Abstract
Rat pups are more resistant to retroactive associative interference 3 hrs after birth than 24 hours later (Cheslock, Sanders, & Spear, 2004). The present experiments tested the effect of age, retention interval and dam presence during the retention interval on odor-induced motor activity subsequent to mere odor exposure. Rats were exposed to an hour of odor immediately after birth or approximately one day later and tested after a given retention interval (3 hrs or 27 hrs [Exp 1]; 0, 30, 75, or 180 min [Exp. 2]). They spent the retention interval either in the presence or absence of a foster dam (Exp. 1 and 3). After the retention interval, pups were tested in a four-minute activity test including a two-minute baseline period and two minutes of odor exposure. Overall activity was scored during tape-playback. Odor-exposed pups were more active than non-exposed pups during reexposure to the odor during testing, but this was true only for P0 pups. In contrast, P1 pups without prior odor exposure were active during testing and behaviorally quieted in the presence of the odor they were previously exposed to. Though one day apart, newborn rats just hours old lack many of the experiences that a one day old has had including nursing, huddling, and being groomed. These experiences are associated with, among other stimuli, a barrage of olfactory cues (e.g., colostrum, saliva, dander, feces, and urine). P0 and P1 pups also differ in their proximity from the birthing experience and associated neurochemical changes. The age-related pattern of responding to odors based on previous odor exposure was discussed in relation to these and other possibilities.
Introduction
Perinatal olfactory learning helps ease the transition from fetal life within the uterus to the external neonatal environment (Coureaud, Schaal, Hudson, Orgeur, & Coudert, 2002). Prenatally, the fetus learns the odor of the amniotic fluid and upon birth is capable of differentiating amniotic fluid from its mother versus from another mother (Hepper, 1987; Robinson & Méndez-Gallardo, 2009). This may assist the neonate in preferentially associating with littermates as well as maintaining proximity to the nest. Furthermore, the amniotic fluid seems to share olfactory components with the colostrum, which is the antibody rich milk available just after birth. Such similarities have been found to scaffold the neonate’s first approaches and meals from the nipple (Coureaud, Schaal, Hudson, Orgeur, & Coudert, 2002). In these examples, the neonate’s adjustment to life outside the uterus is facilitated by prenatal olfactory learning.
Olfactory learning is also robust postnatally and can occur early in life without an associated reinforcer such as milk or stroking. After daily four hour exposures to an odorant for about two weeks, preweanling rats came to huddle preferentially in the presence of this odor (Alberts & May, 1984). With just three minutes of odor exposure to an odor similarly aged preweanling rats came to prefer this odor in a two-way preference test (Caza & Spear, 1984). In human infants, familiarization with an artificial odor for the first day after birth resulted in a preference toward that same odor at least for females (Balogh & Porter, 1986). Previous experiments have shown that mere olfactory exposure for the first 18 days of a rat’s life causes neural changes in the olfactory bulb (Coopersmith, Henderson, & Leon, 1986). Early mere odor exposures can result in long lasting behavioral and neural changes towards that same odor.
Olfactory preference learning during infancy in rats uses special circuitry and has distinct attributes that set it apart from olfactory preference learning later in life (Moriceau & Sullivan, 2004; Sullivan, 2003). Odors are preferred after minimal exposure. Furthermore, there is a sensitive period during the first postnatal week until postnatal day ten, when this preference learning is especially effective (Sullivan & Wilson, 1994). Unique to infants is that they will even learn to prefer an odor paired with mildly aversive stimuli (Sullivan, 2003). If ontogenetic differences in postnatal olfactory learning are related to neurochemical and neurophysiological differences between ages, such developmental studies can provide useful information regarding the basic neuroscience underlying olfactory learning.
Even more striking than these ontogenetic differences between the first two postnatal weeks are differences in learning and memory seen between the first two postnatal days. Cheslock and colleagues (2004) exposed pups on the day of birth (newborns) or one day later to two olfactory associations; one was appetitive and one was an aversive association. This study found that although one-day old pups’ memory for the first association was disrupted by the second association (retroactive interference); newborns’ memory for the first memory was unaffected. The authors discuss the possibility that this resistance to retroactive interference may relate to the neurochemical consequences of the birthing process.
