Abstract
The aim of this study was to evaluate the effects of 7-epiclusianone (7-epi) on specific virulence attributes of Streptococcus mutans in vitro and on development of dental caries in vivo. 7-Epi was obtained and purified from fruits of Rheedia brasiliensis. We investigated its influence on surface-adsorbed glucosyltransferase (Gtf) B activity, acid production, and viability of S. mutans in biofilms, as well as on caries development using a rodent model. 7-Epi (100 μg/mL) significantly reduced the activity of surface-adsorbed GtfB (up to 48.0 ± 1.8 of inhibition at 100 μg/mL) and glycolytic pH-drop by S. mutans in biofilms (125 and 250 μg/mL) (vs. vehicle control, p < 0.05). In contrast, the test compound did not significantly affect the bacterial viability when compared to vehicle control (15% ethanol, p > 0.05). Wistar rats treated topically with 7-epi (twice daily, 60-s exposure) showed significantly smaller number of and less severe smooth- and sulcal-surface carious lesions (p < 0.05), without reducing the S. mutans viable population from the animals’ dental biofilms. In conclusion, the natural compound 7-epiclusianone may be a potentially novel pharmacological agent to prevent and control dental caries disease.
Keywords: 7-epiclusianone, Rheedia brasiliensis, Guttiferae, Streptococcus mutans, GtfB, biofilm, dental caries
Introduction
Dental caries is a common oral infectious disease associated with pathogenic microorganisms, such as mutans streptococci, which form a complex biofilm on tooth surfaces. The biofilm formation occurs as a three-dimensional consortium of microorganisms functionally organized within an extracellular matrix [1].
Streptococcus mutans is regarded as one of the main organisms having a proven role in dental caries initiation and development because of its various virulence attributes [2,3]. S. mutans synthesizes extracellular polysaccharides (mainly glucans) from sucrose using glucosyltransferases (Gtfs), which are critical for the formation and establishment of pathogenic (cariogenic) oral biofilms [4,5]. GtfB is considered one of the most important enzymes in this process due to its ability to synthesize insoluble glucans [5] which promote bacterial accumulation on the tooth surface and contribute to the formation of the extracellular polysaccharide matrix in biofilms [6,7]. A recent clinical study showed that GtfB levels in saliva correlated with the presence of active caries in humans [8]. Furthermore, S. mutans survives at a low pH environment and rapidly produces organic acids from fermentable carbohydrates within the matrix of the biofilms, resulting in selection of aciduric flora and demineralization of the adjacent dental enamel [9,10]. Therefore, disruption of the ability of S. mutans to synthesize glucans and acids would be an effective therapeutic approach to reduce the cariogenicity of this ubiquitous oral pathogen.
Natural products have been used as antimicrobial agents against oral pathogens and/or as inhibitors of virulence factors of S. mutans in an attempt to find new pharmacological agents to prevent and control dental caries [11]. Previous studies investigating the activity of natural compounds against S. mutans and other mutans streptococci have shown that it is possible to reduce caries development in vivo by disrupting the virulence attributes of these microorganisms [12,13].
Recently, we have shown that 7-epiclusianone, a polyprenylated benzophenone naturally found in the fruit of Rheedia brasiliensis (Guttiferae), presents antibacterial and anti-adherence effects on planktonic cells of S. mutans [14,15]. However, it is important to evaluate the biological actions of 7-epiclusianone on biofilms and verify its anticariogenic potential in vivo. Thus, the aim of this study was to investigate the influence of 7-epiclusianone on (1) the activity of surface-adsorbed GtfB, acidogenicity, and viability of S. mutans within biofilms in vitro, and (2) pathogenicity of S. mutans in vivo using a well-established rodent model of dental caries under a high cariogenic challenge.
Material and Methods
Plant material
The fruits of Rheedia brasiliensis Planch. & Triana (Syn. Garcinia brasiliensis Mart., Guttiferae) were collected from trees growing under controlled conditions at the herbarium of the Federal University of Viçosa – UFV (latitude 20°45′14″ south and longitude 42°52′55″ west), Minas Gerais state, Brazil – where the voucher specimen (#VIC2604) is deposited. 7-Epiclusianone [m.p. 92–93°C (MeOH); (c 0.1, CHCl3)] was isolated from the peel (pericarp) hexane extract of R. brasiliensis fruits, as previously described [14,16]. The chemical structure of 7-epiclusianone (7-epi), which was first identified by Dos Santos et al. [16], is represented in Fig. 1.
