Abstract

This paper describes the 1,1-arylacetoxylation of diverse α-olefins using organostannanes and hypervalent iodine oxidants. The reaction provides a convergent approach for generating a C–C and a C-O bond as well as a new stereocenter in a single catalytic transformation.
The coupling of olefins with aryl-metal species in the presence of a terminal oxidant (the oxidative Heck reaction) is an important method for the formation of substituted alkenes.1 This reaction proceeds via a pathway involving: transmetalation, olefin insertion (to generate intermediate C, Scheme 1), β-hydride elimination/olefin dissociation (to release G), and finally oxidation of Pd0 to regenerate the PdII catalyst.1 Work from our group2 and others3,4,5,6 has shown that oxidative Heck intermediate C (or the related species D) can be intercepted to form 1,2- and 1,1-difunctionalized products such as F and H, respectively. These arylfunctionalization reactions combine the C–C bond-forming step of the oxidative Heck reaction with the generation of an additional bond and a new stereocenter. As such, they provide an attractive means for the convergent coupling of three components (A, B, and E) in a single transformation.7
Scheme 1.
Oxidatively Intecepting Heck Intermediates
We have recently utilized this approach for the Pd-catalyzed 1,2- and 1,1-arylhalogenation of unactivated olefins using arylstannanes in conjunction with halide-based oxidants.2 We sought to expand this methodology to analogous aryloxygenations of diverse alkene substrates.8 We report herein the development and scope of a Pd-catalyzed reaction for the 1,1-aryloxygenation of olefins using organostannane transmetallating reagents and hypervalent iodine oxidants.
Iodobenzene diacetate [PhI(OAc)2] has been widely used to oxidatively functionalize PdII alkyl intermediates formed in catalytic sp3 C–H activation,9 olefin nucleopalladation10,11 and Pd-catalyzed cascade reactions.12 We reasoned that this reagent could promote a similar transformation in the context of oxidative Heck intermediates C and/or D (Scheme 1). Thus, the reaction of 2-(hex-3-en-1-yl)isoindoline 1,3-dione (1) with PhSnBu3 was examined in the presence of 2 equiv of PhI(OAc)2. Gratifyingly, the PdII catalyst PdCl2(PhCN)2 provided 1,1-phenylacetoxylated product 2 in 50% yield in diethyl ether at rt (Table 1, entry 1). None of the corresponding 1,2-arylacetoxylated isomer was detected; however, a significant quantity (21% yield) of the Heck product 3 was formed under these conditions.
Table 1.
Optimization of 1,1-Arylacetoxylation Reaction
 | |||||
|---|---|---|---|---|---|
| entry | solvent | temp (°C) | additivea | yield 2b,c | yield 3b |
| 1 | Et2O | rt | none | 50% | 21% |
| 2 | PhMed | rt | none | 22% | 34% |
| 3 | Et2O | rt | LiBr | 59% | 16% |
| 4 | PhMed | rt | LiBr | 35% | 29% |
| 5 | Et2O | −78 °C to rt | none | 51% | 13% |
| 6 | Et2O | −78 °C to rt | LiBr | 25% | 7% |
| 7 | PhMed | −78 °C to rt | none | 66% | 19% |
| 8 | PhMed | −78 °C to rt | LiBr | 62% | 12% |
1 equiv of additive.
Yield of products determined by 1H NMR spectroscopic analysis of crude reaction mixture. In most reactions, the mass balance was 5–20% of the 1,1-arylchlorinated product. The chloride is presumably derived from the Pd catalyst. When LiBr was present, 5–17% of the 1,1-arylbrominated product was observed. See Supporting Information for complete optimization table.
Degassed toluene was used.
Several strategies were examined to limit formation of 3. First, we used LiBr as an additive, since this salt has been shown to suppress β-hydride elimination/alkene dissociation pathways in other Pd-catalyzed transformations.13 Gratifyingly, the addition of 1 equiv of LiBr to the room temperature reaction in Et2O increased the yield of 2 (to 59%), while decreasing that of 3 (to 16%) (Table 1, entry 3). A similar improvement was also observed in toluene (entries 2 and 4).
