Abstract
Aims
Fullerenes are under intensive study for potential biomedical applications. We have previously reported that a C60 fullerene functionalized with three dimethylpyrrolidinium groups (BF6) is a highly active broad-spectrum antimicrobial photosensitizer in vitro when combined with white-light illumination. We asked whether this high degree of in vitro activity would translate into an in vivo therapeutic effect in two potentially lethal mouse models of infected wounds.
Materials & methods
We used stable bioluminescent bacteria and a low light imaging system to follow the progress of the infection noninvasively in real time. An excisional wound on the mouse back was contaminated with one of two bioluminescent Gram-negative species, Proteus mirabilis (2.5 × 107 cells) and Pseudomonas aeruginosa (5 × 106 cells). A solution of BF6 was placed into the wound followed by delivery of up to 180 J/cm2 of broadband white light (400–700 nm).
Results
In both cases there was a light-dose-dependent reduction of bioluminescence from the wound not observed in control groups (light alone or BF6 alone). Fullerene-mediated photodynamic therapy of mice infected with P. mirabilis led to 82% survival compared with 8% survival without treatment (p < 0.001). Photodynamic therapy of mice infected with highly virulent P. aeruginosa did not lead to survival, but when photodynamic therapy was combined with a suboptimal dose of the antibiotic tobramycin (6 mg/kg for 1 day) there was a synergistic therapeutic effect with a survival of 60% compared with a survival of 20% with tobramycin alone (p < 0.01).
Conclusion
These data suggest that cationic fullerenes have clinical potential as an antimicrobial photosensitizer for superficial infections where red light is not needed to penetrate tissue.
Keywords: antimicrobial photodynamic therapy, buckminsterfullerene, mouse model, Proteus mirabilis, Pseudomonas aeruginosa, wound infection
C60 fullerene was nominated as the molecule of the year in 1991 [1]. Since then there has been much speculation that fullerenes may have biomedical applications. Pristine C60 is highly insoluble [2] and most biological investigations have studied functionalized derivatives with water solubility [3]. Potential applications of fullerenes that have been proposed have included:
Free-radical quenching antioxidant [4]
Drug delivery vehicle [5]
Photosensitizer (PS) for photodynamic therapy (PDT) [8]
Photodynamic therapy is a rapidly expanding approach to treating diseases caused by unwanted cells such as malignant cancer cells and infectious microbial cells [9]. PDT involves the combination of nontoxic PS and harmless visible light that excites the PS to a long-lived excited triplet state. In the presence of oxygen the PS triplet state can produce reactive oxygen species (ROS), such as singlet oxygen, by energy transfer, or hydroxyl radicals by electron transfer [10]. These ROS are highly reactive and are able to oxidize biomolecules and thereby kill cells. The use of PDT to treat localized infections generally involves topical application of PS into the infected tissue followed by illumination [11]. Selectivity for bacteria over host tissue can be obtained by the appropriate chemical design of the PS to ensure that the molecule will preferentially bind to bacterial cells rather than mammalian cells, and also by minimizing the drug–light interval to ensure the PS is not highly taken up by host cells by the slow process of endocytosis. It has been determined by many researchers that the most important features of this molecular design are a combination of an overall cationic charge with water solubility [12–15]. Cationic charge is even more important in the case of Gram-negative bacteria, which possess a double-membrane structure that excludes many anionic and uncharged lipophilic molecules that can effectively penetrate Gram-positive bacteria and fungal cells [16].
We previously compared two series of functionalized fullerenes, each series consisting of three compounds with mono-, bi- and tri-substitution patterns [17]. The substituent in the first series was a hydrophilic bis-serinol group, while the substituent in the second series was a positively charged dimethylpyrrolidinium group. All three compounds with cationic charges provided by quaternized dimethylpyrrolidinium groups were effective broad-spectrum antimicrobial PS able to kill Gram-positive bacteria, Gram-negative bacteria and fungal yeast. The order of effectiveness correlated with the number of cationic charges, in other words, tri-substitution > bisubstitution > monosubstitution. We now asked whether this high degree of in vitro activity would translate into an in vivo antibacterial PDT effect in two potentially lethal mouse models of wounds infected with Gram-negative bacteria. Gram-negative bacteria were chosen because they are more resistant to PDT compared with Gram-positive bacteria; therefore, Gram-negative bacteria represent a much more challenging test of antimicrobial PDT mediated by fullerenes. Moreover, many Gram-negative bacteria tend to produce systemic sepsis after infecting mouse wounds, while this is much more difficult to achieve with Gram-positive species.