Fig. 1.
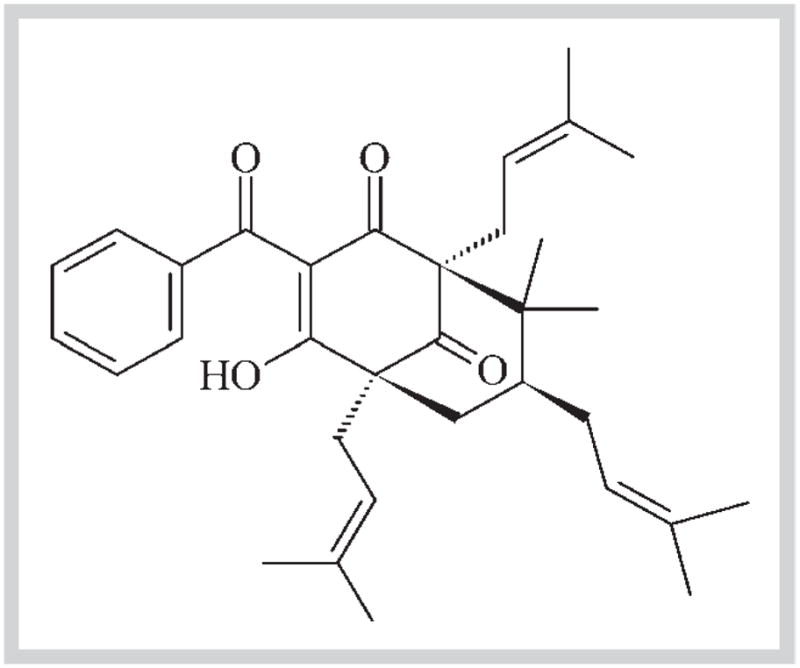
Chemical structure of 7-epiclusianone [(1R,5R,7R)-3-benzoyl-4-hydroxy-8,8-dimethyl-1,5,7-tris (3-methylbut-2-en-1-yl) bicyclo [3.3.1]non-3-ene-2,9-di-one], a polyprenylated benzophenone obtained from Rheedia brasiliensis.
The structure of 7-epi [(1R,5R,7R)-3-benzoyl-4-hydroxy-8,8-di-methyl-1,5,7-tris(3-methylbut-2-en-1-yl)bicyclo[3.3.1]non-3-ene-2,9-dione], a polyprenylated benzophenone, was identified using infrared, ultraviolet, mass spectra data, and nuclear magnetic resonance spectroscopy, and its molecular geometry was established using single crystal X-ray diffraction (XRD) analysis; the purity level was > 98% as determined by HPLC. Immediately prior to carrying out the assays, 7-epi was prepared in a phosphate buffer containing 15% (v/v) ethanol (vehicle) as a stock solution and diluted in vehicle to the final concentrations tested. For the GtfB activity assay, the effect of 7-epi was tested at concentrations ranging from 12.5 to 100 μg/mL. For acid production in biofilms, the concentrations of 7-epi were 125 and 250 μg/mL, which are equivalent to 100 times its minimum inhibitory concentrations [14]. For the animal caries study, 7-epi at 250 μg/mL was used, and a positive control (250 ppm of fluoride) [12,17] was included; both solutions were prepared in phosphate buffer containing 15% (v/v) ethanol.
Bacteria
Streptococcus mutans UA159, a virulent cariogenic pathogen, was used for biofilm and animal studies. S. anginosus KSB8 construct (kindly provided by Howard K. Kuramitsu, State University of New York, Buffalo, NY, USA), which harbors the gtfB gene, was used for the production of GtfB (responsible for the synthesis of insoluble glucans rich in α1,3-linked glucose). The cultures were stored at −80°C in Brain Heart Infusion (BHI) containing 20% glycerol (v/v).