Lowering the temperature of the toluene reaction also decreased formation of Heck product 3. For example, when the reaction mixture was stirred for 4 h at −78 °C and then slowly warmed to rt, product 2 was formed in 66% yield along with only 19% of 3 (entry 7). The combination of LiBr and low temperature further minimized the formation of 3 (entry 8); however, the yield of 2 did not improve, due to the generation of significant quantities of the corresponding arylbrominated product.14
With these optimized conditions in hand (Table 1, entry 7), we next explored the scope of this reaction. A number of electronically different arylstannanes were effective arylating reagents (Table 2). For example, ArSnBu3 derivatives containing both electron-donating (entries 3, 4) and electron withdrawing (entries 6–8) para-substituents provided reasonable to good yields. In comparison, ortho-substituted arylstannanes showed modest reactivity. For example, p-MeOC6H4SnBu3 afforded 75% yield of 1,1-arylacetoxylation (entry 4), while the analogous o-MeO-substituted stannane provided 35% yield of the corresponding product (entry 5).
Table 2.
Scope of Organostannanes and Iodoarene Dicarboxylates
 | |||
|---|---|---|---|
| entry | Aryl | R′ | isolated (crude) yielda |
| 1 | C6H5 | CH3 | 66% (66%)b |
| 2 | 2-napthyl | CH3 | 52% (62%) |
| 3 | p-MeC6H4 | CH3 | 59% (68%) |
| 4 | p-MeOC6H4 | CH3 | 75% (78%) |
| 5 | o-MeOC6H4 | CH3 | 35% (51%) |
| 6 | p-ClC6H4 | CH3 | 56% (63%) |
| 7 | p-BrC6H4 | CH3 | 50% (57%) |
| 8 | p-FC6H4 | CH3 | 49% (56%) |
| 9 | p-MeOC6H4 | CF3 | 41% (49%)c |
| 10 | p-MeOC6H4 | t-Bu | 48% (55%) |
| 11 | p-MeOC6H4 | C6H5 | 60% (72%) |
| 12 | p-MeOC6H4 | p-MeOC6H4 | 53% (52%) |
| 13 | p-MeOC6H4 | p-FC6H4 | 68% (73%) |
Crude yields of products determined by 1H NMR spectroscopic analysis of crude reaction mixture. In most reactions, the mass balance was the Heck product 3 (5–20%) and the 1,1-arylchlorinated product (5–15%).
At 1 mmol scale, the reaction afforded 68% isolated yield
The trifluoroacetate product was observed by 1H NMR analysis of the crude reaction mixture; however, it hydrolyzed upon chromatographic purification and was isolated as the free alcohol.
Iodine(III) reagents of general structure PhI(O2CR′)2 could be used to introduce diverse carboxylates. These oxidants are readily prepared by reacting commercially available PhI(OAc)2 with 2 equiv of R′CO2H in chlorobenzene.15 As shown in Table 2, acetate, trifluoroacetate, pivalate, and benzoate-containing products could be accessed in moderate to good yields (entries 4, 9, 10, and 11). Furthermore, substituted benzoate derivatives (containing both electron withdrawing and electron donating para-substituents) afforded comparable results (entries 12 and 13).
This 1,1-arylacetoxylation reaction was also effective across a wide range of terminal olefin substrates. For example, alkenes containing remote protected alcohol derivatives (Table 3, entries 1–3, 6–7) as well as alkyl bromides (entry 4) and aryl iodides (entry 5) were effective substrates in these transformations. Allylic ethers and acetates (entries 8–9) also afforded 1,1-arylacetoxylated products in good yield. Interestingly, products derived from β-acetoxy elimination (which is typically fast at PdII in the absence of AgI additives)16,17,18 were not detected in these latter systems.
Table 3.
Substrate Scope for 1,1-Arylacetoxylation
 | |||
|---|---|---|---|
| Entry | substrate | product | isolated (crude) yielda |
| 1 |
 (4) |
62% (64%) | |
| 2 |
 (5) |
61% (62%) | |
| 3 |
(6) |
47% (54%) | |
| 4 |
(7) |
53% (61%) | |
| 5 |  |
(8) |
64% (67%) |
| 6 |
(9) |
67% (72%) | |
| 7 |
(10) |
62% (62%) | |
| 8 |
 (11) |
64% (71%) | |
| 9 |
 (12) |
58% (69%) | |
Crude yields of products determined by 1H NMR spectroscopic analysis of crude reaction mixture. In most reactions 5–15% of the corresponding Heck product formation was observed along with <5% of the 1,1-arylchlorinated product.
