Abstract
Background
Regulatory T cells (known as “Treg”) express apyrases (CD39) and ecto-5'-nucleotidase (CD73) and contribute to their inhibitory function by generating adenosine. We investigated the expression of CD39 and CD73 on human T helper (Th) cells and the role of CD73 in regulating Helicobacter felis–induced gastritis and colonization.
Methods
Human CD4+ Th cells, gastric T cells, or Treg subsets were stimulated and assayed for the expression of CD39 and CD73 by means of reverse-transcriptase polymerase chain reaction and flow cytometry. The effect of CD73 on proliferation and cytokine production was assessed, and the presence of gastritis, proinflammatory cytokine expression, or colonization of H. felis was evaluated in CD73-deficient (CD73–/–) mice or recipient mice given control or CD73–/– Treg.
Results
CD4+ T cells expressed CD39 and CD73, particularly in CD25+Foxp3+ Treg from peripheral blood or gastric mucosa. Activation significantly increased CD73 expression on all Th cells. Inhibition of CD73 enhanced production of interferon–γ. Gastritis in H. felis–infected CD73–/– mice was significantly worse than that in wild-type mice and was accompanied by increased levels of proinflammatory cytokines and reduced bacterial colonization, whereas Treg from CD73–/– mice did not inhibit gastritis.
Conclusion
CD39 and CD73 expressed by Th cells contribute to local accumulation of adenosine and attenuation of gastritis, which may favor persistent infection.
Helicobacter pylori causes a lifelong infection that affects >50% of humans. Infection in adults and children is associated with chronic antral gastritis characterized by mucosal infiltration of polymorphonuclear and mononuclear leukocytes [1, 2]. Moreover, this infection causes gastroduodenal ulcer [3, 4], as well as gastric lymphoma and carcinoma [5].
Infection of C57BL/6 mice with H. pylori or Helicobacter felis results in chronic active gastritis [6]. Several studies suggest that T helper (Th) type 1 (Th1) cells are selectively increased in response to gastric infection [7–10]. Other data have demonstrated that different subpopulations of CD4+ T lymphocytes play distinct roles in mediating and regulating H. pylori–induced gastritis. For example, adoptive transfer of CD4+CD45RBhi effector T cells from naive donors to immunodeficient recipients causes severe gastritis in H. pylori–infected recipients, whereas cotransfer of CD4+CD45RBlo regulatory T cells (known as “Treg”) protects against gastritis [11]. Other reports have demonstrated that Treg play an important role in suppressing immune responses to normal flora [12] and pathogens [13, 14].
Treg usually express CD25 [15] and the forkhead transcription factor Foxp3 [16]. Because Treg impair host responses, it has been postulated that they favor persistent infection [17]. This notion is supported by experiments showing that the depletion of Treg in H. pylori–infected mice and the adoptive transfer of lymphocytes depleted of CD4+CD25+ cells to H. pylori–infected recipients promote gastritis and bacterial clearance [18, 19]. Thus, Treg play a central role in the negative regulation of pathological, as well as physiologic, immune responses.
One mediator that has been implicated in the control of the host response to infection is adenosine. Adenosine is a purine nucleoside that accumulates in inflamed or hypoxic tissues largely because of CD39 nucleoside triphosphate dephosphorylase mediating the dephosphorylation of ATP to ADP and then to 5'-AMP and CD73 (ecto-5'-nucleotidase), thereby catalyzing the terminal reaction to convert 5'-AMP to adenosine [20]. The numerous responses controlled by adenosine are mediated by 4 G-protein–coupled receptors (A1, A2A, A2B, and A3). Activation of A2A adenosine receptor (A2AARs) on T cells produces a series of responses that have been categorized as anti-inflammatory [20]. Our laboratory previously reported that Treg-mediated control of colonic inflammation is dependent on the presence of A2AAR [21]. Subsequently, other studies have reported that Treg express CD39 and CD73 and that their presence enhances Treg function through the production of adenosine [22–25]. Currently, little is known about the role of adenosine in Treg function or its role in controlling the host response to infection with Helicobacter species. In the present study, we examined the expression of CD39 and CD73 on human Th cells, including Treg, and evaluated the role of CD73 in regulating gastritis and bacterial burden.
MATERIALS AND METHODS
Reagents
Phorbal myristate acetate, ionomycin, and α,β-methylene ADP (APCP) were purchased from Sigma-Aldrich.
Isolation of peripheral blood mononuclear cells (PBMCs) and purification of CD4+ T cells
Fresh PBMCs were obtained from peripheral blood or buffy coat preparations (Virginia Blood Bank) from healthy human donors, by use of ficollhypaque (Amersham) density centrifugation [26]. For isolation of CD4+ T cells, PBMCs were first depleted of monocytes by use of CD14 magnetic beads. CD4+ T cells were then purified from CD14-negative PBMCs by use of magnetic-activated cell sorting beads (Miltenyi Biotec; Bergisch Gladbach), as described by the manufacturer.
Isolation of lamina propria lymphocytes (LPLs) from gastric biopsy specimens
Biopsy specimens of the gastric antrum were obtained from consenting subjects who were undergoing gastroesophageal duodenoscopy for various clinical indications, as approved by the institutional review board at the University of Virginia (Charlottesville). All subjects were uninfected and were free of gastritis. Gastric T cells were isolated using a modified technique [9]. In brief, biopsy specimens were collected into sterile collection medium (calcium- and magnesium-free Hanks balanced salt solution with 5% fetal calf serum and penicillin plus streptomycin). Biopsy specimens were rinsed with aqueous betadine and immediately rinsed 4 times in collection medium containing 1 mmol/L dithiothreitol and 1 mmol/L EDTA (Sigma). The specimens were agitated for1hat 37°C to remove intraepithelial lymphocytes and epithelial cells, supernatant was discarded, and remaining pieces were washed with complete medium. Subsequently, lamina propria T cells were liberated by treatment with 30 U/mL collagenase (Sigma) in complete RPMI 1640.