To evaluate the potential effects of the birthing process on olfactory learning our laboratory exposed rats to an odor for one hour shortly after birth and tested motor activity and surrogate nipple attachment in the presence of this same odor hours later. Odor exposed newborn rats responded with greater levels of activity to that same odor than pups without odor preexposure. This was true regardless of whether odor exposure occurred immediately, one hour or two hours after birth. This odor preexposure also decreased the time to attach to a similarly scented surrogate nipple particularly when odor exposure was immediately after birth (Miller & Spear, 2008). It is possible that the neurochemical consequences of the birthing process differ more dramatically when comparing newborns and one-day olds than when analyzing within the newborn’s first three hours of life. The goal of the current set of experiments was to test newborn and one-day old pups’ motor responsiveness to a previously experienced odor. Based on previous experiments, we expected prior odor exposure to increase motor activity to the previously experienced odor compared to pups without such odor preexposure. We expected this effect to be greater and longer lasting in newborns than one-day-olds.
General Method
Subjects
Subjects were rat pups cesarean sectioned from Sprague-Dawley (Taconic, Germantown, NY) females bred in large wire hanging cages to a male. When a sperm plug was found (Embryonic day zero, E0) females were removed from the hanging cages and placed in opaque breeder tubs (45 cm long × 23 cm wide × 20 cm high) partially filled with shavings with one to two other pregnant rat(s) until E20 when they were separated into identical individual breeding tubs. Ambient temperatures in the colony room were maintained at 22°C on a 14–10 hour light/dark cycle (lights on at 0700) with ad libitum access to food (Breeders Purina Rat Chow, Lowell, MA) and water. Rats used in the following experiments were maintained and treated in accordance with the guidelines for animal care and use established by the National Institutes of Health (1986) and the Binghamton University Institutional Care and Use Committee.
Cesarean delivery
Near term (E21) pups were delivered by cesarean section. Isoflurane (Baxter, Deerfield, IL; VetEquip, Pleasanton, CA) was used to anesthetize the dam during cesarean delivery. A midline incision was made through the abdominal wall to expose the uterine horns. A small incision into each amniotic sac allowed externalization of the pups. The umbilical cords were ligated with sewing thread and cut. Finally, extra embryonic membranes were removed by gentle rolling of the neonate on a sanitary paper towel. Each pup was placed into a plastic container (12 cm long × 12 cm wide × 6 cm high) lined with moist paper towels. This container held pups in a 35°C ± 1°C incubator before and after odor exposures and testing. Once the cesarean section was completed, the dam was sacrificed via rapid cervical dislocation. The litters’ time of birth was noted when the median pup (e.g., fourth out of seven pups) was delivered.
Fostering
All pups in the present series of experiments were cesarean sectioned. Since the cesarean section is a non survival surgery for the dam, pups to be tested on postnatal day one (P1) were placed with a foster dam, which had given birth within 24 hours. Fostering involved removal of the entire biological litter and replacement with the experimental litter, which always consisted of eight pups. In order to facilitate acceptance, the experimental pups were gently rolled in the shavings of the foster dam to coat them with her scent.
Heating chamber and odor presentation
Since odor exposure procedures persisted for an hour, pups were exposed to stimuli within a temperature controlled heating chamber (Microplate Incubator, Boekel Scientific, Feasterville, PA). These heating chambers were maintained at 35°C ± 1°C. Within the heating chamber, pups were held in a hexagonal shaped shallow cup (8.5 mm wide at the top, 5.5 mm wide at the bottom, 2 mm deep) lined with synthetic fur (for a schematic depiction see Miller & Spear, 2008). All conditions had two pups in each cup, one male and one female. Odors were presented by placing a cotton swab above and in the center of the odor exposure cup. This cotton swab remained there, stationary, for the duration of the odor exposure period.