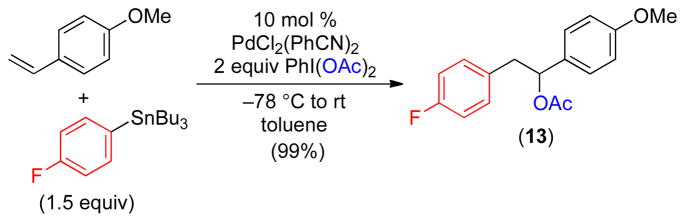
All of the substrates in Table 3 reacted with >20: 1 selectivity for the 1,1-regioisomer. These results suggest that the oxidative functionalization of intermediate C (Scheme 1) with PhI(OAc)2 is significantly slower than β-hydride elimination/equilibration to form PdII-benzyl complex D.2 We reasoned that analogous 1,2-arylacetoxylated products might be accessible if the initially-formed PdII alkyl complex C was more reactive towards oxidative functionalization. Indeed, when p-methoxystyrene was utilized as the alkene substrate with p-FC6H4SnBu3, the 1,2-arylacetoxylation product 13 was obtained with >20: 1 selectivity (Scheme 2). In this case the 1,2-product is formed because intermediate C is a Pd-benzyl complex, which are known to be highly reactive towards oxidative functionalization.2,19
Scheme 2.
1,2-Arylacetoxylation with p-Methoxystyrene
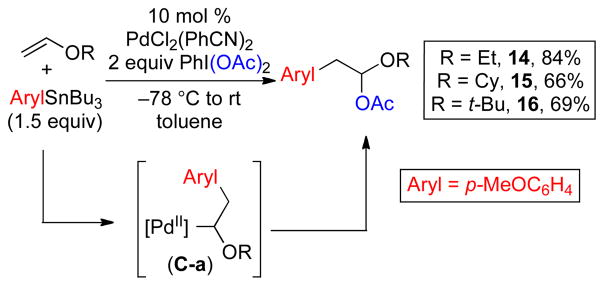
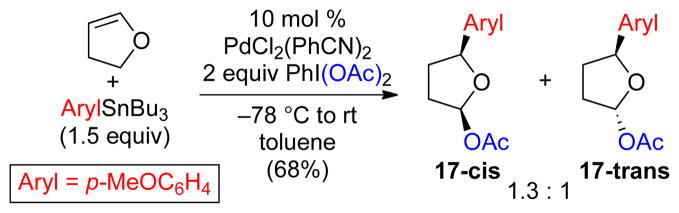
Vinyl ether substrates were also examined in this context. In these systems, the initially formed α-alkoxy- alkyl Pd intermediate C-a (Scheme 3) is expected to be highly electron rich. As such, we anticipated that the relative rate of oxidative functionalization with PhI(OAc)2 (to form the 1,2-product) might be faster than that of β-hydride elimination. Gratifyingly, the use of ethyl, cyclohexyl and t-butyl vinyl ether under our standard reaction conditions afforded excellent yields of the 1,2-arylacetoxylated products 14–16, respectively.20 Interestingly, 2,3-dihydrofuran afforded a somewhat different result, providing the 1,4-arylacetoxylated product 17 in 68% yield as a 1.3: 1 mixture of the cis and trans isomers (Scheme 4). This latter reaction is believed to proceed via initial carbopalladation to place the aryl group α to oxygen. A series of β-H elimination/Pd-H reinsertion reactions then generates an alkyl Pd complex at the 4-position, which undergoes rapid oxidative cleavage with PhI(OAc)2 to afford 17.
Scheme 3.
1,2-Arylacetoxylation with Vinyl Ether Substrates
Yields determined by 1H NMR spectroscopic analysis of crude reaction mixtures; isolated yields were significantly lower due to decomposition of the mixed acetal products on silica gel. See Supporting Information for full details.
Scheme 4.
1,4-Arylacetoxylation of 2,3-Dihydrofuran
The current reaction provides a straightforward route to products such as 3–17. However, the requirement for toxic tin reagents (and the generation of toxic Sn-containing by-products) is a clear limitation. A highly attractive alternative would be to use a simple C–H substrate like benzene as the arene precursor. In this case, intermediate C could be formed via C–H activation of Ph–H followed by olefin insertion (the first two steps of the Fujiwara-Moritani reaction for benzene olefination).1b,6,18c Gratifyingly, a preliminary screen of conditions showed that Pd(acac)2 is an effective catalyst for the coupling of allyl acetate with benzene and PhI(OAc)2. The reaction proceeds efficiently at 60 °C in benzene in the presence of 0.1 equiv of AgOAc to afford the 1,1-phenylacetoxylation product 18 in 51% (Scheme 5). Further studies of the scope and limitations of this transformation are underway.