Flow cytometry
PBMCs, LPLs, or purified Th cells were washed and resuspended in PBS at 1 × 106 cells/mL. Cells were placed on ice and labeled for 30 min in the dark with phycoerythrin (PE)–Cy7/APC–conjugated anti–human CD25 (BD Pharmingen) and PE-Cy7/fluorescein isothiocyanate (FITC)–conjugated anti–human CD4 (BD Pharmingen), PE-conjugated anti–human CD73 (BD Pharmingen), FITC-conjugated CD39 (Serotec), or respective fluorochrome-conjugated isotype-matched control antibodies, as described elsewhere [9]. All antibodies were used at a final concentration of 0.5 μg/million cells. Cells then were washed with 1 mL of cold PBS and resuspended overnight in PBS containing 0.5% paraformaldehyde. The lymphocyte population was selected for acquisition and analysis by gating on forward and side light scatter. In some experiments, intracellular staining of CD4+ T cells with Alexa Fluor 647 anti–human Foxp3 (BioLegend) was also performed after surface staining. Fluorescence intensity was measured using a FACSCalibur flow cytometer (BD). Analysis was performed using Flowjo software (version 6.2.1; Tree Star).
Treg preparation and functional assessment
Purified CD4+ T cells were labeled using anti-CD4, anti-CD25 antibodies, and the CD25hi subset was defined as the highest intensity of CD25 expression. Cells were sorted using FACSVantage (BD), and CD4+ T cells were cultured (5 × 104 cells/well) in round-bottom 96-well plates (Coster; Corning) with RPMI medium supplemented with 2 mmol/L l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies), and 10% AB human serum (Sigma). Cells were activated using a T Cell Activation/Expansion Kit containing beads with anti-CD2, anti-CD3, and anti-CD28 antibodies (Miltenyi Biotec), according to the manufacturer's instructions, in the absence or presence of 5'-AMP (5 μmol/L) and/or the selective CD73 inhibitor APCP (100 μmol/L). Cells were stimulated for 72 h, at which point in time small volumes of supernatant were collected for cytokine assays and 3H-thymidine (1 μCi/well) was added to each well. Cells were harvested 18 h later, and proliferation was analyzed with a β-Trilux scintillation counter.
Cytokine assays
Supernatants collected from the coculture assays were assayed for Th1 cytokines by use of a multiplex bead array (Upstate) and were analyzed using the Bioplex workstation and associated software (Bio-Plex Manager, version 4.0; Bio-Rad).
Mice
C57BL/6 and recombination-activating gene–1 (RAG1)–deficient (RAG1–/–) mice were purchased from The Jackson Laboratory. CD73-deficient (CD73–/–) mice inbred onto the C57BL/6 background were kindly provided by Linda Thompson [27]. All mice were maintained in conventional housing at the University of Virginia, by use of procedures approved by the institutional animal care and use committee.
Helicobacter growth conditions and challenge
H. felis (ATCC strain 51211) was cultured on tryptone soya agar plates containing 5% sheep blood (BBL; Becton and Dickinson) under microaerophilic conditions for 2 days at 37°C. The bacteria were harvested and inoculated into Brucella broth (BD Biosciences) supplemented with 10% heat-inactivated fetal calf serum (Hyclone). The number of bacteria was estimated using spectrometric absorption at A600, with 1 unit of measurement of optical density corresponding to 109 bacteria/mL. To establish primary H. felis infection, mice were inoculated intragastrically with H. felis containing 1 × 108 cfu of the bacteria on 3 consecutive days. Mice were euthanized 4–5 weeks after inoculation.
Collection and histologic examination of gastric samples
Stomachs were dissected along the greater curvature, and they were divided into 3 longitudinal strips and used for tissue DNA extraction for assessment of H. felis UreA by polymerase chain reaction (PCR), histopathologic examination, and RNA extraction for cytokine mRNA analyses. For histopathologic examination, longitudinal segments, including the antrum and corpus plus proximal duodenum, were fixed in Bouin's fixative solution (Ricca Chemical) for 24 h, washed twice with 70% ethanol and embedded in paraffin, cut into 3- to 5-μm sections, and stained with hematoxylin. For immunohistochemical analysis, similar 3-μm gastric sections were stained with polyclonal anti-myeloperoxidase (MPO) antibody (Novus Biochemicals), and tissue-bound peroxidase activity was visualized with 3.3'-diaminobenzidine. Hematoxylin was used for nuclear counter-staining. The number of MPO-positive cells was counted in 10 different fields (original magnification, ×200) and was expressed as the number of positive cells per field. Gastric inflammation was assessed using a modified scoring system described elsewhere [28]. In brief, 2 sections were collected from each stomach, and each region of the stomach (fore stomach, corpus, and antrum) was assessed individually for 3 parameters: (1) thickening, (2) infiltration of polymorphonuclear cells, and (3) infiltration of mononuclear cells. Severity was graded based on the absence (score, 0) or presence (score, 1) of each parameter, with PMN infiltration assessed (absent or present) for focal, diffuse, or abscess involvement. Similarly, mononuclear cell infiltration was assessed for focal, diffuse, or aggregate formation in the lamina propria. For each section, a total score was calculated by totaling the score values for each region of the stomach. Results are reported as the mean total damage score ± SEM.
