Abstract
Purpose
Androgen deprivation therapy is associated with fracture risk in men with prostate cancer. We assessed the effects of toremifene, a selective estrogen receptor modulator, on fracture incidence in men receiving androgen deprivation therapy during a 2-year period.
Materials and Methods
In this double-blind, placebo controlled phase III study 646 men receiving androgen deprivation therapy for prostate cancer were assigned to toremifene (80 mg by mouth daily) and 638 were assigned to placebo. Subjects were followed for 2 years. The primary study end point was new vertebral fractures. Secondary end points included fragility fractures, bone mineral density and lipid changes.
Results
The 2-year incidence of new vertebral fractures was 4.9% in the placebo group vs 2.5% in the toremifene group, a significant relative risk reduction of 50% (95% CI –1.5 to 75.0, p = 0.05). Toremifene significantly increased bone mineral density at the lumbar spine, hip and femoral neck vs placebo (p <0.0001 for all comparisons). There was a concomitant decrease in markers of bone turnover (p <0.05 for all comparisons). Toremifene also significantly improved lipid profiles. Venous thromboembolic events occurred more frequently with toremifene than placebo with 7 subjects (1.1%) in the placebo group experiencing a venous thromboembolic event vs 17 (2.6%) in the toremifene group. Other adverse events were similar between the groups.
Conclusions
Toremifene significantly decreased the incidence of new vertebral fractures in men receiving androgen deprivation therapy for prostate cancer. It also significantly improved bone mineral density, bone turnover markers and serum lipid profiles.
Keywords: osteoporosis, prostatic neoplasms, selective estrogen receptor modulators, toremifene
Prostate cancer is the most frequently diagnosed noncutaneous cancer in men in the United States and the second most common cause of cancer death.1 ADT, with bilateral orchiectomy or the administration of GnRH agonists, is the mainstay of treatment for metastatic prostate cancer.2 Additionally GnRH agonists are frequently administered to men with locally advanced or recurrent disease. Overall approximately a third of the estimated 2 million prostate cancer survivors in the United States currently receive GnRH agonist treatment.3
The intended therapeutic effect of ADT is to reduce testosterone to castrate levels. Because estradiol is derived from the peripheral conversion of testosterone by aromatase, ADT also markedly decreases serum estradiol levels, which may result in unintended estrogen deficiency side effects. Estradiol is critical to bone formation and bone resorption in men.4 The impact of these changes is decreased BMD and increased incidence of clinical fractures.5,6
Fractures are an important cause of morbidity in men worldwide. After age 50 years 1 in 4 men experience a clinical fracture.7 A third of hip fractures occur in men and they are more likely than women to die after hip fracture.8 Loss of BMD can also lead to vertebral fractures with loss of height, respiratory dysfunction, subsequent hip and other clinically important fractures, decreased quality of life and greater mortality.9 Assessment of vertebral fractures has been standardized, and is well accepted by the United States Food and Drug Administration as an end point for clinical trials to assess the efficacy of agents for the reduction of bone fractures.10
Side effects of ADT induced estrogen deficiency are not limited to increased fracture risk. ADT increases serum cholesterol and triglycerides11 by a mechanism that likely involves estrogen receptor mediated changes in hepatic expression of apoprotein genes.12 Furthermore, the relatively larger reduction in testosterone levels compared with estradiol can be clinically manifested as gynecomastia.13
Toremifene is a second-generation SERM.14 In a small prospective study toremifene increased BMD of the hip and spine in men receiving ADT.15 In this large randomized placebo controlled trial we evaluated whether toremifene decreased the incidence of new vertebral fractures in men on ADT. Other key secondary end points included clinical fragility fractures, BMD and lipid changes.
MATERIALS AND METHODS
Study Design
In this 2-year, phase III, randomized, double-blind, placebo controlled, international (United States and Mexico), multicenter trial we evaluated the efficacy and safety of toremifene for the reduction of fractures in men receiving ADT for prostate cancer. Eligible subjects were enrolled between November 2003 (first patient, first visit) and October 2005 (last patient visit October 2007). Subjects were randomly assigned 1:1 to 80 mg toremifene by mouth daily or matching placebo.
The sponsor (GTx, Inc.) and principal investigator (MRS) designed the study. INC Research (Raleigh, North Carolina) provided data management, Synarc (San Francisco, California) provided central radiology services and CRL Medinet (Lenexa, Kansas) provided central laboratory services. An independent data and safety monitoring board monitored subject safety.