Scheme 5.
1,1-Phenylacetoxylation via Benzene C-H Activation
In summary, this paper describes the 1,1-arylacetoxylation of diverse α-olefins using organostannanes and hypervalent iodine oxidants. The reaction provides a convergent approach for merging these three components, and it generates a C–C and a CO bond as well as a new stereocenter in a single catalytic transformation. Preliminary results also indicate that the aryl tin reagents can be replaced by simple arene derivatives. Ongoing work is focused on fully exploring the scope of alternative arylating reagents and oxidants that can be utilized for this and related alkene functionalization reactions.
Supplementary Material
Acknowledgments
We thank Dr. Dipannita Kalyani (a former graduate student) for preliminary studies of this transformation. This work was supported by NIH NIGMS (R01-GM073836).
Footnotes
Supporting Information Available: Experimental details and spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Beletskaya IP, Cheprakov AV. Chem Rev. 2000;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; (b) Oestreich M, editor. The Mizoroki-Heck Reaction. John Wiley & Sons Ltd; Chichester, UK: 2009. [Google Scholar]; (c) Gligorich KM, Sigman MS. Chem Commun. 2009:3854. doi: 10.1039/b902868d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Karimi B, Behzadnia H, Elhamifar D, Akhavan PF, Esfahani FK, Zamani A. Synthesis. 2010:1399. [Google Scholar]
- 2.(a) Kalyani D, Sanford MS. J Am Chem Soc. 2008;130:2150. doi: 10.1021/ja0782798. [DOI] [PubMed] [Google Scholar]; (b) Kalyani D, Satterfield AD, Sanford MS. J Am Chem Soc. 2010;132:8419. doi: 10.1021/ja101851v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Tamaru Y, Hojo M, Higashimura H, Yoshida Z. Angew Chem Int Ed. 1986;25:735. [Google Scholar]; (b) Tamaru Y, Hojo M, Kawamura S, Yoshida Z. J Org Chem. 1986;51:4089. [Google Scholar]
- 4.Parrish JP, Jung YC, Shin SI, Jung KW. J Org Chem. 2002;67:7127. doi: 10.1021/jo020159p. [DOI] [PubMed] [Google Scholar]
- 5.(a) Urkalan KB, Sigman MS. Angew Chem Int Ed. 2009;48:3146. doi: 10.1002/anie.200900218. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Werner EW, Urkalan KB, Sigman MS. Org Lett. 2010;12:2848. doi: 10.1021/ol1009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez A, Moran WJ. Eur J Org Chem. 2009:1313. [Google Scholar]
- 7.For recent reviews on other approaches to the Pd-catalyzed 1,2-difunctionalization of olefins, see: Minatti A, Muniz K. Chem Soc Rev. 2007;36:1142. doi: 10.1039/b607474j.Wolfe JP. Synlett. 2008:2913.
- 8.A related strategy for the aryloxygenation of α, β-unsaturated olefins is reported in ref. 6.
- 9.(a) Dick AR, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:2300. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]; (b) Desai LV, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]; (c) Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Neufeldt SR, Sanford MS. Org Lett. 2010;12:532. doi: 10.1021/ol902720d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhang S, Luo F, Wang W, Jia X, Hu M, Cheng J. Tetrahedron Lett. 2010;51:3317. [Google Scholar]
- 10.(a) Alexanian EJ, Lee C, Sorensen EJ. J Am Chem Soc. 2005;127:7690. doi: 10.1021/ja051406k. [DOI] [PubMed] [Google Scholar]; (b) Liu G, Stahl SS. J Am Chem Soc. 2006;128:7179. doi: 10.1021/ja061706h. [DOI] [PubMed] [Google Scholar]; (c) Desai LV, Sanford MS. Angew Chem Int Ed. 2007;46:5737. doi: 10.1002/anie.200701454. [DOI] [PubMed] [Google Scholar]; (d) Li Y, Song D, Dong VM. J Am Chem Soc. 2008;130:2962. doi: 10.1021/ja711029u. [DOI] [PubMed] [Google Scholar]; (e) Wang W, Wang F, Shi M. Organometallics. 2010;29:928. [Google Scholar]