Surface-adsorbed glucosyltransferase B (GtfB) activity assay
The GtfB enzyme (E.C. 2.4.1.5) was obtained and purified to near homogeneity by hydroxyapatite column chromatography as described by Venkitaraman et al. [18]. GtfB activity was measured by the incorporation of [14C] glucose from labeled sucrose (NEN Research Products) into glucans. The GtfB enzyme added to each sample was equivalent to the amount required to incorporate 1–1.5 μmol of glucose over the 4-hour reaction. To evaluate the activity of GtfB on the surface, hydroxyapatite beads were coated with clarified whole saliva free of Gtf activity in the presence or absence (control) of the test agent (7-epi), as described by Schilling and Bowen [19]. The saliva-coated hydroxyapatite beads were exposed to a sufficient enzyme to saturate the surface as determined experimentally. Following the adsorption of the enzyme, the beads were washed three times with buffer to remove the loosely bound material and then exposed to 7-epi (12.5, 25, 50, and 100 μg/mL) or control for 30 minutes. The excess of 7-epi was removed, and the beads were washed and then exposed to [14C-glucose]-sucrose substrate (100 mM sucrose, final concentration). The formed radiolabeled glucan was collected and quantified by scintillation counting [18]. This evaluation was carried out in triplicate in at least three different experiments.
Biofilm assays
Biofilms were formed on hydroxyapatite discs (HAP ceramic–calcium hydroxyapatite, 0.5″ diameter ceramic; Clarkson Calcium Phosphates) and coated with clarified and filter-sterilized human whole saliva as described previously [17]. The saliva was collected from one donor according to protocols approved by the Ethics Committee in Research of the Piracicaba Dental School, University of Campinas (Protocol #012/2007). The saliva-coated hydroxyapatite (sHA) discs were placed vertically in batch cultures of S. mutans UA159, containing an ultrafiltered (Amicon 10 kDa molecular weight cut-off membrane; Millipore) tryptone-yeast extract with sucrose (1% w/v, final concentration) at 37°C and 5% CO2 [20]. The sHA discs were incubated for 24 h to allow initial establishment of biofilms. At this point, they were transferred to fresh culture medium (for glycolytic pH-drop assay) or treated daily (for bacterial viability assay) until the fifth day, according to the experiments described as follows; the culture medium was changed daily. The biofilm assays were done in duplicate on at least three different experiments.
Glycolytic pH-drop assay
Acid production of S. mutans biofilms was evaluated by standard glycolytic pH-drop assay [21]. Intact 5-day-old biofilms were washed in salt solution (50 mM KCl, 1 mM MgCl2(6H2O, pH 7.0) and exposed to 7-epi (125 and 250 μg/mL) or vehicle control (15% ethanol) for 0, 1, 2, and 3 h. The initial pH (0 h) of the solutions containing biofilms and the test agents was titrated with 0.25 M KOH to a pH 7.2. The effects of 7-epi on glycolytic pH-drop were measured in the presence of excess glucose, which was added to a final concentration of 1% (w/v) to allow bacteria to produce acids. The pH values were monitored with an Orion pH electrode attached to an Orion 290 A+ pHmeter (Thermo Fisher Scientific, Inc.) and measured at 0, 1, 2, and 3 h after introduction of glucose.
Bacterial viability
To evaluate the effect of 7-epi on bacterial viability of S. mutans in biofilms, they were treated with 7-epi (125 and 250 μg/mL) or vehicle control (15% ethanol) twice a day (10 a.m. and 4 p.m.). Biofilms were exposed to the treatments for 1 min, double-dip rinsed in sterile 0.9% NaCl and transferred to fresh culture medium [20]. At the fifth day, the treated biofilms were removed from discs using ultrasonic bath, serially diluted and plated in Tryptic Soy Agar (TSA). The plates were incubated in 5% CO2 at 37°C for 48 h, and then colony forming units per mL (cfu/mL) were determined.
Animal study
This study was approved by the Ethical Committee on Animal Research at the University of Campinas – UNICAMP (Protocol #963–1) and performed as described elsewhere [12,22]. To conduct the experiment, 36 specific pathogen-free female SPF Wistar rats, aged 19 days, were purchased from CEMIB/UNICAMP (credited by ICLAS – International Council for Laboratory Animal Science). Rats were first screened for indigenous mutans streptococci as previously described [22]. At age 19, 20, and 21 days, they were infected with bacterial suspensions obtained from 3-day-old S. mutans UA159 biofilms formed on glass microscope slides as described elsewhere [14].
