Abstract
This article focuses on the role of PAPP-A in mammalian aging. It introduces PAPP-A and a little of its history, briefly discusses the function of PAPP-A in the insulin-like growth factor (IGF) system and the regulators of PAPP-A expression, and then reviews data concerning PAPP-A in aging and age-related diseases especially in regard to the PAPP-A knock-out (KO) mouse. The PAPP-A KO mouse is a valuable new model to test hypotheses concerning the control of the tissue availability of IGF, independent from systemic levels, on healthspan as well as lifespan.
What is PAPP-A?
PAPP-A is the acronym for pregnancy-associated plasma protein-A because the protein was first identified as one of four proteins found at high concentrations in the plasma of pregnant women (Lin et al. 1974). Its function was unknown for 25 years but had, and continues to have, clinical utility in screens for Down Syndrome (van Heesch et al 2010). In the early 1990s, several laboratories noted novel insulin-like growth factor (IGF)-dependent proteolytic activity against IGF binding protein (IGFBP)-4 in culture media from a variety of cells, including fibroblasts, osteoblasts, and vascular smooth muscle cells (Fowlkes & Freemark 1992, Conover et al.1993, Durham et al.1994, Parker et al.1995). The protease was characterized as a highly glycosylated, zinc-binding metalloproteinase with a native molecular weight greater than 200 kD (Lawrence et al. 1999a). However, the protein responsible for this proteolytic activity remained elusive until Lawrence et al. (1999b) successfully isolated it from human fibroblast conditioned media and identified it, using mass spectrometry and molecular probes, as PAPP-A. Subsequent studies demonstrated that PAPP-A clearly had purpose outside of pregnancy.
What does PAPP-A do?
PAPP-A is as an important regulator of local IGF action through proteolysis of IGFBPs (reviewed in Boldt & Conover 2007). Its primary physiological substrate is IGFBP-4, although proteolytic activity against IGFBP-2 and -5 has also been described (Laursen et al. 2001, Monget et al. 2003, Rivera et al. 2003, Kumar et al. 2005). IGFBP-4 is considered an inhibitory IGFBP in that it binds IGF-I and IGF-II with high affinity thereby restricting their interaction with IGF receptors on cells. IGFBP-4 is a substrate for PAPP-A only when it is complexed with IGF, hence the observed IGF-dependent proteolytic activity in cell-free assay (Byun et al. 2000, Laursen et al. 2001)). Moreover, this IGFBP-4/IGF complex can serve as a pericellular reservoir (Ning et al. 2008). PAPP-A cleaves the IGFBP-4 into low binding-affinity fragments, releasing the IGF for receptor activation. In this way, IGF action can be augmented without changes in IGF ligand or receptor expression. PAPP-A regulation of local IGF receptor activation has been assessed by phosphorylation of IGF receptor and downstream signaling intermediate, IGF-stimulated 3H-thymidine incorporation and IGF-responsive gene expression in vitro and in vivo in various systems (Ortiz et al. 2003, Conover et al. 2004, Resch et al. 2006, Harrington et al. 2007, Miller et al. 2007, Conover et al. 2010). Importantly, PAPP-A is secreted and then binds to cell surfaces in an autocrine/paracrine manner (Laursen et al. 2002, Conover et al. 2007). The preserved protease activity of cell-associated PAPP-A would serve to amplify local IGF action. Although IGF-independent actions of PAPP-A are possible and even plausible (Jadlowiec et al. 1995), several studies using different approaches support regulated proteolysis of IGFBP-4 as the major function of PAPP-A in vivo (Bale & Conover 2005, Ning et al. 2008, Phang et al. 2010).
What regulates PAPP-A expression in vitro?
The most potent stimulators of PAPP-A expression in human fibroblasts and in human vascular smooth muscle and endothelial cells are the proinflammatory cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-1β (Resch et al. 2004, Conover et al. 2006, Conover et al. 2008a,). IL-6 is also stimulatory in human coronary artery smooth muscle cells (Boldt & Conover 2007) and transforming growth factor-β is stimulatory in human osteoblasts (Ortiz et al. 2003). Interestingly, PAPP-A expression was found to be markedly increased with human osteoblast senescence (Kveiborg et al. 2000). Direct inhibitors of cytokine-stimulated PAPP-A expression include the polyphenol, resveratrol, and the anti-oxidant, N-acetyl cysteine (Conover et al. 2006, Boldt & Conover 2007). These in vitro findings may be relevant to the regulation and biological function of PAPP-A in vivo, as will be touched upon in a later section.