Subjects
The study included men 50 years old or older who were receiving ADT for histologically documented prostate cancer and who were at increased risk for fracture based on age 70 years or older, or osteopenia at the femoral neck or L1 to L4 lumbar spine. All subjects had a baseline serum PSA of 4 ng/ml or less; adequate bone marrow, liver and renal function; Zubrod performance status 0 or 1; and received continuous ADT for 6 or more months, or intermittent ADT for 12 or more months before enrollment (all subjects received continuous ADT for 24 months after randomization).
The study excluded subjects who received a bisphosphonate, SERM, parathyroid hormone, teriparatide, finasteride, dutasteride, PC-SPES, saw palmetto, calcitonin or oral glucocorticoids within 45 days of randomization. Additional exclusion criteria were prior toremifene treatment; less than 8 evaluable vertebrae from T4 to L4, or 4 or more prevalent vertebral fractures; weight greater than 300 lbs (due to weight limits for densitometry equipment); or chronic hepatitis, cirrhosis or history of thromboembolic disease. Notably 2 subjects who experienced on study VTEs were subsequently found to have a history of thromboembolic disease. The institutional review board for each participating institution approved the study and all subjects provided written informed consent.
End Points
The primary end point was incidence of new vertebral fractures. Secondary efficacy end points included incidence of fragility fractures; BMD at the spine, hip and femoral neck; changes in bone turnover markers (C-telopeptide, bone specific alkaline phosphatase and osteocalcin) and changes in serum lipids (total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides).
Study Procedures
Anteroposterior and lateral radiographs of thoracic and lumbar spine were obtained at baseline, month 12 and month 24 or at early termination. Vertebral fractures were assessed by an expert reader at the central imaging laboratory who evaluated vertebrae from T4 to L4 using the Genant semiquantitative scoring method.10 Prevalent vertebral fractures were defined as those present at screening visit and new vertebral fractures were defined as those in a vertebra without a prevalent fracture. Fragility fractures were defined as those resulting from trauma equivalent to a fall from less than standing height and were confirmed by expert review of radiographs. All fractures were defined as fragility fractures plus new vertebral fractures. Pathological fractures, nonfragility fractures (ie trauma from a fall greater than standing height), or fractures of the skull, face or fingers were excluded from overall fracture analysis.
Dual energy x-ray absorptiometry of the spine, hip and femoral neck was performed at baseline, month 12 and month 24, or at early termination using a Hologic (Bedford, Massachusetts) or Lunar (GE Healthcare, Waukesha, Wisconsin) densitometer. Osteopenia was defined as Hologic L1 to L4 less than 0.926 gm/cm2 and femoral neck less than 0.717 gm/cm2, Lunar L1 to L4 less than 1.050 gm/cm2 and femoral neck less than 0.849 gm/cm2. These values were calculated using a T score of less than –1 as the definition of osteopenia and then converting the T score into BMD (gm/cm2) using the respective machine manufacturer specifications. For safety reasons any subject with a 7% or greater decrease in BMD of the hip or spine was withdrawn from the study. Serum levels of bone turnover markers, lipids and reproductive hormones (testosterone, estradiol, luteinizing hormone and follicle-stimulating hormone) were measured at baseline, and months 12 and 24, and were analyzed at a central laboratory.
Statistical Analyses
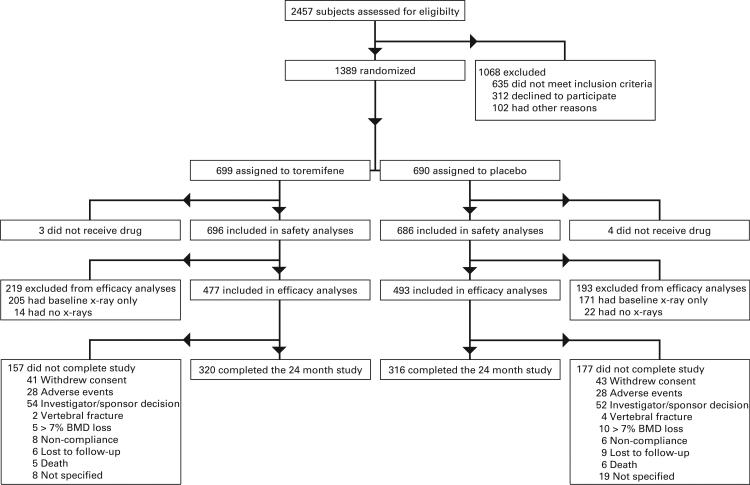
The study design assumed a 75% subject completion rate and a 16% incidence of new vertebral fractures with placebo. Based on these assumptions the planned sample size of 1,200 subjects (600 subjects per arm) provided 80% power (2-sided alpha of 0.05) to detect a 40% reduction in fracture incidence at 24 months. Subjects who received 1 dose or more of medication were included in safety analyses (fig. 1). Subjects who received 1 dose of medication or more and had an on study radiograph were included in efficacy analyses.