At age 25 days, the animals were randomly divided into three groups (n = 12): 1) 7-epi 250 μg/mL; 2) 250 ppm F (positive control); and 3) 15% ethanol (v/v; vehicle control). Their molars were treated using a camel hair brush twice daily (10 a.m. and 4 p.m.) until the last day of experiment (total of 5 weeks) including weekends. The rats, which were placed in individual cages, were provided with cariogenic diet 2000 [23] and 5% sucrose water ad libitum. The animals were weighed weekly, and their behavior, capacity to drink/eat, and physical appearance were checked daily. At the end of the 5-week experimental period, the rats were euthanized by CO2 asphyxiation. The lower left jaw was aseptically dissected, suspended in 5.0 mL of a sterile 0.9% NaCl solution and sonicated (six 10-s pulses at 5-s intervals, at 40 watts, Vibra Cell; Sonics & Material, Inc.). Aliquots (50 μL) of the suspensions were plated on blood agar and on Mitis Salivarius Agar plus bacitracin (Sigma®), using a Spiral Plater (Whitley Automatic Spiral Plater), to respectively determine the number of total cultivable microorganisms and S. mutans populations. The smooth-surface and sulcal caries and their severities were evaluated according to Larson’s modification of Keyes’ system [24]. The determination of the caries score was blind and performed by one calibrated examiner.
Statistical analyses
Data were subjected to ANOVA and Tukey-Kramer HSD test to adjust for multiple comparisons, using JMP version 3.1 software for statistical visualization. The level of significance was set at 5%.
Results
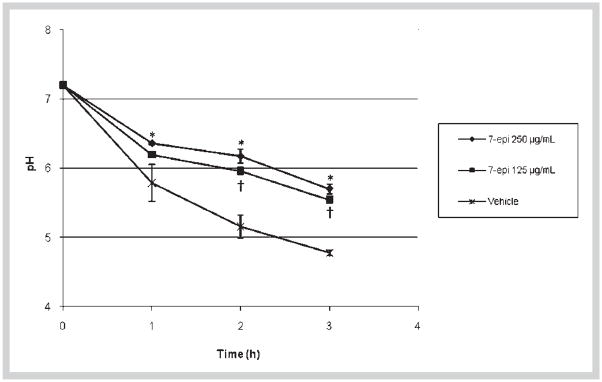
The effects of 7-epi on glucan synthesis by GtfB adsorbed onto saliva-coated hydroxyapatite surface are shown in Table 1. The enzyme activity was diminished when doses of 7-epi were increased. The percentage of inhibition of the enzyme ranged from 9.2 (at 12.5 μg/mL) to 48.0% (at 100 μg/mL) (Table 1). Acid production by S. mutans in biofilms was significantly reduced by 7-epi at both concentrations tested as determined by glycolytic pH-drop in the presence of excess glucose (Fig. 2). The inhibitory effect of 7-epi at 250 μg/mL was statistically different from the vehicle control for all times tested (p < 0.05) whereas the agent at 125 μg/mL inhibited the glycolytic activity only after 2 and 3 h of exposure (vs. vehicle control; p < 0.05). For both concentrations of the test agent, the final pH values were higher than 5.5; in contrast, the final pH for biofilms exposed to vehicle-control was 4.7. Furthermore, the number of viable cells in biofilms treated with 7-epi (at either 125 or 250 μg/mL) did not differ significantly from that of vehicle control treated biofilms (Table 2, p > 0.05).
Table 1.
Effects of 7-epiclusianone (7-epi) on the activity of GtfB on surface.
| Concentration of 7-epi (μg/mL) | GtfB (% of inhibition) |
|---|---|
| 100 | 48.0 ± 1.8 |
| 50 | 33.4 ± 4.7 |
| 25 | 26.6 ± 4.7 |
| 12.5 | 9.2 ± 6.2 |
The percentage of inhibition was calculated considering the vehicle control (15% ethanol) as 100% glucosyltransferase activity (n = 9)
Fig. 2.
Influence of 7-epiclusianone (7-epi) on glycolytic pH-drop in S. mutans UA159 biofilms. *, † indicate a statistically significant difference between vehicle control (15% ethanol) and 7-epi at 250 μg/mL (*) or 7-epi at 125 μg/mL (†) (p < 0.05, ANOVA, comparison for all pairs using Tukey-Kramer HSD; n = 6).