IGFs and aging
According to the theory of antagonistic pleiotropy, there are gene products that can have opposite effects on biological fitness at different ages such that their effects are beneficial early in life but detrimental to the organism later in life (Williams 1957). This appears relevant to the seeming paradox of the IGF system. IGFs are essential for normal body size during fetal development, peak bone mass during puberty, and optimal fecundity in the reproductive period. On the other hand, IGFs are associated with aging and age-related diseases. These observations are generally true for both mice and humans. Since PAPP-A enhances IGF signaling, then the same might be expected for PAPP-A’s effects in early versus late life. And the converse should also hold, i.e., loss of PAPP-A or inhibition of PAPP-A activity should have negative effects early in life and beneficial effects later in life. To test this hypothesis, we developed a mouse with global deletion of PAPP-A gene expression (Conover et al. 2004). The prediction was that loss of PAPP-A would have impact during critical periods when heightened IGF activity is important and in specific tissues where PAPP-A expression is upregulated. Moreover, since PAPP-A’s effects on IGF activity are moderating rather than absolute, the loss of PAPP-A would not be expected to have the dire consequences seen in IGF-I and IGF-I receptor KO mice (Liu et al. 1993).
PAPP-A KO mice
PAPP-A KO mice are born as proportional dwarfs due to the suppression of IGF-II-mediated signaling during early embryogenesis prior to organogenesis (Conover et al. 2004). IGF-II is the predominant fetal IGF, and the dwarf phenotype of the PAPP-A KO mouse could be rescued by increasing Igf2 gene expression during fetal development through disruption of its imprinting (Bale & Conover 2005). The PAPP-A KO mice also have mild deficiencies in bone mass and ovarian steroidogenesis (Tanner et al. 2008, Nyegaard et al. 2010), processes that are known to be regulated by IGFs during puberty and early adulthood. Otherwise, they appear to grow normally while maintaining their small body size. Moreover, and in accordance with antagonistic pleiotropy, PAPP-A KO mice have a dramatic 20–40% increase in median and maximum lifespan compared to wild-type littermates (Conover & Bale 2007, Conover et al. 2010b). Reduced energy expenditure and altered glucose-insulin homeostasis were excluded as key determinants of the enhanced longevity of PAPP-A KO mice (Conover et al. 2008b). Of importance to the model, circulating levels of IGF-I are not different in wild-type and PAPP-A KO mice. Recently, Swindell et al. (2010) evaluated gene expression in various tissues of wild-type and PAPP-A KO mice and found both similar and contrasting expression patterns between PAPP-A KO mice and long-lived dwarf mice with reduced circulating IGF-I. It is not surprising that kidney, with one of the highest PAPP-A expression levels in wild-type mice, had the most marked changes in gene expression in PAPP-A KO mice, whereas liver, with very little PAPP-A expression, showed few changes. The recent review by Bartke (2008) presents other comparisons of PAPP-A KO mice to long-lived mouse models with genetic manipulations of the IGF-I axis. Although many of these long-lived mice are dwarfs, PAPP-A KO mice whose body size was normalized during fetal development did not lose their longevity advantage (Conover et al. 2010a).
A recent report of end-of-life pathology (Conover et al. 2010b) indicated that the incidence of neoplastic disease was not significantly different in wild-type and PAPP-A KO mice. However, it occurred in older-aged PAPP-A KO compared with wild-type mice. Furthermore, PAPP-A KO mice were less likely to show degenerative changes of age even though the average age at death was higher. Co-morbidities were significantly reduced in PAPP-A KO mice, as well. In addition, scheduled sacrifice pathology at 78, 104 and 130 weeks of age indicated that wild-type mice had more degenerative changes earlier than PAPP-A KO mice. Specifically, cardiomyopathy, nephropathy, neurodegenerative lesions, and testicular, ovarian and thymic atrophy were more evident and more severe in wild-type than in PAPP-A KO mice at the time points studied. These results are consistent with a general delay in age-related degenerative disease in PAPP-A KO mice, which likely enables them to maintain a better health status compared with wild-type mice.