RNA extraction and real-time reverse-transcriptase (RT) PCR
Total RNA was extracted from Th cell preparations by use of the QIAamp Blood Mini Kit (Qiagen). RNA was also extracted from mouse gastric tissue by use of the RNeasy Mini Kit (Qiagen). In each case, RNA was reverse transcribed to yield cDNA by use of the SuperScript First Strand synthesis system (Invitrogen). Real-time RT-PCR was performed in a SmartCycler (Cepheid) by use of a primer and dual-labeled probe (Applied Biosystem) for CD39, CD73, and Foxp3 in human Th cells. Real-time RT-PCR performed for the detection of mouse tumor necrosis factor (TNF)–α, interferon (IFN)–γ, and chemokine CXCL1 (KC) was performed and quantified using an SYBR-Green kit (Applied Biosystems). The primers used for sequences were as follows: for TNF-α, 5'-GCGGTGCCTATGTCTCAG-3,' and 5'-GCCATTTGGGAACTTCTCATC-3'; for IFN-γ, 5'-GCCAAGTTTGAGGTCAACAAC-3' and 5'-CCGAATCAGCAGCGACTC-3'; and for KC, 5'-CACCCGCTCGCTTCTCTG-3' and 5'-CTTGAGTGTGGCTATGACTTCG-3'. PCRs were performed, and the threshold cycle number was determined using Opticon software (version 1.07; MJ Research). Normalized levels of each mRNA were determined using the formula 2(Rt–Et), where Rt is the threshold cycle for the reference gene (18S rRNA)) and Et is the threshold cycle for the experimental gene (ΔΔCT method) [29]. Data are expressed as arbitrary units.
Gastric tissue DNA extraction and assessment of H. felis colonization
Stomach DNA from infected mice was prepared using the Maxwell 16 Tissue DNA Purification Kit (Promega), as per the manufacturer's instructions. Gastric colonization with H. felis was estimated by quantifying the H. felis–specific UreA gene in tissue DNA via real-time PCR. The relative quantification of H. felis UreA was expressed relative to mouse gastric glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as determined by the comparative ΔΔCT method mentioned above. Primer sequences for H. felis UreA were 5'-TCGATCGCGCAAAAGCTT-3' and 5'-CGCACCGTTCCAGAT-3'. Primers sequences for GAPDH were 5'-GCTAAGCAGTTGGTGCA-3' and 5'-TCACCACCATGGAGAAGGC-3'.
Adoptive transfers
Splenocytes from C57BL/6 or RAG1–/– mice were enriched using CD4 microbeads (L3T4; Miltenyi Biotec) [21] and then were sorted into subsets on the basis of expression of CD4+ and CD45RB. CD45RBhigh (5 × 105 cells) and CD45RBlow (1 × 105 cells) Th cells from control and CD73–/– mice were intraperitoneally injected into RAG1–/– recipients who were exposed to normal microbial flora within our housing facilities. After 7 weeks, the gastric tissues were collected, and hematoxylin-eosin–stained sections were evaluated.
Statistical analysis
Results are expressed as the mean ± SE. Data were compared using Student's t test (unpaired), and results were considered to be significant if P < .05.
RESULTS
Expression of CD39 and CD73 by human Th cells
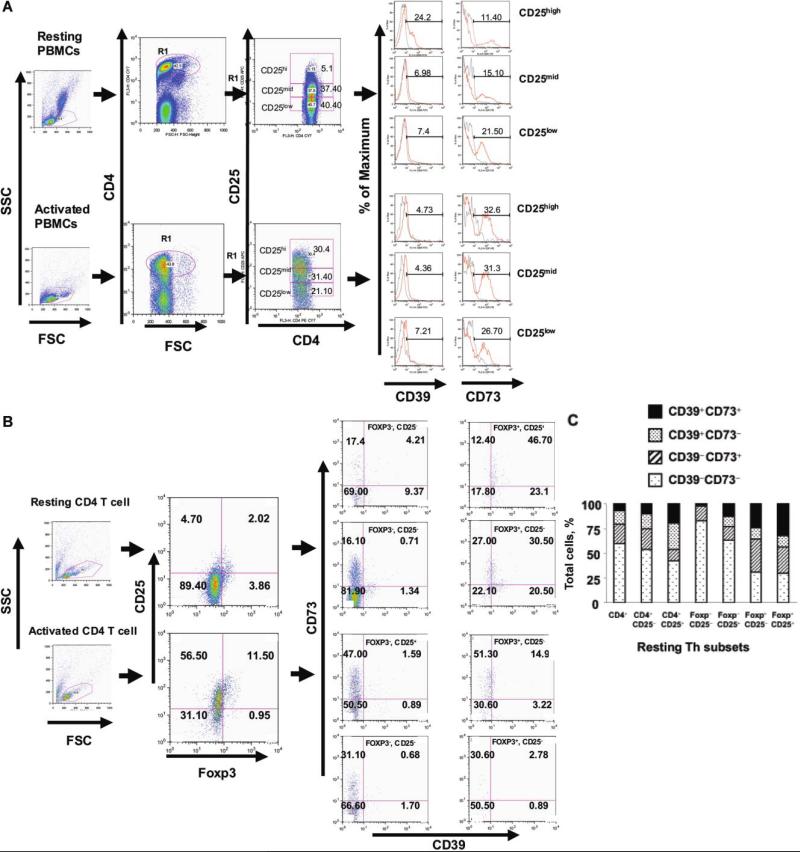
Recent evidence has suggested the involvement of CD39 and CD73 in the functional activity of Treg from systemic tissues [22, 23, 25]. CD39 was abundantly expressed by resting CD4+CD25high Th cells, whereas CD4+CD25low effector Th cells expressed much less CD39 (figures 1A and 2C). Because both activated Th cells and Treg express CD25, we examined the expression of CD39 and CD73 in association with Foxp3 as a more stringent marker of Treg [30]. The Foxp3–CD25–population expressed low levels of CD39 and CD73 (2% and 17% of the population, respectively) (figure 1B and 1C). In contrast, the Foxp3+CD25+population expressed significantly higher levels of CD39 and CD73 (68.8% and 59.1%, respectively), with ~47% bearing both CD39 and CD73 (figure 1B and 1C). CD73 expression was increased significantly in all Th subsets, compared with CD39, in response to activation (figure 1). Figure 1C summarizes the expression of CD39 and CD73 in Th cell subsets and demonstrates the progressive enrichment for CD39, because subsets were analyzed using additional markers that are associated with Treg.