Figure 1.
Enrollment and outcomes
Relative risk reduction due to toremifene treatment for fractures and greater than 7% bone loss with associated 95% CI were estimated using risk estimates from PROC FREQ.16 P values were associated with Cochran-Mantel-Haenszel statistics reflecting stratification by country.17 Homogeneity of odds ratios was tested using the Breslow-Day test.18,19
Percentage changes from baseline to months 12 and 24 were calculated for BMD of the spine, hip and femoral neck; bone metabolism markers; lipids and reproductive hormones. Mean percentage change and associated standard errors are presented for complete cases only with no imputation methods for missing data. Percentage changes were compared between the placebo and toremifene arms using a generalized linear mixed model that included treatment arm and country. Mean differences and their associated 95% CIs were calculated from the least squares mean estimates. All tests were 2-sided and p ≤0.05 was considered statistically significant. There were no adjustments for multiple testing.
RESULTS
Baseline and Followup
The study included 1,284 subjects with 646 assigned to toremifene and 638 to placebo (fig. 1). Safety analyses included 1,284 randomized subjects who received 1 dose of study medication or more, and efficacy analyses included 913 subjects with 1 or more posttreatment radiographs. Approximately 200 patients in each treatment group were excluded from efficacy analyses because baseline or followup radiographs were not available. However, baseline comparisons showed no statistically significant or clinically meaningful differences between men who did and those who did not have available radiographs.
Median subject age was 76 years (range 48 to 93, table 1). Of the subjects in both groups 84% were from the United States and 16% were from Mexico. Mean ADT duration at study entry was 4.0 years for men assigned to placebo and 3.8 years in the toremifene group. Serum total testosterone levels were in castrate range (less than 50 ng/dl) for 92.1% and 93.6% of the toremifene and placebo group, respectively, and remained in castrate range at all study times for 94.3% and 95.9% of subjects, respectively (Fisher exact test p = 0.26).
Table 1.
Subject characteristics
| Toremifene | Placebo | |
|---|---|---|
| Median age (range) | 76 (51, 93) | 76 (48, 92) |
| No. older than 70 yrs (%) | 534 (82.7) | 554 (86.8) |
| No. race (%): | ||
| White | 456 (70.6) | 453 (71.0) |
| Hispanic | 113 (17.5) | 109 (17.1) |
| Black | 69 (10.7) | 68 (10.7) |
| Asian | 5 (0.8) | 4 (0.6) |
| Other | 3 (0.5) | 4 (0.6) |
| No. country of residence (%): | ||
| United States | 541 (83.8) | 534 (83.7) |
| Mexico | 105 (16.2) | 104 (16.3) |
| Mean ± SD kg/m2 body mass index | 28.3 ± 4.4 | 28.1 ± 4.1 |
| No. bilat orchiectomy (%) | 37 (5.7) | 45 (7.0) |
| Mean ± SD yrs ADT | 3.8 ± 3.1 | 4.0 ± 3.4 |
| No. prevalent vertebral fracture (%) | 77 (11.9) | 85 (13.3) |
| Mean ± SD gm/cm2 BMD: | ||
| Lumbar spine | 1.09 ± 0.22 | 1.08 ± 0.22 |
| Total hip | 0.93 ± 0.16 | 0.92 ± 0.15 |
| Femur | 0.79 ± 0.16 | 0.78 ± 0.15 |