As reported previously, the thymi of PAPP-A KO mice are relatively resistant to the normal age-dependent atrophy seen in mice and in humans (Vallejo et al. 2009). At 78 weeks of age the thymi of wild-type mice were very small in size with extensive fatty tissue infiltrates and few thymocytes. In contrast, PAPP-A KO mice maintained thymic structure with normal histology and cellularity and a pool of diverse and functionally competent T cells. However, at 104 weeks, obvious thymic tissue was not present in most of the PAPP-A KO mice, suggesting delay rather than absolute resistance to thymic involution later in life (Conover et al. 2010b). It is intriguing that this delay in thymic involution paralleled the delayed occurrence of neoplasia, especially lymphomas, in PAPP-A KO mice. However, further study is necessary to determine whether-or-not there is a direct relationship.
PAPP-A KO mice are also relatively resistant to the development of atherosclerosis (Harrington et al. 2007). In these studies, PAPP-A KO mice were crossed with apolipoprotein E (ApoE) KO mice, the latter being an established mouse model of atherosclerosis susceptibility (Meir & Letersdorf 2004). In the absence of PAPP-A, mice on the ApoE-null background (double KO) had a 70–80% reduction in lesion area after 10 weeks on a high fat diet compared to ApoE KO mice with wild-type PAPP-A gene expression. This was in spite of similar elevations in serum cholesterol and triglyceride levels in the two groups of mice. ApoE KO mice had a progressive increase in aortic lesion complexity, whereas double KO mice had lesions resembling more early stage fatty streaks predominantly composed of lipid-enriched macrophages. Interestingly, the absolute amount of macrophage staining in the lesions of ApoE KO and double KO mice did not differ. In addition, there were no significant differences in expression of macrophage-derived cytokines, TNF-α and IL-1β. Sulchanov et al. (2007) found that systemic infusion of human recombinant IGF-I into ApoE KO mice fed a high fat diet for 12 weeks was associated with a 30% reduction in atherosclerotic lesion size, decreased macrophage accumulation within lesions, and decreased markers of inflammation. Although these findings appear to be contrary to the findings in the PAPP-A KO mice, they likely reflect differences in local versus circulating IGF-I action. In a recent study (Conover et al. 2010), overexpression of PAPP-A in arterial smooth muscle accelerated atherosclerosis in ApoE KO mice, further evidence for the importance of PAPP-A in the cardiovascular system.
PAPP-A and inflammatory stress
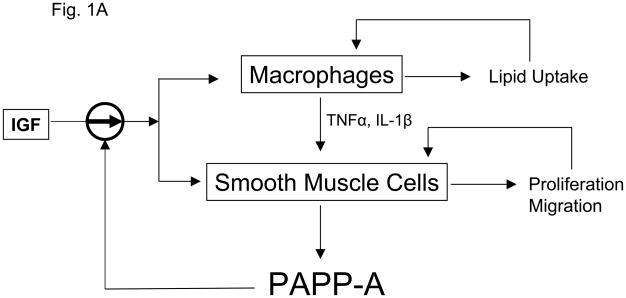
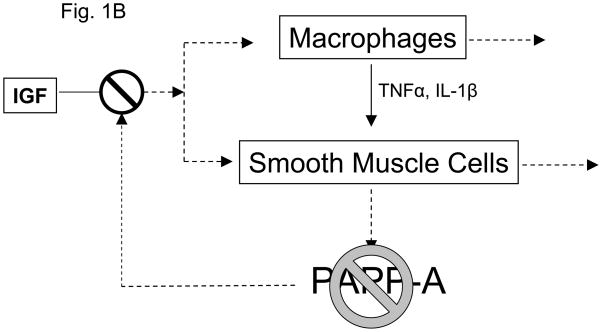
Both atherosclerosis and aging are characterized by chronic inflammatory stress. Immunostaining of human atherosclerotic plaque for PAPP-A revealed intense staining in the inflammatory regions of unstable plaque associated with activated macrophages and smooth muscle cells (Bayes-Genis et al. 2001). Macrophages do not express PAPP-A (Conover et al. 2007). However, our working model is that activated macrophages synthesize proinflammatory cytokines that stimulate vascular smooth muscle cells to synthesize and secrete PAPP-A. PAPP-A can function as both an autocrine and paracrine factor by binding to cells in the plaque. Cell-associated PAPP-A thereby enhances local IGF actions on smooth muscle cells and macrophages, representing an important amplification point in atherosclerotic plaque progression (Fig. 1A). With the loss of PAPP-A as a target of these cytokines, the vicious cycle would be blunted in spite of the continued presence of macrophages and similar levels of TNF-α and IL-1β expression (Fig. 1B). If validated, this model of PAPP-A and inflammatory stress could also apply to other situations. Aging is one such situation (Goto 2008, Vasto et al. 2009). Adipogenesis is another. PAPP-A is highly expressed in human, baboon, and mouse preadipocytes from visceral fat depots (Tchkonia et al. 2007, Tchoukalova et al. 2009, our unpublished data) which is known to be an inflammatory environment especially in obese subjects.