Materials & methods
Tricationic fullerene
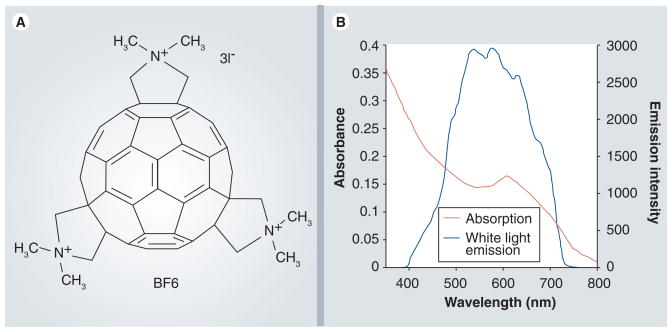
The synthesis, purification and characterization of BF6 has been previously described [17]. Briefly, C60 was treated with sarcosine and paraformaldehyde in refluxing toluene, and the products were purified by silica gel chromatography, quaternized with methyl iodide and characterized by mass spectrometry. There are 46 possible regioisomers of a trisubstituted fullerene and it was not practicable to separate them. The compound was kept in the dark as a 2.5-mM solution in dimethylsulfoxide and diluted in phosphate-buffered saline (PBS) for in vitro or in vivo application. The structure is shown in Figure 1A, together with the visible absorption spectrum and superimposed thereon the spectral output of the white-light filtered lamp (Figure 1B).
Figure 1. Details of BF6 photosensitizer.
(A) Chemical structure of BF6 (one of several positional isomers is displayed). (B) Absorption spectrum of BF6 in water (10 μM) and output spectrum of the broadband white-light source.
Strains & culture conditions
The Pseudomonas aeruginosa strain employed was ATCC 19660 (strain 180), which causes septicemia after intraperitoneal injection [18] and has been shown to be invasive in mice with skin burns [19]. The Proteus mirabilis strain used was ATCC 51393. This strain has been reported to cause burn infections and sepsis in rats that receive scald wounds to 30% of body surface area [20]. The stable bioluminescent variants of these strains carried the entire bacterial lux operon integrated in their chromosomes for stable luciferase expression that allowed them to be used for bioluminescent imaging (strains Xen 44, and Xen 5; kind donations from Xenogen Inc. [Alameda, CA, USA]) [21]. Bacteria were grown in brain–heart infusion medium supplemented with 50 μg/ml kanamycin in an orbital incubator (37°C; 100 rpm) to an optical density of 0.6–0.8 at 600 nm that corresponds with 108 cells/ml (mid-log phase). This suspension was centrifuged, washed with PBS, and resuspended in PBS at the same density. Luminescence was routinely measured on 100 μl aliquots of bacterial suspensions in 96-well black-sided plates by a luminescence plate-reader (MicroBeta Trilux 1450, PerkinElmer Life and Analytical Sciences Inc., Wellesley, MA, USA).
In vitro PDT
P. aeruginosa and P. mirabilis were used at cell densities of 108 cells/ml [22]. Bacterial suspensions in PBS (1 ml) were incubated with BF6 (concentrations 0.01, 0.1, 1, 3, 10, 30 and 100 μM) for 15 min at room temperature in the dark, 500 μl aliquots were transferred to a 24-well plate and illuminated from the top, or not illuminated (covered by aluminum foil). We used a noncoherent lamp with 400–700 nm white-light band-pass filter (LumaCare LC122, MBG Technologies, Inc. Newport Beach, CA, USA) to provide illumination. The lamp was adjusted to give a uniform spot of 4 cm diameter with an irradiance of 200 mW/cm2 as measured with a power meter (model DMM 199 with 201 Standard head, Coherent, Santa Clara, CA, USA). The contents of the wells were mixed and after delivery of 10 J/cm2 (50 s) aliquots were removed from each illuminated and nonilluminated well, serially tenfold diluted in PBS and streaked on square brain–heart infusion agar plates according to the method of Jett et al. [23]. Survival fractions were calculated with reference to cells incubated in PBS alone. Light alone had no effect on bacterial viability.