Figure 1.
A, Expression of surface apyrases (CD39) and ecto-5'-nucleotidase (CD73) in human CD4+ T helper (Th) cells subsets. Control or activated peripheral blood Th cells were stained with PerCP-conjugated anti–human CD4+, APC-conjugated anti–human CD25, phycoerythrin (PE)–conjugated anti–human CD73, and fluorescein isothiocyanate (FITC)–conjugated anti–human CD39. Cells were gated on forward vs. side light scatter and analyzed for CD4+ and CD25. Three different populations (CD25high, CD25mid, and CD25low) were chosen based on the expression of CD25 and subsequently were assayed for CD39 and CD73. Quadrants are drawn based on isotype-matched control antibodies that gave <1% background. Data denote findings for ≥6 independent experiments. B, Resting or stimulated purified CD4+ T cells stained with phycoerythrin (PE)–Cy7–conjugated anti–human CD25, PE-conjugated anti–human CD73, and FITC-conjugated anti–human CD39 and, later, intracellularly labeled with Alexa Fluor 647 Foxp3. Th cells were gated on forward vs. side light scatter and analyzed for CD25 and Foxp3. Different populations (Q1–Q4) were chosen based on the expression of Foxp3 and were assayed for CD39 and CD73. Quadrants are drawn based on isotype-matched control antibodies that gave <1% background. Data are representative of ≥3 independent experiments. C, Summary graph showing the percentile distribution of CD39 and CD73 in the different subpopulations of resting Th cells from 6 different buffy-coat–derived peripheral blood mononuclear cells (PBMCs). Percentile values in each bar denote the mean value for 6 PBMC samples analyzed by multicolor fluorescence-activated cell sorter (FACS) staining mentioned earlier and show the enrichment for Th cells that express both CD39 and CD73 in association with the markers associated with regulatory T cells (i.e., Treg). FSC, forward scatter; SSC, side scatter.
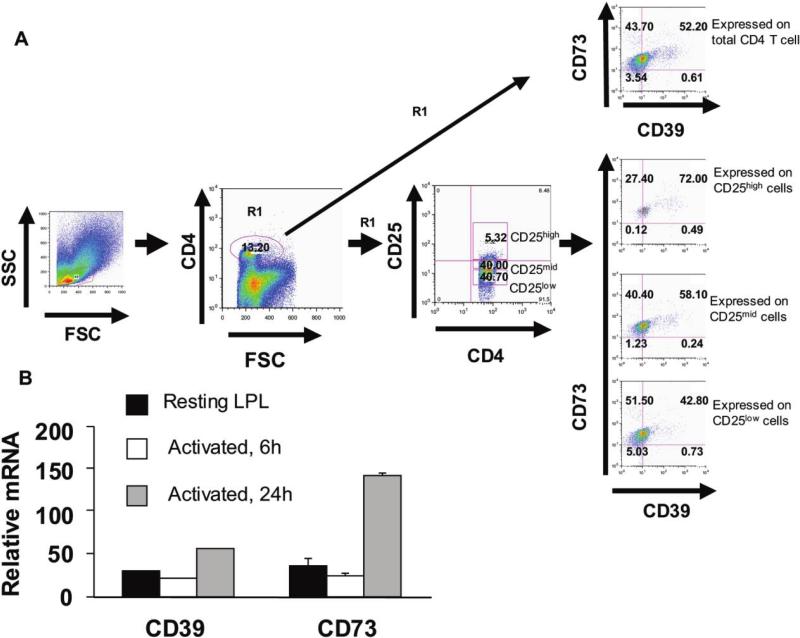
Figure 2.
Expression of CD39 and CD73 by human gastric T cells subsets from lamina propria lymphocytes (LPLs). A, LPS were isolated from human gastric antral biopsy specimens and stained with phycoerythrin (PE)–Cy7–conjugated anti–human CD4, APC-conjugated anti–human CD25, PE-conjugated anti–human CD73, and fluorescein isothiocyanate (FITC)–conjugated anti–human CD39. Lymphocytes were gated on forward vs. side light scatter, were first analyzed for CD39 and CD73, and then were analyzed for CD4+ and CD25. Three different populations (CD25high, CD25mid, and CD25low) were chosen based on the expression of CD25 and assayed for CD39 and CD73. Quadrants are drawn based on isotype-matched control antibodies that gave <1% background. Data are representative of ≥4 separate experiments. B, Gastric LPLs were treated with media (i.e., resting) or activated with phorbal myristate acetate and ionomycin (50 ng/mL + 500 ng/mL) at 37°C for varying lengths of time and analyzed for mRNA expression of CD39 and CD73 measured by real-time reverse-transcriptase polymerase chain reaction. Data are representative of ≥3 separate experiments. FSC, forward scatter; SSC, side scatter.
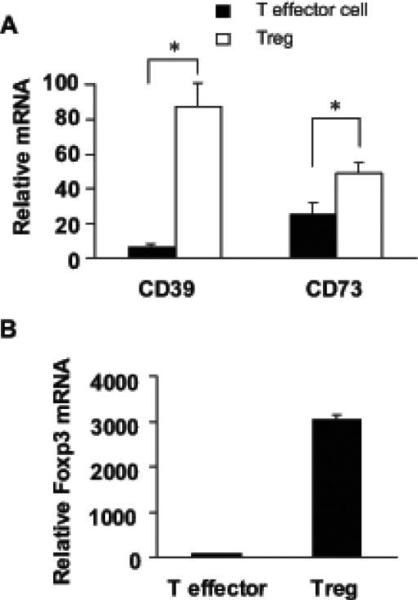
Consistent with the results of flow cytometry, activation of CD4+ T cells increased CD73 mRNA expression (data not shown). When Th cells were sorted into CD4+CD25+ Treg or CD4+CD25– effector T cells and analyzed, Treg expressed more CD39 and CD73 mRNA than did effector Th cells (figure 3A). The enrichment for Treg was confirmed by assaying Foxp3 mRNA expression, and, as expected, the enriched Treg subset had the highest expression of Foxp3 (figure 3B).