Table 2.
Influence of 7-epiclusianone on viability of Streptococcus mutans UA159 biofilms: means (± SD).
| Treatments | cfu/mL (× 106) |
|---|---|
| Vehicle | 1.5 ± 0.7 |
| 7-epi 250 μg/mL | 1.1 ± 0.3 |
| 7-epi 125 μg/mL | 1.5 ± 0.4 |
No statistical difference was found among the treatments (p > 0.05, ANOVA, comparison for all pairs using Tukey-Kramer HSD; n = 6)
In the in vivo assay, the animals remained in good health and gained weight during the experimental period; weight gains were not significantly different among groups (p > 0.05, data not shown). Table 3 shows the total cultivable microflora, S. mutans viable populations, and percentage of S. mutans recovered from the animals’ biofilms (as calculated from the total cultivable microflora and S. mutans population). Although the 7-epi treated groups presented lower total flora counts (vs. vehicle control, p < 0.05), there was no statistically significant difference regarding the number and percentage of S. mutans in the animals’ biofilms among the tested groups (p > 0.05) (Table 3).
Table 3.
Effects of 7-epiclusianone (7-epi) as well as positive (250 ppm F) and vehicle (15% ethanol) controls after a five-week experiment on oral microbiota in rats: means (± SD).
| Treatment | Total microorganisms (× 104 cfu/mL) | Streptococcus mutans UA159 (× 104 cfu/mL) | Streptococcus mutans UA159 (%) |
|---|---|---|---|
| Vehicle | 3.6 (1.7)a | 2.6 (1.9)a | 63.8 (21.3)a |
| 7-epi | 1.8 (0.7)b | 1.2 (0.5)a | 66.7 (17.2)a |
| 250 μg/mL | |||
| 250 ppm F | 1.9 (0.5)b | 1.6 (0.3)a | 62.4 (14.1)a |
Values followed by the same letters (vertical) are not significantly different from each other (p > 0.05; n = 12; ANOVA, comparison for all pairs using Tukey-Kramer HSD)
The effects of the treatments on the incidence and severity of smooth-surface and sulcal caries are shown in Table 4. The animals treated with 7-epi (250 μg/mL) displayed significantly less total smooth and sulcal carious lesions than those treated with vehicle control (p < 0.05). Furthermore, 7-epi treatment remarkably reduced the severity of both smooth- and sulcal-caries when compared to the vehicle control group (50 to 80% of reduction at Dm and Dx levels, p < 0.05). The positive control group (250 ppm F) showed the lowest scores for both incidence and severity of smooth-surface and sulcal caries. However, the cariostatic effect of 250 ppm F treatments was not statistically different from that of 7-epi (250 μg/mL) regarding severity of smooth-surface caries.
Table 4.
Effects of 7-epiclusianone (7-epi) and respective controls on caries development (smooth-surface and sulcal caries) and severity (Ds, slight dentinal caries; Dm, moderate dentinal caries; Dx, extensive dentinal caries) in rats, after a five-week experiment: means (± SD) of Keyes’ scores modified by Larson.
| GROUP | Smooth surface | Sulcal | ||||||
|---|---|---|---|---|---|---|---|---|
| TOTAL | Severity | TOTAL | Severity | |||||
| Ds | Dm | Dx | Ds | Dm | Dx | |||
| Vehicle | 78.6a (5.8) | 44.8a (8.1) | 19.9a (7.0) | 7.0a (7.2) | 47.1a (4.0) | 38.9a (3.0) | 30.5a (5.3) | 22.6a (5.4) |
| 7-epi 250 μg/mL | 63.9b (6.7) | 25.7b (8.1) | 3.4b (5.0) | 2.1b (3.6) | 40.6b (5.0) | 25.2b (5.4) | 14.9b (5.4) | 5.4b (4.7) |
| 250 ppm F | 34.8c (4.1) | 22.8b (6.7) | 0.5b (0.8) | 0.0b (0.0) | 28.6c (3.3) | 16.1c (3.4) | 2.6c (1.6) | 0.4c (0.7) |
Values followed by the same letters (vertical) are not significantly different from each other (p > 0.05; n = 12; ANOVA, comparison for all pairs using Tukey-Kramer HSD)
Discussion
The high incidence of dental caries and its associated morbidity in the oral cavity require novel strategies for its prevention and control [25]. Our previous studies have demonstrated that specific compounds isolated from natural products exhibit pharmacological activities against mutans streptococci and possess anti-caries properties in vivo using animal models [12,13]. The present study demonstrated that the purified 7-epiclusianone, a benzophenone, interferes with key virulence attributes of S. mutans, i.e. enzymatic activity of surface-adsorbed GtfB and acid production in vitro, while also reducing the development of dental caries in our in vivo model of disease.