Animal experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), Subcommittee on Research Animal Care of Massachusetts General Hospital and were in accordance with US NIH guidelines. A total of 72 male Balb/c mice weighing 20–25 g were shaved on the back and depilated with Nair (Carter-Wallace Inc, New York, NY, USA) the day before the experiment. Mice were anesthetized with an intraperitoneal injection of ketamine/xylazine cocktail (90 mg/kg ketamine, 10 mg/kg xylazine) for surgery, infection and imaging. The operative area of skin was cleaned with alcohol and surgical scissors and forceps were used to construct a full-thickness excisional wound down to but not through the panniculus carnosus measuring 5 × 5 mm. There was no visible bleeding within the wounds. Mice were divided into six groups of 12 animals each (three groups for P. mirabilis and three groups for P. aeruginosa infection). Infection was carried out by applying 50 μl of a suspension of bacteria in PBS containing 2.5 × 107 log-phase colony-forming units (CFU) of either P. mirabilis or P. aeruginosa. PDT was carried out after a 15-min interval to allow the bacteria to bind to the wound tissue.
Bioluminescence imaging
The low-light imaging system (Hamamatsu Photonics KK, Bridgewater, NJ, USA) has been described elsewhere in detail [24]. In short, it consists of an intensified change-coupled device (CCD) camera mounted in a light-tight specimen chamber, fitted with a light-emitting diode, a set-up that allowed for a background gray-scale image of the entire mouse to be captured. In the photon-counting mode, an image of the emitted light from the bacteria was captured using an integration time of 2 min, at a maximum setting on the image-intensifier control module. By use of ARGUS software (Hamamatsu Photonics KK, Bridgewater, NJ, USA), the luminescence image was presented as a false-color image superimposed on top of the grayscale reference image. The image-processing component of the software calculated the total pixel values from the luminescence images of the infected wound area. Before undergoing bioluminescence imaging, mice were anesthetized with intraperitoneal injections of 20 μl ketamine/xylazine (10:1) cocktail.
In vivo PDT
BF6 was added at 15 min after infection as 50 μl of a 1-mM solution in PBS/10% dimethylsulfoxide mixture, which was added to the wounds that would be PDT treated or PS dark controls. After a further 15 min to allow the fullerene to bind to and penetrate the bacteria, the mice were again imaged using luminescence camera to quantify any dark toxicity of the BF6 solution to the bacteria. Mice were then illuminated with white light as described in the in vitro studies. Mice were given the following light doses (fluences) with a bioluminescence imaging session taking place after each fluence; 12, 36, 84 and 180 J/cm2. Immediately after the end of PDT, the mice were resuscitated with an intraperitoneal injection of 1 ml sterile saline to prevent dehydration.
Mouse follow-up
Bacterial luminescence from mouse wounds was recorded daily until the bioluminescence disappeared or fatalities occurred. Mice were euthanized according to protocol when their condition was judged to be moribund. When fatalities or euthanasia occurred, blood samples were taken from the heart of the dead mice and streaked on brain–heart infusion agar plates.
Statistical methods
Means of in vitro survival fractions and fractions of bioluminescence remaining were analyzed with one-way analysis of variance (ANOVA). Survival analysis was performed using the Kaplan–Meier method [25]. Survival curves were compared, and differences in survival rate were tested for significance using a log rank test [26]. Any p-values of less than 0.05 were considered significant.
Results
In vitro PDT with BF6
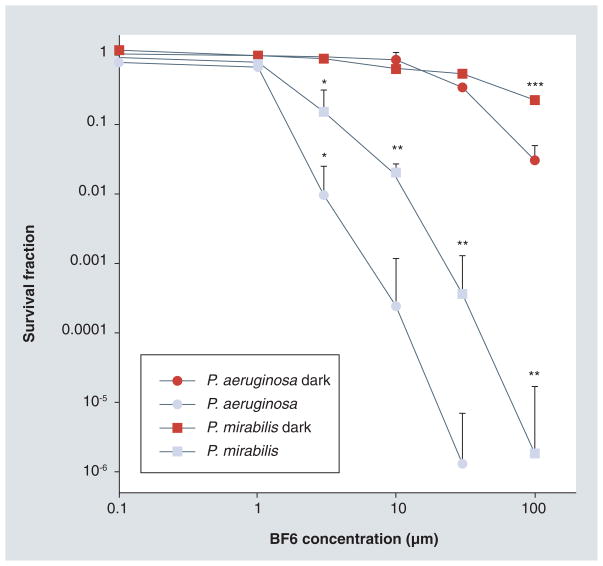
The in vitro PDT killing of the two Gram-negative bacterial species is shown in Figure 2. Since we wanted to compare susceptibilities of the two different bacteria, we used an increasing concentration of BF6 and a single light dose. There was no dark toxicity (killing of bacteria by the fullerene in the absence of light) apparent until the BF6 concentration reached 30 μM and even at 100 μM it was only approximately 1-log. P. aeruginosa displayed rather more dark toxicity than P. mirabilis (p < 0.05).