Test box
Testing occurred in a transparent glove box (63 cm long × 50 cm wide × 25 cm high). The pup was placed on a mirror atop a heating pad maintained at 35.5°C ± 0.5°C. The mirror allows the 35.5°C ± 0.5°C temperature to radiate up and around the pup. For this reason the ambient temperature in the box was kept at a lower temperature (28.0°C± 1.0°C). These temperatures have been used for these ages in many similar paradigms (Cheslock et al., 2004; Cheslock, Varlinskaya, Petrov, & Spear, 2000; Miller & Spear, 2008; Petrov, Varlinksaya, & Spear, 2001). Heating pad temperature was maintained using a temperature controller (Model 40-90-8B, Frederick Haer, Inc., Brunswick, ME). Ambient temperature was obtained with the use of commercial heating pads. In order to facilitate and standardize the odor exposure procedure the individual subject was strapped and fastened into a ‘vest’ made from ultra-thin, elastic rubber (Petrov, Varlinskaya, & Spear, 2001). The vest was designed to hold the pup in a semi-supine posture, which simulates the natural position of neonatal rats suckling at the maternal nipple (Eilam & Smotherman, 1998). This gentle restraint also prevents a righting reflex but does not otherwise produce apparent discomfort or behavioral incompetence.
Test
Duration of behavioral activation was measured by a blind experimenter (via video playback) in a continuous fashion for the entire four-minute test (inter-rater reliability: Pearson’s correlation coefficient > 0.93). Pups were placed into a vest and then into the testing box for a two-minute baseline period. Next, exposure to the test odor was presented on a cotton swab lightly waved 1 cm in front of the pups’ snout for two more minutes for a total of a four-minute motor activity test. Duration of head and full body or bursting movements was scored, with a stopwatch, as a single motor activity score (Miller & Spear, 2008).
Data analysis
The dependent variable was the duration of overall motor activity during minute one and minute two of test odor exposure. Thus, a mixed design ANOVA was used for these analyses. Since baseline levels of motor activity were recorded the dependent variable was adjusted according to baseline measurements. Differences in baseline activity also were tested with between groups ANOVAs. The mean motor activity duration score from the first two minutes of the test (baseline) was subtracted from each of the odor exposure minutes for baseline-adjusted data. For example: motor activity during Minute 3 − [(motor activity during Minute 1 + motor activity during Minute 2)/2]. The same was done for Minute 4. In the following experiments, Minute was a within-subjects independent variable (Minute 1 and Minute 2 of odor exposure) and duration of motor activity in seconds was the dependent measure. Post hoc analyses were conducted using Fisher’s least significant difference test. Results were considered significant if p < 0.05.
Experiment 1
The goal of Exp 1 was to assess odor-induced motor activity in newborn rats after prior exposure to the odor. Two different ages and two different retention intervals were employed. Previous experiments have found differences in the olfactory learning and/or retention of rats three hours after birth (P0) compared to pups just one day later (Cheslock, Sanders, & Spear, 2004). The present experiment is unique in that it used a mere odor exposure paradigm rather than associative conditioning. Furthermore, the newborn rats used in the experiments described below were exposed to an odor immediately after birth rather than three hours later.
Method
Subjects
Eighty cesarean sectioned rat pups from 13 different dams were used in the current experiment. The present design was a 2 (exposure condition, odor vs no odor) × 2 (exposure day, P0 vs P1) × 2 (retention interval, 3hr vs 27hr), which resulted in 8 groups. There were 10 pups per group with no more than one male and one female per litter represented in each group.
Procedures
Previous experiments did not find consistent sex effects in olfactory learning immediately after birth (Miller & Spear, 2008, 2009). Therefore, although sex was evenly distributed across groups, this was not a factor in the present design. All cesarean deliveries were conducted between 900 and 1100 hours. At this time, eight experimental pups were delivered via cesarean. Extra pups were delivered via cesarean section to ensure that pups were not isolated in the incubator and to maintain a foster litter of eight pups. Pups were marked by wrapping a thin piece of adhesive tape around the pups’ tail. Half (four) of the pups were assigned to the odor condition and were exposed to 0.1 mL of lemon oil immediately after delivery (postnatal day zero, P0) or the following day, on P1. The other half was treated similarly but with a non-odorized cotton swab (no odor). On the appropriate exposure day, two pups (one male, one female) were exposed to lemon odor and two to a non odorized cotton swab in a heated chamber for one hour. Half of the pups exposed on P0 were held in an incubator for a 3 hr retention interval (P0-3 hr) prior to testing. The other half were placed into an incubator for 3 hours and then fostered for testing 27 hrs after odor exposure (P0-27 hr). Pups to be exposed on P1 were placed into an incubator immediately after birth for four hours and then fostered. Pups exposed on P1 were returned to their foster dam during the 3 hr retention interval (P1-3 hr) prior to testing or for the 27 hr retention interval (P1-27 hr). Thus, P0 and P1 pups spent their 3 hr retention interval in different locations, in the incubator and with the foster dam respectively. Previous studies using these ages have conducted their procedures in this manner. More specifically, P0 rats are placed into incubators (Petrov, Nizhnikov, Varlinskaya, & Spear, 2006; Cheslock, Varlinskya, Petrov, & Spear, 2000) whereas P1 pups are returned to their dam during a retention interval (Bordner & Spear, 2006). Testing occurred after the retention interval as described above (see section “test”).