We have previously reported that 7-epiclusianone inhibits GtfB activity in solution in a noncompetitive manner (mixed inhibition), indicating that the compound interacts with the enzyme and/or the enzyme-substrate complex [15]. In the present investigation, we continued our investigations of GtfB, this time using the same enzyme adsorbed to the surface of saliva-coated hydroxyapatite beads. Studies with surface-adsorbed Gtfs are biologically and clinically relevant. GtfB can be incorporated into the salivary pellicle in its active form, synthesizing large amounts of insoluble glucans rich in α1,3-linked glucose [18]. In situ, these GtfB-synthesized glucans serve as specific bacterial binding sites for colonization and accumulation of S. mutans on apatitic surfaces [4]. At the same time, these contribute to the formation and structural integrity/stability of the biofilm matrix [7,26]. The presence of extracellular polysaccharide matrix also increases the resistance of biofilms to antimicrobial agents and other environmental assaults [27]. Because the development of dental caries can be attributed to events that occur at the tooth pellicle-biofilm interface, it is desirable to determine potential inhibitors of adsorbed Gtfs [6]. Moreover, surface-adsorbed Gtf enzymes display an increased resistance to most of the common inhibiting agents, including currently commercially available anti-plaque/anti-caries agents when compared to the same enzymes in solution [27]. Thus, inhibition of surface-adsorbed Gtfs may play a significant (and more relevant) role in preventing the formation of pathogenic biofilms related to dental caries.
Our experiments clearly show that 7-epiclusianone inhibited the activity of surface-adsorbed GtfB at the same concentrations used in the solution assay [15]. This effective anti-GtfB activity (both in solution and on surface) of 7-epiclusianone is highly relevant considering that: (1) GtfB-glucans are essential in modulating the formation of tightly adherent microcolonies by S. mutans, thereby promoting the initial steps of biofilm development by this cariogenic organism [26], (2) GtfB is associated with caries activity in animals and humans [5,8], and (3) fluoride and chlorhexidine (current anti-caries and anti-plaque gold standards) are not effective inhibitors of surface-adsorbed GtfB [12,27]. Thus, 7-epiclusianone can be considered a novel substance capable of modulating the activity of this important virulence factor associated with the pathogenesis of dental caries.
Furthermore, 7-epiclusianone affected both the acid production and acid tolerance of S. mutans cells within biofilms as observed in the glycolytic pH-drop assay, which confirms and extends a previous observation showing disruptive effects of the agent against planktonic cells of S. mutans [15]. The test agent sensitized the biofilms’ cells to acidification to the point that the final pH values (i) were significantly higher than those in the presence of the vehicle control, and (ii) were above the critical pH (5.5) for tooth enamel demineralization [9]. These effects may be related to disturbances of net membrane permeability to protons based on pH-drop curves and lack of any biocidal activity observed in previous [15] and current studies; increased proton-permeability by 7-epiclusianone causes cytoplasmic acidification, which disrupts the activity of acid-sensitive enolase and other enzymes of the glycolytic pathway [15]. Considering that biofilms display increased resistance, and the presence of an exopolysaccharide matrix acts as a barrier to diffusion of antimicrobial agents [28], it is noteworthy that 7-epiclusianone was still capable of acting against acid production/acid tolerance of S. mutans within mature (5-day-old) biofilms.
The effects of 7-epiclusianone on GtfB and acidogenicity (two major caries-related virulence factors) may provide beneficial effects against the formation of cariogenic biofilms in vivo. Thus, we further investigated the anti-caries activity of 7-epiclusia-none (at 250 μg/mL) using a rodent model of dental caries. The concentration of 250 μg/mL was selected for the animal study because it was the most effective concentration in reducing the accumulation and glycolytic activity of S. mutans biofilms in vitro as indicated in the present and previous [15] studies.