Figure 2. In vitro BF6-photodynamic therapy.
Proteus mirabilis and Pseudomonas aeruginosa suspensions were incubated for 15 min using various concentrations of BF6 and then illuminated or not with 10 J/cm2 of 400–700 nm light. Values are means of three separate experiments and bars are standard deviations.
*p < 0.05 versus dark; **p < 0.05 versus P. aeruginosa light; ***p < 0.05 versus P. aeruginosa dark.
P. aeruginosa was more sensitive to BF6-mediated PDT than P. mirabilis. The light-mediated killing became significant at 3 μM, and was 2–4 logs at 10 μM. P. aeruginosa was eradicated (>6 logs) at 30 μM and P. mirabilis was eradicated at 100 μM. All the PDT survival fractions were significantly (p < 0.05) different from the respective dark survival fractions. P. aeruginosa survival fractions were significantly different from those for P. mirabilis at 10, 30 and 100 μM.
PDT of P. mirabilis wound infection
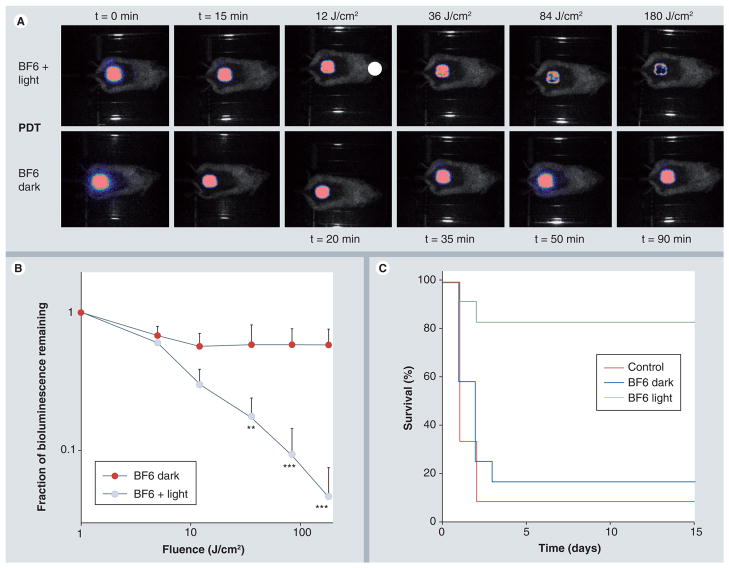
We developed a model in which 2.5 × 107 log-phase bacterial cells were introduced into an excisional wound on the back of BALB/c mice. This wound infection model had a high mortality of 92% and blood cultures taken from the heart and liver of mice that were sacrificed after becoming moribund were all positive for bioluminescent P. mirabilis. When a solution of BF6 was placed in the wound in the dark there was a small reduction in bioluminescence observed immediately Figure 3A & 3B, but this did not progress any further during the time of the experiment. The survival of these mice was 16% compared with 8% for no treatment group, but these survivals were not statistically different (Figure 3C). When white-light illumination was combined with the BF6 solution added to the infected wound, there was a steady, accumulating, light-dose-dependent reduction in bioluminescence signal that reached a maximum of 96% reduction after 180 J/cm2 had been delivered (Figure 3A & 3B). This translated into a highly significant increase in mouse survival of 82% (Figure 3C; BF6 in white light vs no treatment [survival 8%]: p < 0.0005; BF6 in white light vs BF6 in dark [survival 16%]: p < 0.01).
Figure 3. BF6-photodynamic therapy of Proteus mirabilis wound-infected mice.
(A) Bioluminescence images of mice infected with P. mirabilis, treated after 15 min with BF6 solution and either successive fluences of white light (top row) or kept in dark (bottom row). (B) Quantification of luminescence values from the images shown in Figure 3A together with values obtained from corresponding images from the no treatment mice (data not shown): *p < 0.05; **p < 0.01; ***p < 0.001 versus BF6 in dark. (C) Kaplan–Meier survival curves for the groups of mice; no treatment control (n = 12); BF6 in dark (n = 12); BF6 plus light (n = 11).