Figure 1.
Working model for (A) PAPP-A enhancement of atherosclerotic lesion development and (B) the consequence of loss of PAPP-A in this context.
PAPP-A and oxidative stress
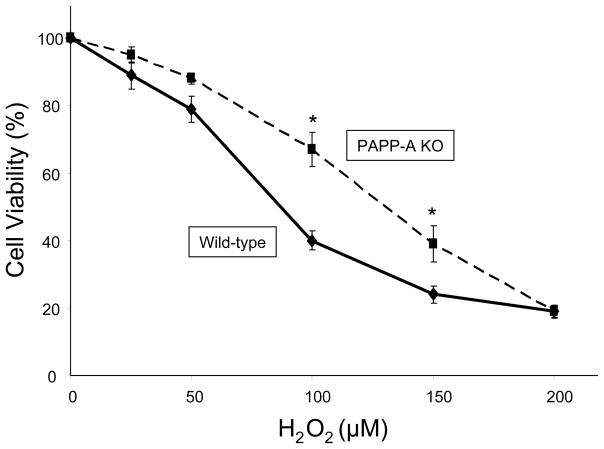
At this time, little is known about PAPP-A and oxidative stress, a principal cause of aging (Finkel & Holbrook 2000). Activation of NFκB, a transcription factor involved in oxidative stress, mediated cytokine stimulation of PAPP-A expression in human fibroblasts (Resch et al. 2004). Preliminary data suggest that skin fibroblasts from PAPP-A KO mice are more resistant to hydrogen peroxide-induced cell death than fibroblasts from wild-type mice (Fig. 2). Also, levels of PAPP-A have been shown to be elevated in renal transplant patients and correlated with levels of F2-isoprostanes, markers of in vivo oxidative stress (Coskun et al. 2007, Lauzurica et al. 2005). Further studies will lead to a better understanding of the relationship between PAPP-A, oxidative stress, and aging.
Figure 2.
Skin fibroblasts from wild-type and PAPP-A KO mice were treated with phenol red-free DMEM/10% fetal bovine serum and the indicated concentrations of hydrogen peroxide for 6 h. Cell viability was assessed by trypan blue exclusion. Values are mean ± SEM, N = 4, *P < 0.05.
What’s next?
Accumulating data attest to the importance of PAPP-A in mammalian aging. Nevertheless, more research is needed to fully define its role in the aging process. This review suggests some specific areas that warrant further investigation, among them, immune function, inflammatory response, oxidative stress, adipogenesis, and age-related cancer, atherosclerosis and kidney disease. And the impact of PAPP-A on brain function has yet to be studied. The availability of tissue-specific PAPP-A KO and overexpressing mice will be important for identifying the tissues responsible for the longevity phenotype. Conditional PAPP-A KO mice would allow assessment of adult-specific loss of PAPP-A on lifespan without affecting important aspects of early life physiology. Insights gained by such studies will provide for a deeper understanding of the aging process and contribute valuable information in the further exploration of PAPP-A as a novel drug target for aging and age-related diseases.
Acknowledgments
This work was supported by grants from the National Institute on Aging (AG028141), National Institutes of Health (HL074871), and The Ellison Medical Foundation.