Topical application of 7-epiclusianone (twice daily, 60-s exposure) was able to reduce the incidence and severity of carious lesions in rats without significantly affecting viable populations and percentage of S. mutans in the animals’ biofilms. 7-Epiclusianone effectively reduced smooth-caries number and severity when compared to the vehicle control, and the results were similar to those of the positive control (250 ppm F). In sulcal caries, where the cariogenic challenge is higher, the compound also reduced dental caries, but it was not as effective as those displayed by 250 ppm F (positive control). Fluoride at 250 ppm is a clinically proven anti-caries agent, and such a concentration is found in most of the currently available fluoride-based mouth rinses [29]; and thereby, it is commonly used as a positive control for dental caries studies in animal models [12,17]. Clearly, 7-epiclusianone is a highly effective agent capable of diminishing dental caries development in vivo, even under a high cariogenic challenge (i.e., animals infected with high levels of a virulent organism and fed with high-sucrose diet) used in this study.
Our data suggest that the anti-caries mechanisms of 7-epiclusia-none could be attributed to multiple inhibitory activities, including disruption of exopolysaccharide synthesis (particularly GtfB-glucans), and factors related to glycolytic activity and acidurance of S. mutans. Although the test agent did not display bactericidal activity, it is also possible that the effects on the bacterial metabolism could impact the physiological responses and growth rate of this organism in biofilms contributing to the overall cariostatic activity.
Substances that act on S. mutans virulence could be used to control dental caries or even to enhance the anticariogenic effect of other recognized agents, such as fluoride [17,30]. Such agents could be used to enhance the effectiveness of the protective effects of fluoride, which (despite some antibacterial effects) does not effectively address the infectious character of the disease. In this context, it would be valuable to investigate the possible additive or synergistic anti-caries effects of 7-epiclusianone and fluoride, since these agents have distinct (but potentially complementary) mechanisms of action. In addition to the improvement of fluoride’s anti-caries effects, the association could also effectively diminish the concentration of fluoride required for therapy (without affecting its cariostatic effectiveness).
In conclusion, 7-epiclusianone may be considered a novel and promising alternative agent to prevent and control dental caries. Further studies are needed to investigate its toxicological effects.
Acknowledgments
The authors thank FAPESP (State of São Paulo Research Foundation, Brazil) for financial support (#06/56379-4), CNPq (National Council for Scientific and Technological Development, Brazil) for fellowships to L.S.B.A. (#131751/2006-9) and R.M.M. (#141253/2005-3), and CAPES (PDEE/CAPES: BEX 0154/06-7, Brazil) for a fellowship to R.M.M. This research was supported in part by USPHS Research grant 1R01 DE018023 from the National Institute of Dental and Craniofacial Research (National Institutes of Health). We are grateful to Jose Carlos Gregorio and Katia Borges Batista for technical assistance. This publication is part of the thesis submitted by the first author to the Piracicaba Dental School.
References
- 1.Marsh PD. Plaque as a biofilm: pharmacological principles of drug delivery and action in the sub- and supragingival enviroment. Oral Dis. 2003;9:16–22. doi: 10.1034/j.1601-0825.9.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 2.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Houte J. Role of microorganisms in caries etiology. J Dent Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 4.Schilling KM, Bowen WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992;60:284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen WH. Do we need to be concerned about dental caries in the coming millennium? Crit Rev Oral Biol Med. 2002;13:126–131. doi: 10.1177/154411130201300203. [DOI] [PubMed] [Google Scholar]
- 7.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. The role of sucrose in cariogenic dental biofilm formation – new insight. J Dent Res. 2006;85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vacca Smith AM, Scott-Anne KM, Whelehan MT, Berkowitz RJ, Feng C, Bowen WH. Salivary glucosyltransferase B as a possible marker for caries activity. Caries Res. 2007;41:445–450. doi: 10.1159/000107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowden GH. Microbiology of root surface caries in humans. J Dent Res. 1990;69:1205–1210. doi: 10.1177/00220345900690051701. [DOI] [PubMed] [Google Scholar]