Figure 3.
Expression of CD39, CD73, and Foxp3 transcripts in CD4+ Th cells. A and B, mRNA expression for CD39, CD73, and Foxp3 in sorted T effector or regulatory T cells (i.e., Treg) measured by real-time reverse-transcriptase polymerase chain reaction. Data are representative of ≥3 independent experiments.
Human gastric lamina propria Th cells express CD39 and CD73
To directly characterize CD39 and CD73 in gastric Th cells, we examined LPLs isolated from human gastric biopsy specimens. LPLs were stained and analyzed for the expression of CD39 and CD73 in various Th cell subsets. Gastric CD4+ T cells expressed both enzymes. Moreover, the levels expressed by Th cells bearing markers normally associated with Treg expressed the highest levels of CD39 and CD73 (figure 2A).
Inhibition of CD73 impairs suppression of IFN-γ by Treg
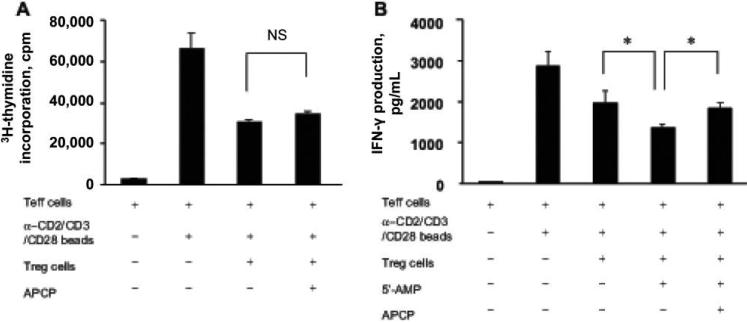
Th cells from peripheral blood or the gastric lamina propria expressed CD73, but yields from the mucosa were too low to study functionally. Therefore, Treg were separated from peripheral blood and used to assess CD73 function. Th cells were activated with anti-CD3/CD28 or beads coated with anti-CD2, anti-CD3, and anti-CD28 antibodies. Subsequently, the cultures were assayed for proliferative responses and IFN-γ secretion in the presence or absence of APCP, a specific inhibitor of CD73 enzymatic activity [23]. Other cultures were tested with or without the CD73 substrate 5'-AMP. As shown in figure 4A and 4B, the addition of APCP in a coculture assay with Treg and effector Th cells did not reverse the suppressive action of Treg on proliferation but was able to impair suppression of IFN-γ production (figure 4B). In another assay, anti-CD3/CD28–stimulated autologous PBMCs were cultured with sorted Treg with or without 5'-AMP. This also suppressed IFN-γ production, which was reversed by the addition of APCP (data not shown). These functional assays proved that, like murine Treg, human Th cells express CD73 and appear to be able to generate adenosine that suppresses IFN-γ production.
Figure 4.
Partial reversal of regulatory T cell (Treg)–mediated suppression of interferon (IFN)–γ production by a specific inhibitor of CD73, α,β-methylene ADP (APCP). A, Peripheral blood mononuclear cells from peripheral blood were sorted based on CD4 and CD25 expression into Treg (CD4+CD25+) or T effector (CD4+CD25–) subsets. A total of 50,000 sorted Treg were combined with an equal number of effector Th cells and were stimulated with beads with anti-CD2, anti-CD3, and anti-CD28 monoclonal antibodies in a coculture assay, in the absence or presence of APCP (100 μmol/L). Cells were stimulated for 3 days, and, on the last day, they were labeled with 3H-thymidine and harvested after 16 h. Proliferation was estimated using a β-cell–plate scintillation counter. NS, not significant. B, IFN-γ production (at 72 h) measured in supernatants in which culture were supplemented with 5'-AMP (5 μmol/L) with or without APCP treatment. Data are the mean ± SE of triplicate wells. *P < .05. Data are representative of ≥3 independent experiments.