PDT: Photodynamic therapy.
PDT of P. aeruginosa wound infection
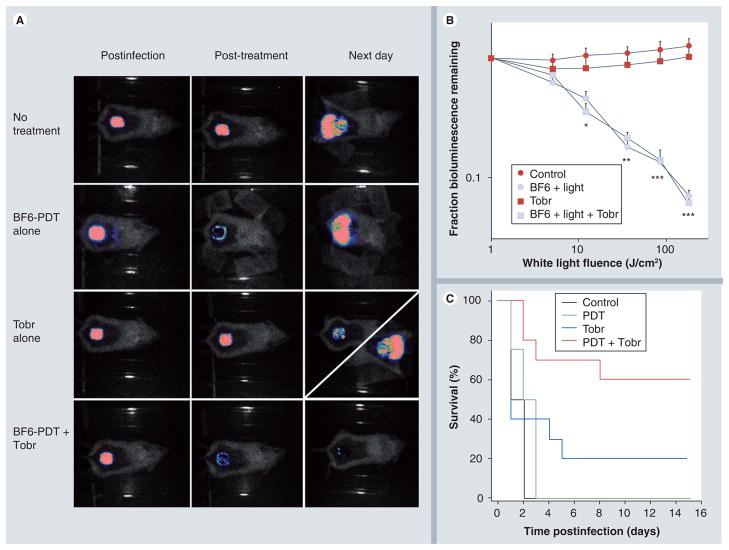
P. aeruginosa proved to be more virulent than P. mirabilis and an initial inoculum of 5 × 106 cells proved fatal in 100% of the mice (Figure 4C). Again all mice had positive blood cultures for bioluminescent Gram-negative bacteria. Similar bioluminescence imaging results were obtained when PDT was conducted on P. aeruginosa wound infections as had been found with P. mirabilis. There was a small reduction with BF6 solution in the dark (data not shown), and a steady fluence-dependent decrease after white-light illumination reaching a maximum reduction of 95% (Figure 4A & 4B). However, there was no beneficial effect on mouse survival in the PDT-treated mice, with 100% of the mice dying within 3 days of the infection with positive blood cultures. The reason for this lack of survival benefit was apparent when examining the bioluminescence image of a PDT-treated mouse the next day after no treatment of after PDT (Figure 4A). It can be seen that in both the no treatment mouse and also in the PDT mouse, the luminescence signal the next day had increased significantly, and moreover had spread laterally into the normal skin surrounding the wound. The highly virulent P. aeruginosa was able to regrow after 95% of the bacteria were killed by PDT, invade the mouse tissues and causer death by sepsis. Therefore, we combined BF6-mediated PDT with a modest antibiotic regimen (6 mg/kg tobramycin for 1 day). Tobramycin alone did not produce a reduction in bioluminescence (Figure 4A & 4B) but did lead to a modest increase in survival of 20% (Figure 4C). The next day images after tobramycin alone took one of two patterns (Figure 4A). Either the luminescence showed a modest drop (top diagonal) and the mouse survived, or (more likely) the luminescence increased and spread laterally (bottom diagonal) and the mouse died. The combination of PDT and tobramycin gave the same fluence-dependent reduction in bioluminescence signal as the PDT alone (Figure 4A & 4B), but now on the next day there was no regrowth and no lateral spreading of the luminescence signal. In the case of the combination (PDT plus tobramycin) the mouse survival was increased to 60% (Figure 4C) and this was significantly higher than survival for the other groups (PDT plus tobramycin vs no treatment/PDT alone: p < 0.005; PDT plus tobramycin vs tobramycin alone: p < 0.01).
Figure 4. BF6-PdT and tobramycin treatment of Pseudomona aeruginosa wound-infected mice.
(A) Representative bioluminescence images of P. aeruginosa-infected mice (captured immediately postinfection, immediately post-treatment and 24 h post-treatment), receiving: no treatment (top row); treated with BF6-PDT alone (180 J/cm2; second row); treated with Tobr alone (6 mg/kg for 1 day; third row, diagonal panel 24 h post-treatment shows two possible outcomes); and treated with a combination of BF6-PDT and 1 day Tobr (bottom row). (B) Quantification of luminescence values from bioluminescence images (not shown) obtained during the PDT process, or at equivalent times for non-PDT mice. *p < 0.05; **p < 0.01; ***p < 0.001; BF6 plus light (with and without Tobr) versus BF6 in dark and versus Tobr alone. (C) Kaplan–Meier survival curves for the groups of mice in Figure 4A; no treatment control (n = 10); PDT alone (n = 12); Tobr alone (n = 2); PDT plus Tobr (n = 10).
