1007/BF00193815. [DOI] [PubMed] [Google Scholar]
- 190.Krishnamurthy GT, Tubis M, Hiss J, Blahd WH. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA. 1977;237:2504–2506. [PubMed] [Google Scholar]
- 191.Zelinka T, Timmers HJ, Kozupa A, et al. Role of positron emission tomography and bone scintigraphy in the evaluation of bone involvement in metastatic pheochromocytoma and paraganglioma: specific implications for succinate dehydrogenase enzyme subunit B gene mutations. Endocr Relat Cancer. 2008;15:311–323. doi: 10.1677/ERC-07-0217. [DOI] [PubMed] [Google Scholar]
- 192.Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. Am J Roentgenol. 2001;177:229–236. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 193.Ohta M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun. 2001;22:875–879. doi: 10.1097/00006231-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 194.Kao CH, Hsieh JF, Tsai SC, et al. Comparison and discrepancy of 18F-2-deoxyglucose positron emission tomography and Tc-99m MDP bone scan to detect bone metastases. Anticancer Res. 2000;20:2189–2192. [PubMed] [Google Scholar]
- 195.Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 196.Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 197.Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 198.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 199.van den Hout WB, van der Linden YM, Steenland E, et al. Single-versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95:222–229. doi: 10.1093/jnci/95.3.222. [DOI] [PubMed] [Google Scholar]
- 200.Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15:373–385. doi: 10.1007/s00520-006-0161-3. [DOI] [PubMed] [Google Scholar]
- 201.Gerszten PC, Burton SA, Welch WC, et al. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104:2244–2254. doi: 10.1002/cncr.21467. [DOI] [PubMed] [Google Scholar]
- 202.Quilty PM, Kirk D, Bolger JJ, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33–40. doi: 10.1016/0167-8140(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 203.Porter AT, McEwan AJB, Powe JE, et al. Results of a randomized phase III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805–813. doi: 10.1016/0360-3016(93)90309-j. [DOI] [PubMed] [Google Scholar]
- 204.Robinson RG. Strontium-89-precursor targeted therapy for pain relief of blastic metastatic disease. Cancer. 1993;72:3433–3435. doi: 10.1002/1097-0142(19931201)72:11+<3433::aid-cncr2820721609>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 205.Sartor O, Reid RH, Hoskin PJ, et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–945. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 206.Maxon HR, Schroder LE, Hertzberg VS, et al. Rhenium-186(Sn)-HEDP for treatment of painful osseous metastases: results of a double blind crossover comparison with placebo. J Nucl Med. 1991;32:1877–1881. [PubMed] [Google Scholar]
- 207.Palmedo H, Manka-Waluch A, Albers P, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol. 2003;21:2869–2875. doi: 10.1200/JCO.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 208.Tu S, Millikan RE, Mengistu B, et al. Bone targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 209.Ward WG, Holsenbeck S, Dorey FJ, et al. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003:S230–244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 210.Redmond BJ, Biermann JS, Blasier RB. Interlocking intramedullary nailing of pathological fractures of the shaft of the humerus. J Bone Joint Surg Am. 1996;78A:891–896. doi: 10.2106/00004623-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 211.Franck WM, Olivieri M, Jannasch O, Hennig FF. An expandable nailing system for the management of pathological humerus fractures. Arch Orthop Trauma Surg. 2002;122:400–405. doi: 10.1007/s00402-002-0428-1. [DOI] [PubMed] [Google Scholar]
- 212.Samsani SR, Panikkar V, Venu KM, et al. Breast cancer bone metastasis in femur: surgical considerations and reconstruction with Long Gamma Nail. Eur J Surg Oncol. 2004;30:993–997. doi: 10.1016/j.ejso.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 213.Moholkar K, Mohan R, Grigoris P. The Long Gamma Nail for stabilisation of existing and impending pathological fractures of the femur: an analysis of 48 cases. Acta Orthop Belg. 2004;70:429–434. [PubMed] [Google Scholar]
- 214.Marco RA, Sheth DS, Boland PJ, et al. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82:642–651. doi: 10.2106/00004623-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 215.Benevenia J, Cyran FP, Biermann JS, et al. Treatment of advanced metastatic lesions of the acetabulum using the saddle prosthesis. Clin Orthop Relat Res. 2004:23–31. doi: 10.1097/01.blo.0000141387.03035.3e. [DOI] [PubMed] [Google Scholar]
- 216.Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 217.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 218.Lane JM, Hong R, Koob J, et al. Kyphoplasty enhances function and structural alignment in multiple myeloma. Clin Orthop Relat Res. 2004;426:49–53. doi: 10.1097/01.blo.0000131642.96984.74. [DOI] [PubMed] [Google Scholar]
- 219.Kelekis A, Lovblad KO, Mehdizade A, et al. Pelvic osteoplasty in osteolytic metastases: technical approach under fluoroscopic guidance and early clinical results. J Vasc Interv Radiol. 2005;16:81–88. doi: 10.1097/01.RVI.0000141717.84515.92. [DOI] [PubMed] [Google Scholar]
- 220.Callstrom MR, Charboneau JW, Goetz MP, et al. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15. doi: 10.1007/s00256-005-0003-2. [DOI] [PubMed] [Google Scholar]
- 221.Kalantaridou SN, Davis SR, Nelson LM. Premature ovarian failure. Endocrinol Metab Clin North Am. 1998;27:989–1006. doi: 10.1016/s0889-8529(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 222.Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 223.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]