CD73–/– mice have more H. felis–induced gastritis
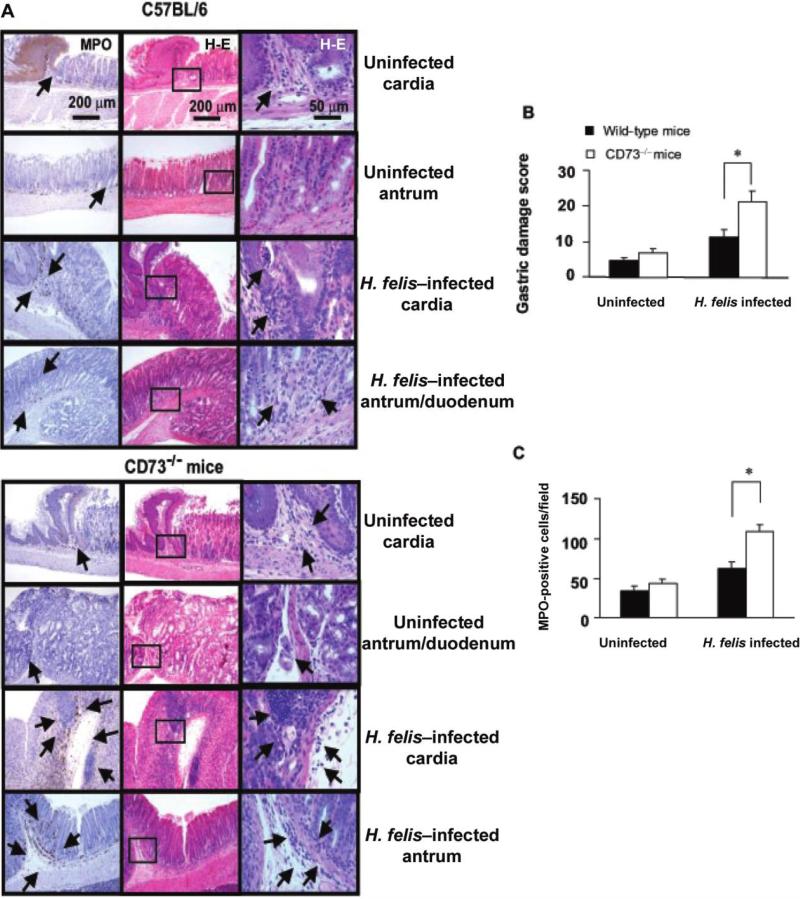
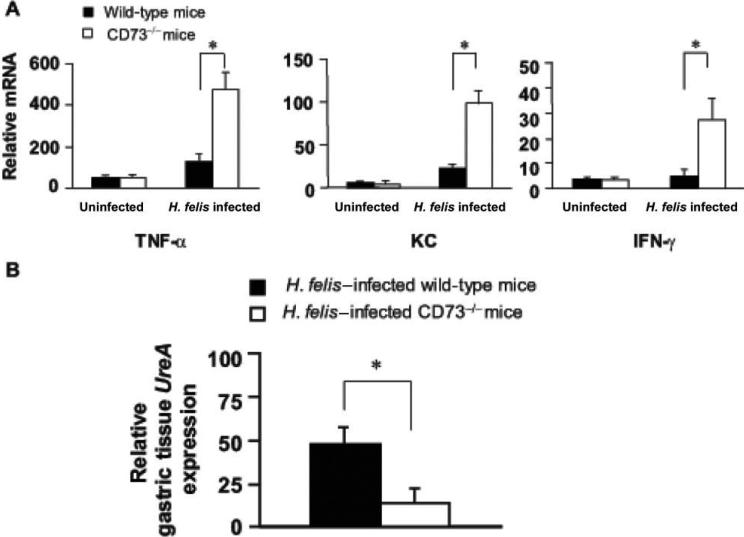
Although in vitro results suggested that Th cells in the peripheral blood and stomach express CD73, an in vivo model was used to study the role of this enzyme in the development of gastritis and bacterial colonization. There was no significant difference in gastritis or cytokine expression in uninfected mice lacking CD73. In contrast, the inflammation score and inflammatory cytokine (TNF-α, IFN-γ, and KC) mRNA responses were significantly higher in the CD73–/– mice at 28 days after infection (figure 5), and inflammation was virtually identical at 60 days after infection (data not shown). Consistent with reports in other models in which gastritis is more severe [19, 31], bacterial colonization was lower in the CD73–/– mice at 28 days after infection (figure 6B) and, again, was comparable at 60 days after infection.
Figure 5.
Gastritis is more severe in Helicobacter felis–infected CD73-deficient (CD73–/–) mice. Mice were infected by gavage with 1 × 108 cfu of H. felis per inoculation every other day for 3 separate inoculations. Mice were euthanized 4 weeks later, and gastric tissue was processed for histologic examination. A, Myeloperoxidase (MPO) (left column) and hematoxylin-eosin–stained (middle and right columns) of gastric sections from representative uninfected or infected C57BL/6 mice (top), and uninfected or infected CD73–/– mice (bottom). Arrows denote cells expressing MPO (original magnification, ×100). Only a few scattered mononuclear cell (MNCs) and MPO-positive granulocytes can be seen in the submucosa and lamina propria, with no abnormal thickening of the gastric wall noted in uninfected control mice. Severe gastritis with dense MNC infiltration and defused MPO-positive granulocytes were noted in the submucosa and mucosa of the CD73–/– mice in both the cardia and antrum regions. MNC aggregates between the glands are spanning the entire width of the mucosa. A concomitant hyperplasia with widespread thickening of the gastric wall, as well as gastric atrophy, can be observed. B, Histologic scoring of gastritis in uninfected and infected wild-type and CD73–/– mice. C, Quantitative expression of MPO-positive cells in uninfected and infected control and CD73–/– mice per field (original magnification, ×200). Data are the mean ± SE for pooled data, including 2 identical experiments with 4 mice per group. *P < .05.
Figure 6.
Increased expression of proinflammatory cytokines and decreased Helicobacter felis colonization in CD73-deficient (CD73–/–) mice. Both wild-type and CD73–/– mice were infected with the H. felis. Mice were euthanized, and gastric tissue was processed for H. felis colonization and measurement of mucosal cytokine transcripts. A, mRNA expression of cytokines and the chemokine CXCL1 in infected wild-type and CD73–/– mice, as determined by real-time reverse-transcriptase polymerase chain reaction. B, H. felis colonization in gastric tissue analyzed by the presence of the H. felis–specific UreA gene. Data are the mean ± SE from pooled data, including 2 identical experiments with 4 mice per group. *P < .05.