| Trochanter | 0.75 ± 0.16 | 0.74 ± 0.16 |
| No. osteoporosis at any site (%) | 140 (21.7) | 163 (25.6) |
| Mean ± SD ng/dl serum testosterone* | 31.8 ± 72.9 | 28.1 ± 51.0 |
| No. castrate testosterone level (%) | 595 (92.1) | 597 (93.6) |
| Mean ± SD ng/dl serum estradiol† | 12.4 ± 8.5 | 11.8 ± 7.7 |
| Mean ± SD IU/l serum follicle-stimulating hormone | 7.7 ± 11.9 | 8.4 ± 14.6 |
| Mean ± SD μg/l serum PSA | 0.5 ± 1.0 | 0.4 ± 0.8 |
| Mean ± SD U/l serum bone specific alkaline phosphatase | 29.6 ± 12.4 | 29.7 ± 12.6 |
| Mean ± SD ng/ml serum osteocalcin‡ | 11.5 ± 5.8 | 11.2 ± 5.0 |
| Mean ± SD ng/ml serum C-telopeptide | 0.6 ± 0.3 | 0.6 ± 0.3 |
| Mean ± SD mg/dl total cholesterol§ | 194.5 ± 39.9 | 196.5 ± 37.5 |
| Mean ± SD mg/dl HDL cholesterol§ | 49.7 ± 13.4 | 50.4 ± 13.0 |
| Mean ± SD mg/dl LDL cholesterol§ | 111.4 ± 33.1 | 114.5 ± 31.6 |
| Mean ± SD mg/dl triglycerides∥ | 165.2 ± 90.9 | 159.0 ± 90.4 |
To convert testosterone from ng/dl to nmol/l multiply by 0.0347.
To convert estradiol from pg/ml to pmol/l multiply by 3.671.
To convert osteocalcin from mg/dl to mmol/l multiply by 0.1705.
To convert cholesterol from mg/dl to mmol/l multiply by 0.0259.
To convert triglycerides from mg/dl to mmol/l multiply by 0.0113.
Fractures
The 2-year incidence of new vertebral fractures was 4.9% (23 patients) with placebo vs 2.5% (11) with toremifene, a significant relative risk reduction of 50% (95% CI –1.5 to –75.0, p <0.05). The incidence of all fractures was 10.1% (47 patients) with placebo and 6.3% with toremifene (28), a significant relative risk reduction of 38% (95% CI 2.2 to 60.2, p = 0.036).
Bone Mineral Density
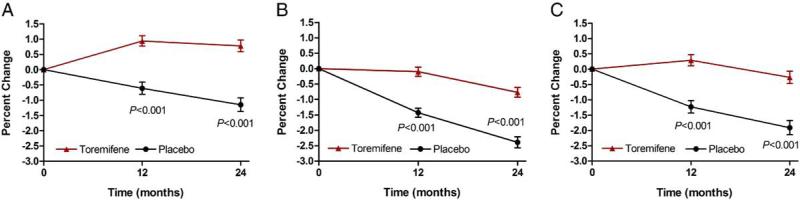
Compared with placebo BMD increased significantly at the lumbar spine (2.3%, 95% CI 1.6 to 3.1), total hip (1.9%, 95% CI 1.3 to 2.4) and femoral neck (1.9%, 95% CI 1.2 to 2.7) in the toremifene group (p <0.0001 for each comparison, fig. 2). There were 13 subjects (2.8%) receiving placebo who discontinued the study after 1 year because of a greater than 7% decrease in BMD of the hip or spine vs 6 (1.3%) receiving toremifene (relative risk reduction 51.7%, 95% CI –2.6 to 81.5, p = 0.129).
Figure 2.
Changes in BMD of lumbar spine (A), total hip (B) and femoral neck (C)
Bone Turnover Markers
Biochemical markers of bone turnover decreased significantly in subjects receiving toremifene vs placebo. At 2 years serum bone specific alkaline phosphatase, osteocalcin and C-telopeptide levels decreased by 18.3% (95% CI –22.4 to –14.1, p <0.0001), 23.9% (95% CI –31.2 to –16.7, p <0.0001) and 14.5% (95% CI –27.7 to –1.4, p = 0.031), respectively, for toremifene vs placebo.
Serum Lipids
Changes in serum lipoproteins differed significantly between the placebo and toremifene groups. At 2 years total cholesterol, LDL cholesterol and triglycerides decreased 4.7% (95% CI –7.5 to –1.9), 7.0% (95% CI –11.0 to –2.9) and 17.6% (95% CI –25.9 to –9.3), respectively, in the toremifene group vs placebo (p <0.001 for each comparison). In contrast HDL cholesterol increased 7.2% (95% CI 4.2 to 10.3, p <0.0001) with toremifene compared to placebo.