Results
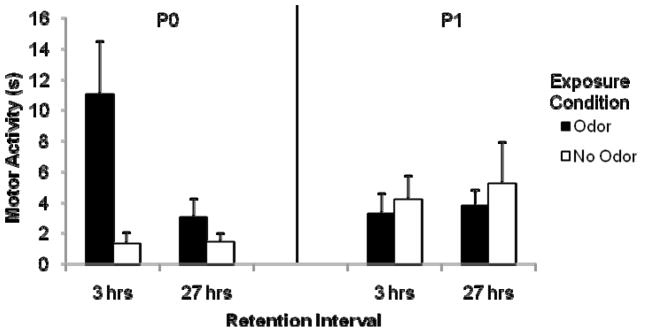
Baseline levels of activity did not differ based on exposure condition, exposure day or retention interval. Using baseline adjusted data, a significant exposure condition by exposure day interaction was found, F (1, 72) = 8.10, p = .00575. Post hoc tests revealed that P0-odor pups were activated more by the previously experienced odor than were P0-no odor and P1-odor pups (see Figure 1). In other words, pups exposed to an odor on P0 showed elevated levels of activity upon reexposure to that odor at test compared to non-exposed pups. This was not the case when odor exposure occurred on P1. Although the lack of difference on P1 between odor and no odor pups seems not only due to the fact that P1 odor pups activated less than P0 odor pups, but also that P1 no odor pups responded more to the odor at test without prior odor experience than P0 pups did, this post hoc comparison failed to reach significance (p = .059). There were no other significant effects. This experiment indicated that odor-induced motor activity at test was evident when odor exposure took place immediately after birth but not if given 24 hrs later.
Figure 1.
P0 pups, but not P1 pups, respond with greater motor activity to an odor after prior odor exposure (black bars) compared to pups with no prior odor exposure (white bars). Data is adjusted for baseline levels of activity. Vertical lines represent the standard error of the mean.
Experiment 2
Previous experiments with P0 rats have maintained the pups in an incubator between odor exposure and testing, assuming they were not fostered (the cesarean section procedure is a non-survival surgery). In experiments conducted with P1 rats, however, pups have been returned to their dam during the retention interval (at this age, pups were typically vaginally delivered). For comparison of P0 and P1 pups’ olfactory learning, all pups were cesarean sectioned in the current series. Nevertheless, as in previous similar experiments, P0 pups were kept in an incubator and P1 pups with a foster dam during the 3 hr retention interval in Exp 1. While being in an incubator for 3 hours is typical for studies using P0 pups that have never experienced a dam, this 3-hr period far exceeds the maternal deprivation ordinarily allowed for P1 pups in our laboratory. Before explicitly testing the effect of dam presence during the retention interval (Exp 3) the present experiment was designed to find a shorter retention interval that would still allow odor preexposure effects to be expressed. Since learning was only found in P0 rats (Exp 1), the current experiment was conducted with this age only.
Method
Subjects
One hundred and six cesarean sectioned rat pups from 16 different dams were used in the current experiment. The present design was a 2 (odor exposure, odor vs no odor) x 4 (retention interval, 0, 0.5, 1.25, or 3 hr), which resulted in 8 groups. Sex was evenly distributed across groups. There were 11–15 pups per group, with no more than one male and one female per litter represented in each group.
Procedures
Cesarean sections were performed between 900 and 1100 hours and eight pups (four males and four females) were delivered. All pups were placed into heated chambers immediately after birth (P0). Half of the pups (two males, two females) were exposed to lemon odor (odor) and the other half were exposed to a non-odorized cotton swab (no odor) for 60 minutes. Pups were held in a warm incubator for a 0, 0.5, 1.25 or 3 hr retention interval. Testing occurred after this retention interval in a manner identical to Exp 1.