CD45RBlow Treg from CD73–/– mice failed to prevent gastritis
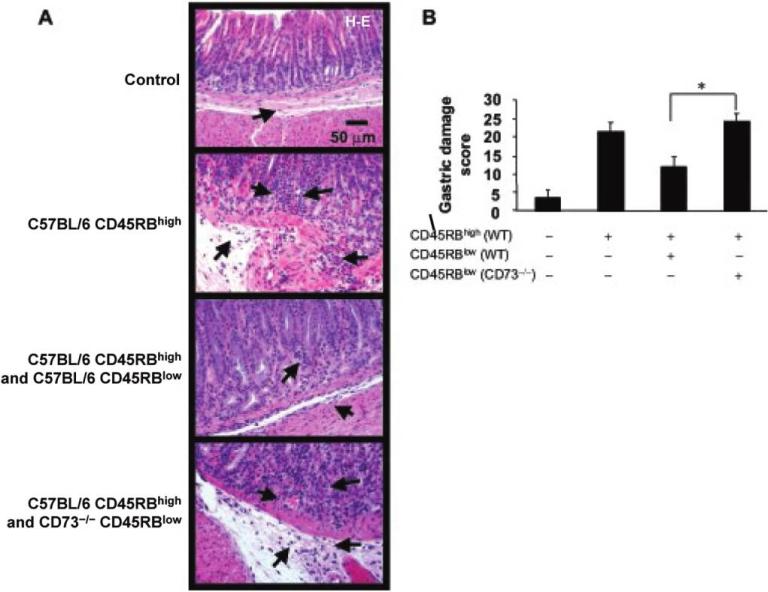
To examine the functional relevance of CD73 on Treg in vivo, we used a mouse adoptive transfer model [11, 32]. It is known that adoptive transfer of pathogenic CD4+ Th cells to RAG1-deficient mice causes severe gastritis and that this transfer can be prevented by the simultaneous cotransfer of Treg [11, 32]. We confirmed CD73 expression by murine Treg (both CD45RBlow and CD4+CD25+ cells) from gastric or splenic lymphocyte preparations (data not shown), whereas the cotransfer of Treg from CD73–/– mice did not prevent gastritis (figure 7A and 7B).
Figure 7.
Failure of CD45RBlow regulatory T cells (Treg) from CD73-deficient (CD73–/–) mice to prevent gastritis. RAG1–/– mice received CD4+CD45RBhigh cells from C57BL/6 mice, CD4+CD45RBhigh cells and CD4+CD45RBlow from C57BL/6 mice, or CD4+CD45RBhigh cells from C57BL/6 mice, as well as CD4+CD45RBlow cells from CD73–/– mice. The histologic findings for the gastric tissue specimens from recipient mice were scored 7 weeks after transfer. A, Photomicrographs show the hematoxylin-eosin (H-E)–stained sections of the gastric corpus-antrum region of representative recipient mice. Severe gastritis with dense mononuclear cell infiltration and diffuse granulocyte accumulation in the submucosa and mucosa of both the corpus and antrum, with widespread thickening of the gastric wall, as well as the presence of gastric atrophy, can be observed in recipient mice that received Treg from CD73–/– mice. B, Inflammation was scored and summarized. Data are the mean ± SE from pooled animals from 2 identical experiments. *P < .05. WT, wild type.
DISCUSSION
Adenosine is a ubiquitous nucleoside that is typically found at low concentrations in the extracellular space. Levels can increase substantially in response to tissue injury or metabolic stress, even reaching as high as 100 μmol/L in some areas of inflammation [33]. Increased levels of extracellular adenosine are produced in part from extracellular ATP and govern various cellular responses by acting on the adenosine receptors (ARs). Thus, the expression of the ectoapyrase CD39 and the ecto-5'-nucleotidase CD73 levels on subsets of Th cells is important to increasing our understanding of how metabolism of ATP to adenosine regulates inflammation in response to gastric infection. The present study demonstrates that human CD4+CD25+Foxp3+ Treg from peripheral blood or gastric tissue are enriched for the expression of CD39 and CD73, suggesting that such Treg contribute to adenosine synthesis which, in turn, mediates an anti-inflammatory function. Moreover, the attenuation of the inflammatory response may favor Helicobacter persistence.
Deaglio et al. [22] recently reported that, in murine Th cells, the expression of CD39 and CD73 by Treg and the presence of A2AAR on activated effector Th cells generate immunosuppressive loops whereby Treg generate adenosine that inhibits the function of effector Th cells. Similarly, Kobie et al. [23] showed that murine Treg express CD73 that converts 5'-AMP from extracellular sources into adenosine, which, in turn, suppresses effector Th cells. We found that CD73 is widely expressed and highly inducible with activation and that CD39 is more closely associated with Treg but is still somewhat inducible during Th cell activation. Unlike in the murine model, CD39 is not expressed on all Treg in humans, a finding that is in agreement with a previous report [25]. Because CD39 and CD73 are rate limiting for extracellular adenosine generation, the ability of Treg to synthesize adenosine from ATP and ADP can significantly contribute to their ability to suppress effector cells during infection-induced inflammation. Furthermore, the expression of CD73 alone by effector Th cells or other cells may contribute to the adenosine pool that serves to limit host responsiveness.
Normally, T cells in the stomach are biased predominantly toward the Th1 phenotype, as is evidenced by their ability to produce IFN-γ but little interleukin (IL)–4 [8, 9, 34]. Despite the robust gastritis associated with Helicobacter infection, infection persists for life. Failure to clear the infection may lead to compensatory induction of Th cells with regulatory function to protect the gastric mucosa. Indeed, there are now several reports that Th cells resembling Treg are present in the gastric mucosa during infection of humans and mice [35–38]. Moreover, Rad et al. [19] have proposed that these Treg contribute to persistence. Treg may produce sufficient levels of IL-10 and transforming growth factor (TGF)–β to attenuate responses rather than to prevent gastritis totally. In the present report, we have shown that Treg prepared from mice lacking CD73 fail to regulate gastritis, compared with wild-type Treg, suggesting that adenosine too, may contribute to the pool of mediators that give Treg their function.