PDT: Photodynamic therapy; Tobr: Tobramycin.
Discussion
This is the first report to our knowledge in which experimental animals have been cured of a fatal disease by a therapy mediated by a fullerene or a fullerene derivative. It is also the first report in which antimicrobial PDT has been shown to be synergistic with traditional antibiotics. Antimicrobial PDT is a new approach to the destruction of pathogenic microorganisms located within infected tissue [11,12,27]. Its investigation is driven by the relentless worldwide increase in multiple antibiotic resistance in many species of human pathogens [28]. Fullerenes have both particular advantages and particular disadvantages as antimicrobial PS [9]. The advantages of fullerenes as PS include:
Overall high activity when derivatized with multiple quaternary ammonium groups to give a constitutive cationic charge [17];
An unusual photochemical mechanism that operates mainly via generation of type I ROS, such as superoxide and hydroxyl radical [8], compared with the type II mechanism that generates singlet oxygen and is more common among tetrapyrrole-based PS [29];
A high degree of photostability, which means considerable amounts of light energy can be delivered before the PS is irreversibly degraded by photobleaching.
It is possible that type I ROS are more efficient in killing microorganisms when compared with Type II [30]. The main disadvantage of fullerenes as antimicrobial PS lies in their absorption spectrum, that, in addition to substantial UV absorption, is mainly in the blue and green visible wavelengths at which the penetration of light into tissue is relatively poor. Nevertheless, fullerenes may still be useful as antimicrobial PS for the treatment of relatively superficial infections, where the light does not need to penetrate more than 1 mm or more.
Our laboratory has previously demonstrated that PDT mediated by traditional cationic PS, such as polymer-conjugated tetrapyrroles [31] and phenothiazinium dyes [32], can be used to treat excisional wound infections in mice [27]. We have used stably transduced bioluminescent bacteria that emit light without the need for addition of any luciferase substrate to follow the progress of the infection by low-light imaging. This technique has the advantage of being noninvasive and hence able to monitor each mouse longitudinally in real time [33].
Photodynamic therapy in vitro with the tricationic BF6 was able to eliminate the populations (kill 6 logs of both P. mirabilis and P. aeruginosa) with modest concentrations of fullerene (up to 100 μM) and small fluences of broadband white light that could be delivered in less than 1 min. A short incubation time of 15 min was used in order to be able to minimize the time that the fullerene was in contact with the mouse cells in the wound. Using a short incubation time of the fullerene in the infected wound allowed selective destruction of the bacteria without unacceptable damage to the host tissue. Interestingly P. aeruginosa was found to be more susceptible to PDT killing than P. mirabilis, although in our previous study P. aeruginosa was the most resistant of the four species tested [17].
When PDT using BF6 was performed on mice with P. mirabilis wound infection there was a light-dose-dependent reduction of bioluminescence in the wound. This was not seen when the BF6 was used without illumination. In common with other reported studies [32], higher concentrations of PS and higher fluences of light were needed in vivo to produce a certain loss of luminescence than to produce an equivalent loss of luminescence or equivalent loss of CFU in vitro. The reason for this discrepancy is considered to be that in vivo there is a large excess of host tissue over bacterial tissue (biomass) that could be as high as 1 million to one [34]. The host tissue will compete to some extent for binding the PS, despite the selectivity provided by the cationic character of the PS molecule. The bactericidal effect observed by luminescence imaging during the light delivery was translated into a beneficial therapeutic effect, with 82% of PDT-treated mice surviving compared with control groups (no treatment or BF6 in dark) where at most 20% survived. There have been reports about P. mirabilis clinical infections [35] and recent acquisition of antibiotic resistance has led to problems in effective therapy [36].