Results
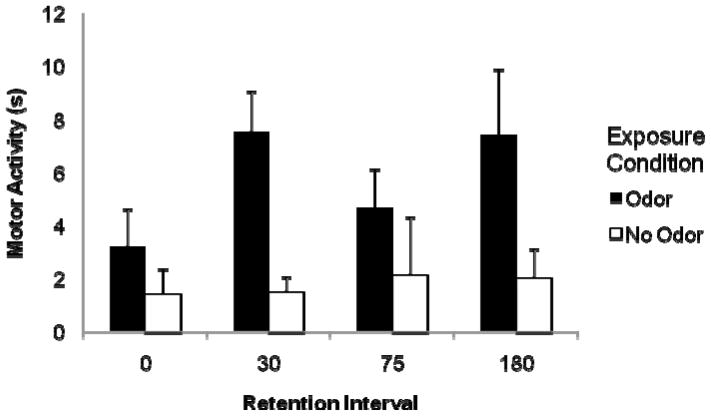
Baseline levels of activity did not differ as a function of exposure condition or retention interval. Using baseline adjusted data, there was a significant effect of exposure condition, F (1, 98) = 19.17, p < .05, and of the repeated measure, minute, F (1, 98) = 6.22, p < .05, but no effect of retention interval, F (3, 98) = 1.49, p = .22 nor an interaction of any of these variables (ps > 0.1). Pups exposed to the lemon odor were more active at test during reexposure to this odor, regardless of retention interval (see Figure 2). Odor-evoked activity was higher during the first minute of odor exposure than the second minute. Despite the fact that increased motor activity was seen in pups given previous odor exposure regardless of retention interval, we chose to use the 30 minute retention interval in Exp 3 instead of the 0 minute retention interval since levels of responding appeared higher in this group (Figure 2).
Figure 2.
P0 pups that were exposed to an odor (black bars) immediately after birth responded with greater levels of motor activity after a 0, 30, 75, and 180 minute retention interval compared to pups without this exposure (white bars). Data is adjusted for baseline levels of activity. Vertical lines represent the standard error of the mean.
Experiment 3
In Exp 1 we found that responding to a previously exposed odor was greater for pups that had this experience immediately after birth compared to 24 hours later. Nevertheless, there was a confounding of age by differences in maternal deprivation. P0 pups spent their 3 hour retention interval in an incubator whereas P1 pups spent this time with a foster dam. Although both ages also had littermates present, the presence of a dam could have played a role in the pups’ response to the previously experienced odor. The goal of the present experiment was to explicitly manipulate dam presence during the retention interval.
Method
Subjects
Eighty two cesarean sectioned rat pups from 13 different dams were used in the current experiment. The present design was a 2 (exposure condition, odor vs no odor) × 2 (exposure day, P0 vs P1) × 2 (dam presence, yes vs no), which resulted in 8 groups. There were 8–12 pups per group with no more than one male and one female per litter represented in each group.
Procedures
As in Exp 1 and 2, sex was evenly distributed across all groups. Cesarean delivery, fostering and odor exposure procedures were identical to Exp 1. After exposure to the odor or not for an hour on P0 or P1 pups were placed either in an incubator or with a foster dam for a 30 minute retention interval prior to testing. Testing procedures were identical to Exp 1 and 2.
Results
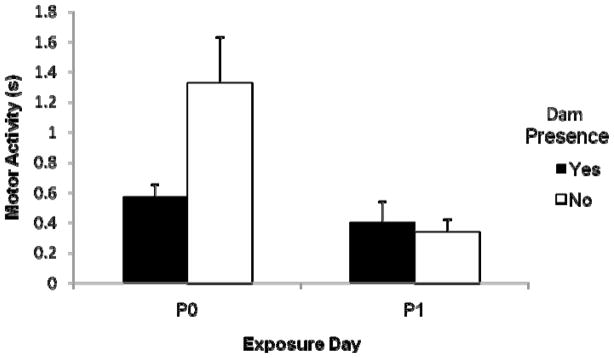
Unlike Exp 1 and 2, baseline activity levels differed across groups. There was a significant effect of exposure day, F (1, 74) = 10.04, p < .05, a borderline effect of dam presence, F (1, 74) = 3.78, p = .056, and a significant interaction betwen these two variables, F (1, 74) = 4.64, p < .05, see Figure 3. P0 pups were more active during baseline than P1 pups. Without the dam presence during the retention interval pups were moderately more active at baseline than pups that had spent this time with a dam. The interaction was a result of low baseline activity levels for P1 pups regardless of dam presence, whereas P0 rats were relatively active after being in the incubator with only littermates during the retention interval although behaviorally quieted when the dam was present.