References
- Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol. 2005;186:325–331. doi: 10.1677/joe.1.06259. [DOI] [PubMed] [Google Scholar]
- Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–1029. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Byun D, Mohan S, Kim C, Suh K, Yoo M, Lee H, Baylink DJ, Qin X. Studies on human pregnancy-induced insulin-like growth factor (IGF)-binding protein-4 proteases in serum: determination of IGF-II dependency and localization of cleavage site. J Clin Endocrinol Metab. 2000;85:373–381. doi: 10.1210/jcem.85.1.6319. [DOI] [PubMed] [Google Scholar]
- Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab. 2001;86:847–854. doi: 10.1210/jcem.86.2.7223. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Grell JA, Mader JR, Mason MA. Longevity is not influenced by prenatal programming of body size. Aging Cell. 2010a doi: 10.1111/j.1474-9726.2010.00589.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerongol: Biol Sci Med Sci. 2010b;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer E-M, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy associated plasma protein-A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. Am J Physiol Cell Physiol. 2006;290:C183–C188. doi: 10.1152/ajpcell.00199.2005. [DOI] [PubMed] [Google Scholar]
- Conover CA, Harrington SC, Bale LK, Oxvig C. Surface association of pregnancy-associated plasma protein-A accounts for its colocalization with activated macrophages. Am J Physiol Heart Circ Physiol. 2007;292:H994–H1000. doi: 10.1152/ajpheart.00798.2006. [DOI] [PubMed] [Google Scholar]
- Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res. 2008a;18:213–220. doi: 10.1016/j.ghir.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Kiefer MC, Zapf J. Posttranslational regulation of insulin-like growth factor binding protein-4 in normal and transformed human fibroblasts: insulin-like growth factor dependence and biological studies. J Clin Invest. 1993;91:1129–1137. doi: 10.1172/JCI116272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Mason MA, Bale LK, Harrington SC, Nyegaard M, Oxvig C, Overgaard MT. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol. 2010;299:H284–H291. doi: 10.1152/ajpheart.00904.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Mason MA, Levine JA, Novak CM. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J Endocrinol. 2008b;198:599–605. doi: 10.1677/JOE-08-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun A, Duran S, Apaydin S, Bulut I, Sariyar M. Pregnancy-associated plasma protein-A: evaluation of a new biomarker in renal transplant patients. Transplant Proc. 2007;39:3072–3076. doi: 10.1016/j.transproceed.2007.08.111. [DOI] [PubMed] [Google Scholar]
- Durham SK, Kiefer MC, Riggs BL, Conover CA. Regulation of insulin-like growth factor binding protein 4 by a specific insulin-like growth factor binding protein 4 proteinase in normal human osteoblast-like cells: implications in bone cell physiology. J Bone Miner Res. 1994;9:111–117. doi: 10.1002/jbmr.5650090115. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fowlkes J, Freemark M. Evidence for a novel insulin-like growth factor (IGF)-dependent protease regulating IGF-binding protein-4 in dermal fibroblasts. Endocrinology. 1992;131:2071–2076. doi: 10.1210/endo.131.5.1385096. [DOI] [PubMed] [Google Scholar]
- Goto M. Inflammaging (inflammation + aging): a driving force for human aging based on an evolutionary antagonistic pleiotropy theory? Bioscience Trends. 2008;2:218–230. [PubMed] [Google Scholar]
- Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- van Heesch PN, Struijk PC, Laudy JAM, Steegers EAP, Wildschut HIJ. Estimating the effect of gestational age on test performance of combined first-trimester screening for Down syndrome: a preliminary study. J Perinat Med. 2010;38:305–309. doi: 10.1515/jpm.2010.033. [DOI] [PubMed] [Google Scholar]
- Jadlowiec J, Dongell D, Smith J, Conover CA, Campbell P. PAPP-A is involved in matrix mineralization of human adult mesenchymal stem cells and angiogenesis in the chick CAM. Endocrinology. 2005;146:3765–3772. doi: 10.1210/en.2004-1351. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mohan S, Newton J, Rehage M, Tran K, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A regulates myoblast proliferation and differentiation through an insulin-like growth factor-dependent mechanism. J Biol Chem. 2005;280:37782–37789. doi: 10.1074/jbc.M505278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveiborg M, Flyvbjerg A, Rattan SIS, Kassem M. Changes in the insulin-like growth factor-system may contribute to in vitro age-related impaired osteoblast functions. Exp Gerontol. 2000;35:1061–1074. doi: 10.1016/s0531-5565(00)00125-x. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem. 2002;277:47225–47234. doi: 10.1074/jbc.M209155200. [DOI] [PubMed] [Google Scholar]