- 11.(a) Streuff J, Hovelmann CH, Nieger M, Muniz K. J Am Chem Soc. 2005;127:14586. doi: 10.1021/ja055190y. [DOI] [PubMed] [Google Scholar]; (b) Muniz K. J Am Chem Soc. 2007;129:14542. doi: 10.1021/ja075655f. [DOI] [PubMed] [Google Scholar]; (c) Muniz K, Hovelmann CH, Streuff J. J Am Chem Soc. 2008;130:763. doi: 10.1021/ja075041a. [DOI] [PubMed] [Google Scholar]; (d) Muniz K, Hovelmann CH, Streuff J, Campos-Gomez E. Pure Appl Chem. 2008;80:1089. [Google Scholar]; (e) Iglesias A, Perez EG, Muniz K. Angew Chem Int Ed. 2010;49:8109. doi: 10.1002/anie.201003653. [DOI] [PubMed] [Google Scholar]
- 12.(a) Tong X, Beller M, Tse MK. J Am Chem Soc. 2007;129:4906. doi: 10.1021/ja070919j. [DOI] [PubMed] [Google Scholar]; (b) Welbes LL, Lyons TW, Cychosz KA, Sanford MS. J Am Soc. 2007;129:5836. doi: 10.1021/ja071204j. [DOI] [PubMed] [Google Scholar]; (c) Liu H, Yu J, Wang L, Tong X. Tetrahedron Lett. 2008;49:6924. [Google Scholar]; (d) Lyons TW, Sanford MS. Tetrahedron. 2009;65:3211. doi: 10.1016/j.tet.2008.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tsujihara T, Takenaka K, Onitsuka K, Hatanaka M, Sasai H. J Am Chem Soc. 2009;131:3452. doi: 10.1021/ja809965e. [DOI] [PubMed] [Google Scholar]; (f) Jaegli S, Dufour J, Wei HI, Piou T, Duan XH, Vors JP, Neuville L, Zhu J. Org Lett. 2010;12:4498. doi: 10.1021/ol101778c. [DOI] [PubMed] [Google Scholar]; (g) Fujino D, Yorimitsu H, Oshima K. Chem Asian J. 2010;5:1758. doi: 10.1002/asia.201000194. [DOI] [PubMed] [Google Scholar]
- 13.Lu X. Top Catal. 2005;35:73. [Google Scholar]
- 14.The use of degassed toluene also enhanced the yield of 2. For example, when the reaction in Table 1, entry 7 was run in non-degassed toluene, 2 was formed in 42% yield along with 29% of 3.
- 15.Stang PJ, Boehshar M, Wingert H, Kitamura T. J Am Chem Soc. 1988;110:3272. [Google Scholar]
- 16.Zhu G, Lu X. Organometallics. 1995;14:4899. [Google Scholar]
- 17.Pan D, Jiao N. Synlett. 2010:1577. [Google Scholar]
- 18.For examples of selective β-H elimination in Heck-type reactions using allyl acetate substrates see: Pan D, Chen A, Su Y, Zhou W, Li S, Jia W, Xiao J, Liu Q, Zhang L, Jiao N. Angew Chem Int Ed. 2008;47:4729. doi: 10.1002/anie.200800966.Su Y, Jiao N. Org Lett. 2009;11:2980. doi: 10.1021/ol9009865.Pan D, Yu M, Chen W, Jiao N. Chem Asian J. 2010;5:1090. doi: 10.1002/asia.200900558.For examples of selective β-OAc elimination in Heck-type reactions using allyl acetate substrates see: Mariampillai B, Herse C, Lautens M. Org Lett. 2005;7:4745. doi: 10.1021/ol051947e.Ohmiya H, Makida Y, Tanaka T, Sawamura M. J Am Chem Soc. 2008;130:17276. doi: 10.1021/ja808673n.
- 19.(a) Becker Y, Stille JK. J Am Chem Soc. 1978;100:845. [Google Scholar]; (b) Johns AM, Utsunomiya M, Incarvito CD, Hartwig JF. J Am Chem Soc. 2006;128:1828. doi: 10.1021/ja056003z. [DOI] [PubMed] [Google Scholar]; (c) Johns AM, Tye JW, Hartwig JF. J Am Chem Soc. 2006;128:16010. doi: 10.1021/ja067084h. [DOI] [PubMed] [Google Scholar]
- 20.For an alternative approach to the 1,2-aryloxygenation of olefins, see: Heinrich MR, Wetzel A, Kirschstein M. Org Lett. 2007;9:3833. doi: 10.1021/ol701622d.Lazzaroni S, Protti S, Fagnoni M, Albini A. Org Lett. 2008;10:349. doi: 10.1021/ol802560t.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.