We initially observed a similar response to BF6-mediated PDT in mice whose wounds were infected with P. aeruginosa as we observed in the P. mirabilis-infected wounds, and delivery of 180 J/cm2 of white light led to a similar or even greater loss of bioluminescence from the wound. However, when we imaged the mice the next day it was apparent that the infection has recurred with possibly even greater intensity than was observed on day 0. Moreover, the luminescence signal had spread laterally around the wound, indicating that the bacteria had invaded previously healthy tissue and that the mice were at high risk of bacteremia, sepsis and death. This observation prompted us to test a novel combination therapy, BF6–PDT plus a suboptimal antibiotic regimen, in order to see if a dose of antibiotics that was insufficient to cure a full-blown infection might be sufficient to prevent regrowth of the P. aeruginosa after 95% of them had been eliminated by PDT. This hypothesis was proved by a dramatic increase in survival to 60% from survivals of 0 or 20% for the individual treatments alone. It should be emphasized that a full course (10 mg/kg for 8 days) of tobramycin antibiotic therapy in these mice was able to cure the majority of the P. aeruginosa wound infections (data not shown). It is known that Pseudomonas infections today pose a major clinical problem, with resistance spreading throughout the world [37].
In conclusion, we have demonstrated that antimicrobial PDT with a cationic fullerene and white light is a highly effective antimicrobial therapy both in vitro and in vivo. Wounds infected with virulent species of Gram-negative bacteria could be treated with fullerene-mediated PDT and mice saved from death. In the case of the highly invasive P. aeruginosa fullerene–PDT was able to synergize with a suboptimal antibiotic regimen to prevent regrowth and produce significantly higher survival.
Future perspective
The encouraging results obtained using a cationic fullerene as an antimicrobial PS both in vitro and in vivo suggest that further work is justified. We believe that the present study may be one of the few existing reports that have saved the life of mice suffering from any disease by a treatment using a fullerene molecule. Possible avenues for improving the effectiveness of fullerene–PDT as a therapy for localized infection include the following:
Synthesis of new polycationic fullerene derivatives with better selectivity for microbial cells;
Synthesis of fullerene derivatives with light-harvesting antennae to broaden the range of activating light that can be used, hence increasing light penetration depth into tissue;
Understanding the mechanisms that govern the balance between type I and II ROS with the aim of increasing the microbicidal efficacy of fullerene-mediated PDT;
Further exploring the synergy between fullerene-mediated PDT and low subtherapeutic doses of antibiotics;
Expanding the range of localized infections that can be treated with fullerene-mediated PDT to include burn infections, abrasions and fungal infections.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
At the time of writing, Tim Wharton and Ashlee Jahnke were employees of Lynntech Inc. Lynntech Inc. has licensed a patent on which Tim Wharton and Michael R Hamblin are inventors. This work was supported by the NIH (grants R43AI68400 to Lynntech Inc and R01AI050875 to Michael R Hamblin) and by the US Air Force Medical Free Electron Laser Program (FA9550-04-1-0079). Tianhong Dai was supported by the Bullock Wellman Postdoctoral Fellowship. George P Tegos was partly supported by a Massachusetts Technology Transfer Center Award. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1▪.Culotta L, Koshland DE., Jr Buckyballs: wide open playing field for chemists. Science. 1991;254(5039):1706–1709. doi: 10.1126/science.254.5039.1706. Introduction to chemical and physical properties of fullerenes. [DOI] [PubMed] [Google Scholar]
- 2.Duncan LK, Jinschek JR, Vikesland PJ. C60 colloid formation in aqueous systems: effects of preparation method on size, structure, and surface charge. Environ Sci Technol. 2008;42(1):173–178. doi: 10.1021/es071248s. [DOI] [PubMed] [Google Scholar]
- 3▪.Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc Chem Res. 2003;36(11):807–815. doi: 10.1021/ar030027y. Review of synthesis and biomedical applications of functionalized fullerenes. [DOI] [PubMed] [Google Scholar]
- 4.Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60] fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5(12):2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 5.Zakharian TY, Seryshev A, Sitharaman B, Gilbert BE, Knight V, Wilson LJ. A fullerene-paclitaxel chemotherapeutic: synthesis, characterization, and study of biological activity in tissue culture. J Am Chem Soc. 2005;127(36):12508–12509. doi: 10.1021/ja0546525. [DOI] [PubMed] [Google Scholar]
- 6.Isobe H, Nakanishi W, Tomita N, Jinno S, Okayama H, Nakamura E. Nonviral gene delivery by tetraamino fullerene. Mol Pharm. 2006;3(2):124–134. doi: 10.1021/mp050068r. [DOI] [PubMed] [Google Scholar]