Serum Hormone Levels
At baseline mean serum total testosterone level was in the castrate range (less than 50 ng/dl [1.7 nmol/l]) in both groups (table 1). Changes in serum total testosterone, estradiol and luteinizing hormone levels did not differ significantly between the groups. In contrast, serum follicle-stimulating hormone decreased 65.0% (95% CI –54.5 to –75.5, p <0.001) in the toremifene group vs the placebo group.
Adverse Events
The number of subjects who died, had a serious AE, or had any AE or discontinued participation in the study because of an adverse event did not significantly differ between groups (table 2). The most commonly reported AEs for both groups were arthralgia, back pain, peripheral edema, constipation and dizziness. Rates for each of these AEs were similar for men assigned to toremifene or placebo.
Table 2.
Incidence of adverse events
| No. Toremifene (%) | No. Placebo (%) | |
|---|---|---|
| General: | ||
| Any AE | 482 (74.6) | 481 (75.4) |
| Serious AE | 136 (21.1) | 128 (20.1) |
| Death from AE | 14 (2.2) | 10 (1.6) |
| Discontinuation due to AE | 127 (19.7) | 110 (17.2) |
| Most common AEs (more than 5% in either group): | ||
| Arthralgia | 47 (7.3) | 75 (11.8) |
| Dizziness | 41 (6.3) | 33 (5.2) |
| Back pain | 39 (6.0) | 33 (5.2) |
| Hot flush | 25 (3.9) | 34 (5.3) |
| Hematuria | 24 (3.7) | 34 (5.3) |
| Fatigue | 24 (3.7) | 32 (5.0) |
| Diarrhea | 20 (3.1) | 33 (5.2) |
| VTEs: | ||
| Any | 17 (2.6) | 7 (1.1) |
| Fatal | 0 (0) | 0 (0) |
| Yr 1 | 13 (2.0) | 4 (0.6) |
| Yr 2 | 4 (0.6) | 3 (0.5) |
| Cardiovascular events: | ||
| Myocardial infarction | 6 (0.9) | 8 (1.3) |
| Stroke | 4 (0.7) | 4 (0.7) |
VTEs were reported in 17 subjects (2.6%; 12 deep venous thrombosis, 8 pulmonary embolism) in the toremifene group and 7 (1.1%; 6 deep venous thrombosis, 3 pulmonary embolism) in the placebo group. There were no fatal VTEs. During year 1 VTEs occurred in 13 subjects in the toremifene group and 4 in the placebo group. During year 2 VTEs were balanced (4 toremifene, 3 placebo). VTEs were more common in men 80 years old or older and those who experienced prolonged immobilization. There were no differences in the incidence of stroke (0.5% in the toremifene group vs 0.3% in the placebo group) or myocardial infarction (0.6% placebo and 0.3% toremifene, table 2). There was no observed difference in tumor progression based on bone scan, PSA or physical examination (data not shown).
DISCUSSION
During a 2-year period toremifene significantly reduced the incidence of new vertebral fractures in men receiving ADT for prostate cancer. The 50% reduction in new vertebral fractures is similar to that previously observed in postmenopausal women with osteoporosis treated with raloxifene, another SERM.20 Toremifene significantly increased BMD of the hip and spine, and decreased bone turnover markers. Effects of toremifene on BMD and bone metabolism markers were similar to those reported for toremifene and other SERMs in postmenopausal women.
This study was designed to address the prevention of fractures, an important unmet medical need in men receiving ADT for prostate cancer. A few agents have been tested for effects on BMD and bone turnover markers in men with prostate cancer receiving ADT. In a small, open label study of men receiving ADT for prostate cancer raloxifene increased BMD and decreased bone turnover markers.21 In addition, bisphosphonates including zoledronic acid22 and alendronate23 increased BMD and decreased bone turnover markers in men receiving ADT for prostate cancer. Nonetheless these agents have not demonstrated a primary fracture benefit in men receiving ADT. Recently the injectable monoclonal antibody denosumab demonstrated an improvement in BMD in men with prostate cancer on ADT.24 Denosumab also reduced new vertebral fractures, a secondary study end point.24 The current study is the first prospective study to our knowledge to evaluate targeting the estrogen receptor in men with prostate cancer on ADT with fracture incidence as the primary end point. In this study 80 mg toremifene showed a fracture benefit, and improvement in bone turnover markers and BMD. This finding is important because vertebral fractures are predictive of future clinical fractures,25 particularly hip fractures.26
Men treated with ADT for advanced prostate cancer have been shown to be at greater risk for coronary heart disease and hospital admission for myocardial infarction.27 Several mechanisms may contribute to a greater risk of cardiovascular disease including increased fat mass, decreased insulin sensitivity, and increased serum LDL cholesterol and triglycerides.28 In this study toremifene significantly improved serum lipoprotein profiles during ADT. Compared with placebo toremifene significantly increased HDL cholesterol, and significantly decreased total cholesterol, LDL cholesterol and triglycerides. Additional studies are necessary to characterize the potential impact of these changes on cardiovascular outcomes.