We observed that Th cells in human blood or gastric tissue expressing markers associated with Treg have a significantly higher expression of both CD39 and CD73. Moreover, there is an even higher percentage of Th cells resembling activated Th cells and Treg, compared with peripheral T cells (figure 2). Murine Th cells expressing CD73 act on the 5'-AMP substrate to generate sufficient adenosine to suppress effector Th cell function [23]. The importance of CD73 on Treg was demonstrated by the observation that Treg from CD73–/– mice failed to prevent gastritis. The current study confirms the importance of CD73 in regulating immune and inflammatory responses and extends this principle to human Th cells in both systemic and mucosal tissues.
As observed in IL-10–deficient mice, marked inflammatory responses are sufficient to clear infection with Helicobacter species [31, 39]. Thus, attenuating these responses favors persistence. Moreover, infection of CD73–/– mice led to markedly greater inflammation and decreased colonization with H. felis, compared with infection of wild-type mice. These data are consistent with the fact that activation of A2AAR by a specific agonist attenuates gastritis in rats [40]. Therefore, adenosine can be added to the list of mediators that have the potential to control gastritis in response to a local infection with Helicobacter species and, in so doing, regulate colonization.
In summary, the expression of CD39 and CD73 by Th cells and the enrichment of both enzymes in Treg in humans suggest that Th cells contribute to local adenosine accumulation and the control of inflammation. Moreover, diminished generation of adenosine in CD73–/– mice was associated with impaired Treg function, enhanced gastric inflammation, and reduced levels of colonization on challenge. Together, these observations support the notion that the production of adenosine and its ability to limit inflammation may contribute to the persistence of Helicobacter infection.
Acknowledgments
We thank Joanne Lannigan, Mike Solga, William Ross, Erik Keller, and Sharon Hoang for technical assistance and Margarita Mishina for designing primers for real-time polymerase chain reaction. We appreciate the thoughtful discussion from O. Rötzschke and Linda Thompson.
Financial support: Crohn's and Colitis Foundation of America; National Institutes of Health (grants DK50980, AI069880, AI70491, and RR00175 [to P.B.E.]); the Immunology and Cell Isolation Core, the Molecular Biology Core, and the Morphology/Imaging Core of the University of Virginia Digestive Health Research Center (grant DK 56703).
Footnotes
Potential conflicts of interest: J.L. is a share holder in Adenosine Therapeutics. All other authors: no conflicts.
Presented in part: Digestive Diseases Week, Washington, DC, 19–24 May 2007 (poster M1675).
References
- 1.Dixon MF. Pathophysiology of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:7–10. [PubMed] [Google Scholar]
- 2.Ernst PB, Gold B. The disease spectrum of H. pylori: the immunopatho-genesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–40. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–52. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 4.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Miller MJ. Carcinogenesis, apoptosis and cell proliferation. Br Med Bull. 1998;54:151–62. doi: 10.1093/oxfordjournals.bmb.a011665. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–23. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 7.Karttunen R, Andersson G, Poikonen K, et al. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin Exp Immunol. 1990;82:485–8. doi: 10.1111/j.1365-2249.1990.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Elios MM, Manghetti M, De Carli M, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 9.Bamford KB, Fan XJ, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 10.Ahlstedt I, Lindholm C, Lonroth H, Hamlet A, Svennerholm AM, Quiding-Jarbrink M. Role of local cytokines in increased gastric expression of the secretory component in Helicobacter pylori infection. Infect Immun. 1999;67:4921–5. doi: 10.1128/iai.67.9.4921-4925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–61. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 12.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 13.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 14.McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002;23:450–5. doi: 10.1016/s1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FOXP3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 17.Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 18.Raghavan S, Fredriksson M, Svennerholm AM, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin Exp Immunol. 2003;132:393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rad R, Brenner L, Bauer S, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–37. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–82. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 21.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for adenosine A2A receptors in the T cell mediated regulation of colitis. J Immunol. 2006;177:2765–9. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 22.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 24.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–84. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 25.Borsellino G, Kleinewietfeld M, Di MD, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 26.Haeberle HA, Kubin M, Bamford KB, et al. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–35. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson LF, Eltzschig HK, Ibla JC, et al. Crucial role for ecto-5’-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail HF, Zhang J, Lynch RG, Wang Y, Berg DJ. Role for complement in development of Helicobacter-induced gastritis in interleukin-10-deficient mice. Infect Immun. 2003;71:7140–8. doi: 10.1128/IAI.71.12.7140-7148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Berg DJ, Lynch NA, Lynch RG, Lauricella DM. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10−/− mice. Am J Pathol. 1998;152:1377–86. [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CW, Rao VP, Rogers AB, et al. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2−/− mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Karttunen R, Karttunen T, Ekre H-PT, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–5. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goll R, Gruber F, Olsen T, et al. Helicobacter pylori stimulates a mixed adaptive immune response with a strong T-regulatory component in human gastric mucosa. Helicobacter. 2007;12:185–92. doi: 10.1111/j.1523-5378.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 36.Enarsson K, Lundgren A, Kindlund B, et al. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin Immunol. 2006;121:358–68. doi: 10.1016/j.clim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Stromberg E, Edebo A, Lundin BS, et al. Down-regulation of epithelial IL-8 responses in Helicobacter pylori-infected duodenal ulcer patients depends on host factors, rather than bacterial factors. Clin Exp Immunol. 2005;140:117–25. doi: 10.1111/j.1365-2249.2005.02736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundgren A, Strömberg E, Sjöling A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–31. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail HF, Zhang J, Lynch RG, Wang Y, Berg DJ. Role for complement in development of Helicobacter-induced gastritis in IL-10-deficient mice. Infect Immun. 2003;71:7140–8. doi: 10.1128/IAI.71.12.7140-7148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odashima M, Otaka M, Jin M, et al. Selective adenosine A receptor agonist, ATL-146e, attenuates stress-induced gastric lesions in rats. J Gastroenterol Hepatol. 2005;20:275–80. doi: 10.1111/j.1440-1746.2004.03555.x. [DOI] [PubMed] [Google Scholar]