- 7.Sitharaman B, Zakharian TY, Saraf A, et al. Water-soluble fullerene (c60) derivatives as nonviral gene-delivery vectors. Mol Pharm. 2008;5(4):567–578. doi: 10.1021/mp700106w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochem Photobiol Sci. 2007;6(11):1139–1149. doi: 10.1039/b711141j. Review of fullerene-mediated photodynamic therapy (PDT) covering mechanism, solution, in vitro and in vivo studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mroz P, Hamblin MR, editors. Advances In Photodynamic Therapy: Basic, Translational and Clinical. Artech House; Norwood, MA, USA: 2008. [Google Scholar]
- 10.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one – photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1(4):279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. Review of the history, state-of-the art and future possibilities of antimicrobial PDT and its use for localized infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jori G, Fabris C, Soncin M, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38(5):468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 13.Merchat M, Spikes JD, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B. 1996;35(3):149–157. doi: 10.1016/s1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 14.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42(10):2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. In vitro resistance selection studies of rlp068/cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54(2):637–642. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44(3):522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Tegos GP, Demidova TN, Arcila-Lopez D, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12(10):1127–1135. doi: 10.1016/j.chembiol.2005.08.014. First description of the fullerene used in the present study as a highly active light-mediated broad spectrum antimicrobial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal SM. Local and systemic therapy of Pseudomonas septicemia in burned mice. Ann Surg. 1967;165(1):97–103. doi: 10.1097/00000658-196701000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markley K, Smallman E. Protection by vaccination against pseudomonas infection after thermal injury. J Bacteriol. 1968;96(4):867–874. doi: 10.1128/jb.96.4.867-874.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McManus AT, McLeod CG, Jr, Mason AD., Jr Experimental Proteus mirabilis burn surface infection. Arch Surg. 1982;117(2):187–191. doi: 10.1001/archsurg.1982.01380260057010. [DOI] [PubMed] [Google Scholar]
- 21.Rocchetta HL, Boylan CJ, Foley JW, et al. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45(1):129–137. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demidova TN, Hamblin MR. Effect of cell–photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49(6):2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23(4):648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 24▪.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75(1):51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. First use of bioluminescent bacteria and in vivo optical imaging to monitor antimicrobial PDT in vivo. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–448. [Google Scholar]
- 26.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Royal Stat Soc A. 1972;135:185–207. [Google Scholar]
- 27.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections – state of the art. Photodiagnosis Photodyn Ther. 2009;6(3–4):170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schito GC. Is antimicrobial resistance also subject to globalization? Clin Microbiol Infect. 2002;8(Suppl 3):1–8. doi: 10.1046/j.1469-0691.8.s.3.1.x. discussion 33–35. [DOI] [PubMed] [Google Scholar]
- 29.Maisch T, Baier J, Franz B, et al. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci USA. 2007;104(17):7223–7228. doi: 10.1073/pnas.0611328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪.Martin JP, Logsdon N. The role of oxygen radicals in dye-mediated photodynamic effects in Escherichia coli B. J Biol Chem. 1987;262(15):7213–7219. Emphasizes the importance of type I reactive oxygen species and hydroxyl radicals in antimicrobial PDT. [PubMed] [Google Scholar]
- 31▪.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187(11):1717–1726. doi: 10.1086/375244. Demonstrates that antimicrobial PDT with cationic photosensitizer conjugates can save the life of mice with potentially lethal wound infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragas X, Dai T, Tegos GP, Agut M, Nonell S, Hamblin MR. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in-vitro and in-vivo studies. Lasers Surg Med. 2010;42(5):384–390. doi: 10.1002/lsm.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamblin MR, Dai T. Can photodynamic therapy be used to treat surgical site infections? Photodiagn Photodyn Ther. 2010 doi: 10.1016/j.pdpdt.2010.04.004. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endimiani A, Luzzaro F, Brigante G, et al. Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum β-lactamases. Antimicrob Agents Chemother. 2005;49(7):2598–2605. doi: 10.1128/AAC.49.7.2598-2605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falcone M, Perilli M, Mezzatesta ML, et al. Prolonged bacteraemia caused by vim-1 metallo-β-lactamase-producing Proteus mirabilis: first report from Italy. Clin Microbiol Infect. 2010;16(2):179–181. doi: 10.1111/j.1469-0691.2009.02781.x. [DOI] [PubMed] [Google Scholar]
- 37.Lister PD. Chromosomally-encoded resistance mechanisms of pseudomonas aeruginosa: therapeutic implications. Am J Pharmacogenomics. 2002;2(4):235–243. doi: 10.2165/00129785-200202040-00003. [DOI] [PubMed] [Google Scholar]