- 10.Belli WA, Marquis RE. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo H, Jeon JG. Naturally occurring molecules as alternative therapeutic agents against cariogenic biofilms. Adv Dent Res. 2009;21:63–68. doi: 10.1177/0895937409335629. [DOI] [PubMed] [Google Scholar]
- 12.Koo H, Pearson SK, Scott-Anne K, Abranches J, Cury JA, Rosalen PL, Park YK, Marquis RE, Bowen WH. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol. 2003;17:337–343. doi: 10.1034/j.1399-302x.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- 13.Duarte S, Rosalen PL, Hayacibara MF, Cury JA, Bowen WH, Marquis RE, Rehder VL, Sartoratto A, Ikegaki M, Koo H. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Arch Oral Biol. 2006;51:15–22. doi: 10.1016/j.archoralbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Almeida LS, Murata RM, Yatsuda R, Dos Santos MH, Nagem TJ, Alencar SM, Koo H, Rosalen PL. Antimicrobial activity of Rheedia brasiliensis and 7-epiclusianone against Streptococcus mutans. Phytomedicine. 2008;15:886–891. doi: 10.1016/j.phymed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Murata RM, Branco de Almeida LS, Yatsuda R, Dos Santos MH, Nagem TJ, Rosalen PL, Koo H. Inhibitory effects of 7-epiclusianone on glucan synthesis, acidogenicity and biofilm formation by Streptococcus mutans. FEMS Microbiol Lett. 2008;282:174–181. doi: 10.1111/j.1574-6968.2008.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos MH, Nagem TJ, Oliveira TT, Braz-Filho R. 7-Epiclusianona: a nova benzofenona tetraprenilada e outros constituintes químicos dos frutos de Rheedia gardneriana. Quim Nova. 1999;22:654–660. [Google Scholar]
- 17.Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, Rosalen PL, Park YK. Apigenin and tt-farnesol with fluoride on S. mutans biofilms and dental caries. J Dent Res. 2005;84:1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkitaraman AR, Vacca-Smith AM, Kopec LK, Bowen WH. Characterization of glucosyltransferaseB, GtfC, and GtfD in solution and on the surface of hydroxyapatite. J Dent Res. 1995;74:1695–1701. doi: 10.1177/00220345950740101101. [DOI] [PubMed] [Google Scholar]
- 19.Schilling KM, Bowen WH. The activity of glucosyltransferases adsorbed onto saliva-coated hydroxyapatite. J Dent Res. 1988;67:2–8. doi: 10.1177/00220345880670010201. [DOI] [PubMed] [Google Scholar]
- 20.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 21.Belli WA, Buckley DH, Marquis RE. Weak acid effects and fluoride inhibition of glycolysis by Streptococcus mutans GS35. Can J Microbiol. 1995;41:785–791. doi: 10.1139/m95-108. [DOI] [PubMed] [Google Scholar]
- 22.Bowen WH, Madison KM, Pearson SK. Influence of desalivation in rats on incidence of caries in intact cagemates. J Dent Res. 1988;67:1316–1318. doi: 10.1177/00220345880670101401. [DOI] [PubMed] [Google Scholar]
- 23.Keyes PH. Dental caries in the molar teeth of rats. I. Distribution of lesions induced by high carbohydrate low-fat diets. J Dent Res. 1958;37:1077–1087. doi: 10.1177/00220345580370060801. [DOI] [PubMed] [Google Scholar]
- 24.Larson RM. Merits and modifications of scoring rat dental caries by Keyes3 method. In: Tanzer JM, editor. Animal models in cariology. Sp. supp. microbiology abstracts. Washington, DC: IRL Press; 1981. pp. 195–203. [Google Scholar]
- 25.Antunes JFL, Narvai PC, Nugent ZJ. Measuring inequalities in the distribution of dental caries. Community Dent Oral Epidemiol. 2004;32:41–48. doi: 10.1111/j.1600-0528.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 26.Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192:3024–3032. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wunder D, Bowen WH. Action of agents on glucosyltransferases from Streptococcus mutans in solution and adsorbed to experimental pellicle. Arch Oral Biol. 1999;44:203–214. doi: 10.1016/s0003-9969(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 28.Stewart PS. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 1996;40:2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zero DT. Dentifrices, mouthwashes, and remineralization/caries arrestment strategies. BMC Oral Health. 2006;6 (Suppl 1):S9. doi: 10.1186/1472-6831-6-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008;20:17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]