Men receiving toremifene had an increased incidence of VTEs compared to those receiving placebo. The relative risk of VTEs was approximately 2.4, similar to the relative risk of approximately 3.0 reported for postmenopausal women receiving raloxifene.20 Rates of other AEs were similar for both groups.
CONCLUSIONS
In men receiving ADT for prostate cancer toremifene significantly decreased new vertebral fractures, increased BMD of the hip and spine, and decreased bone turnover markers. Toremifene also significantly increased serum HDL cholesterol, and decreased serum LDL cholesterol and triglycerides. Targeting the estrogen receptor toremifene may provide a promising new treatment to prevent fractures during ADT for prostate cancer.
ACKNOWLEDGMENTS
Dr. Michael Malia from Complete Publication Solutions provided editorial assistance supported by GTx, Inc.
Supported by GTx Inc.
Clinical Trial Registration NCT00129142 (www.clinicaltrials.gov).
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- AE
adverse event
- BMD
bone mineral density
- GnRH
gonadotropin-releasing hormone
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- PSA
prostate specific antigen
- SERM
selective estrogen receptor modulator
- VTE
venous thromboembolic event
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 4.Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 6.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 7.National Osteoporosis Foundation: Men and osteoporosis [July 14, 2010]; Available at www.nof.org/men.
- 8.Seeman E. The structural basis of bone fragility in men. Bone. 1999;25:143. doi: 10.1016/s8756-3282(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 9.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 10.Genant HK. Assessment of vertebral fractures in osteoporosis research. J Rheumatol. 1997;24:1212. [PubMed] [Google Scholar]
- 11.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 13.Schwandt A, Garcia JA. Complications of androgen deprivation therapy in prostate cancer. Curr Opin Urol. 2009;19:322. doi: 10.1097/MOU.0b013e32832a082c. [DOI] [PubMed] [Google Scholar]
- 14.Wiseman LR, Goa KL. Toremifene. A review of its pharmacological properties and clinical efficacy in the management of advanced breast cancer. Drugs. 1997;54:141. doi: 10.2165/00003495-199754010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Steiner MS, Patterson A, Israeli R, et al. Toremifene citrate versus placebo for treatment of bone loss and other complications of androgen deprivation therapy in patients with prostate cancer. J Clin Oncol. 2004;22 abstract 4597. [Google Scholar]
- 16.SAS Institute, Inc . SAS/STAT 9.1 User's Guide. SAS Institute, Inc.; Cary, North Carolina: 2004. [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719. [PubMed] [Google Scholar]
- 18.Breslow NE, Day NE. Statistical Methods in Cancer Research Volume 1- the Analysis of Case-Control Studies. IARC Scientific Publications No. 32. International Agency for Research on Cancer; Lyon, France: 1980. [PubMed] [Google Scholar]
- 19.Zelen M. The analysis of several2x2 contingency tables. Biometrika. 1971;58:129. [Google Scholar]
- 20.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Eastham J, Gleason D, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 24.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melton LJ, 3rd, Atkinson EJ, Cooper C, et al. Vertebral fractures predict subsequent fractures. Osteoporos Int. 1999;10:214. doi: 10.1007/s001980050218. [DOI] [PubMed] [Google Scholar]
- 26.Naves M, Diaz-Lopez JB, Gomez C, et al. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14:520. doi: 10.1007/s00198-003-1405-4. [DOI] [PubMed] [Google Scholar]
- 27.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation for prostate cancer. J Clin Oncol. 2006;24:4448. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 28.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998. doi: 10.1016/j.juro.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]