- Lauzurica R, Pastor MC, Bayes B, Hernandez JM, Bonet J, Llopis MA, Carrera L, Romero R. F2-Isoprostanes in kidney transplant patients: relationship with inflammatory markers. Transplant Proc. 2005;37:3842–3843. doi: 10.1016/j.transproceed.2005.09.106. [DOI] [PubMed] [Google Scholar]
- Lawrence JB, Bale LK, Haddad TC, Clarkson JT, Conover CA. Characterization and partial purification of the insulin-like growth factor (IGF)-dependent IGF binding protein-4-specific protease from human fibroblast conditioned media. Growth Horm IGF Res. 1999a;9:25–34. doi: 10.1054/ghir.1998.0083. [DOI] [PubMed] [Google Scholar]
- Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, III, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy associated plasma protein-A. Proc Natl Acad Sci USA. 1999b;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Galbert SP, Kiefer D, Spellacy WN, Gall S. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol. 1974;118:223–236. doi: 10.1016/0002-9378(74)90553-5. [DOI] [PubMed] [Google Scholar]
- Liu J-P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- Miller BS, Bronk JT, Nishiyama T, Yamagiwa H, Srivastava A, Bolander ME, Conover CA. Pregnancy associated plasma protein-A is necessary for expeditious fracture healing in mice. J Endocrinol. 2007;192:505–513. doi: 10.1677/JOE-06-0011. [DOI] [PubMed] [Google Scholar]
- Monget P, Mazerbourg S, Delpuech T, Maurel MC, Manière S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod. 2003;68:77–86. doi: 10.1095/biolreprod.102.007609. [DOI] [PubMed] [Google Scholar]
- Ning Y, Schuller AGP, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol. 2008;22:1213–1225. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard M, Overgaard MT, Su Y-Q, Hamilton AE, Kwintkiewicz J, Hsieh M, Nayak NR, Conti M, Conover CA, Giudice LC. Lack of functional pregnancy-associated plasma protein-A (PAPP-A) compromises mouse ovarian steroidogenesis and female fertility. Biol Reprod. 2010;82:1129–1138. doi: 10.1095/biolreprod.109.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz CO, Chen B-K, Bale LK, Overgaard MT, Ovig C, Conover CA. Transforming growth factor-β regulation of the insulin-like growth factor binding protein-4 protease system in cultured human osteoblasts. J Bone Miner Res. 2003;18:1066–1072. doi: 10.1359/jbmr.2003.18.6.1066. [DOI] [PubMed] [Google Scholar]
- Parker A, Gockerman A, Busby WH, Clemmons DR. Properties of an insulin-like growth factor-binding protein-4 protease that is secreted by smooth muscle cells. Endocrinology. 1995;136:2470–2476. doi: 10.1210/endo.136.6.7538463. [DOI] [PubMed] [Google Scholar]
- Phang D, Rehage M, Bonafede B, Hou D, Xing W, Mohan S, Wergedal JE, Zin X. Inactivation of insulin-like growth factors diminished the anabolic effects of pregnancy-associated plasma protein-A (PAPP-A) on bone in mice. Growth Horm IGF Res. 2010;20:192–200. doi: 10.1016/j.ghir.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch ZT, Oxvig C, Bale LK, Conover CA. Stress-activated signaling pathways mediate the stimulation of pregnancy-associated plasma protein-A expression in cultured human fibroblasts. Endocrinology. 2006;147:885–890. doi: 10.1210/en.2005-0908. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology. 2006;147:5634–5640. doi: 10.1210/en.2006-0493. [DOI] [PubMed] [Google Scholar]
- Rivera GM, Fortune JE. Selection of the dominant follicle and insulin-like growth factor (IGF)-binding proteins: evidence that pregnancy-associated plasma protein A contributes to proteolysis of IGF-binding protein-5 in bovine follicular fluid. Endocrinology. 2003;144:437–446. doi: 10.1210/en.2002-220657. [DOI] [PubMed] [Google Scholar]
- Sulchanov S, Higashi Y, Shai S-Y, Vaugn C, Mohler J, Li Y, Song Y-H, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Masternak MM, Bartke A. In vivo analysis of gene expression in long-lived mice lacking the pregnancy-associated plasma protein A (PappA) gene. Exp Gerontol. 2010;45:366–374. doi: 10.1016/j.exger.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-A deletion on the adult murine skeleton. J Bone Miner Res. 2008;23:655–662. doi: 10.1359/JBMR.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Nathanielsz PW, Conover CA, Smith SR, Ravussin E. Regional variation in adipogenesis and IGF regulatory proteins in the fetal baboon. Biochem Biophys Res Commun. 2009;380:679–683. doi: 10.1016/j.bbrc.2009.01.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci USA. 2009;106:11252–11257. doi: 10.1073/pnas.0807025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G, Caruso C. Inflammation, ageing and cancer. Mech Ageing & Devel. 2009;130:40–45. doi: 10.1016/j.mad.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotrophy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]