Figure 3.
Baseline activity levels were suppressed in P0 pups after exposure to the dam during the retention interval compared to P0 pups, which were not returned to the dam. P1 pups’ baseline activity levels did not differ based on dam presence during the retention interval. Vertical lines represent the standard error of the mean.
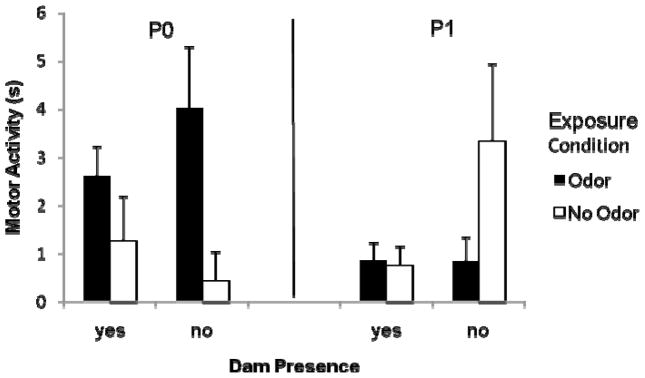
Using baseline adjusted data, there were no main effects but there was a significant interaction of exposure condition by exposure day, F (1, 74) = 11.65, p < .05. P0 pups previously exposed to an odor were significantly more active upon reexposure to that odor at test than pups without previous odor exposure. P1 pups, however, showed the opposite pattern. After previous odor exposure, P0 pups were significantly more active at test than P1 pups with this same exposure. There was an interaction of exposure condition by exposure day by dam presence, F (1, 74) = 5.05, p < .05, which tempered the aforementioned interaction (see Figure 4). The pattern described above occurs largely when there is no dam present during the retention interval. When pups are placed with a dam during the retention interval, P0 pups with odor exposure continue to respond more to that odor at testing than P0 pups without prior odor exposure whereas P1 pups show no differences based on previous odor exposure, replicating results from Exp 1.
Figure 4.
Motor activity to an odor after prior odor exposure (black bars) or not (white bars) differs based on age of pup and, for P1 pups, on dam presence during the retention interval. Data is adjusted for baseline levels of activity. Vertical lines represent the standard error of the mean.
Although P1 pups’ baseline activity was unaffected by dam presence during the retention interval, after spending 30 minutes with the foster dam P0 pups were much less active than P0 pups that spent the retention interval in an incubator. Dam presence, however could not explain the age-related differences found in Exp 1. In the present experiment P0 pups but not P1 pups were again more active than controls in the presence of a previously exposed odor regardless of dam presence during the retention interval. In the absence of the dam during the retention interval, P1 pups responded to the odor at test only when they had no prior odor experience.
General Discussion
In Exp 1, P0 but not P1 pups displayed heightened motor activity in response to an odor experienced three hours earlier compared to pups without this odor preexposure. After odor exposure, P0 pups were placed into an incubator with littermates and P1 pups were placed with a foster dam for the three hour retention interval. To test the importance of dam presence during the retention interval, Exp 2 identified a shorter but still effective retention interval in order to avoid prolonged maternal deprivation. P0 pups were capable of expressing prior odor exposure after a 0, 30, 75 or 180 minute retention interval. Using the 30 minute retention interval, results from Exp 3 indicated that P0 pups behaved similarly whether the dam had been present during the retention interval or not. P1 pups that spent the 30 min retention interval with their dam showed no effect of their previous odor exposure, replicating findings from Exp 1. Without the dam present, however, P1 pups seemed to be affected by the prior odor exposure but in a manner opposite that of P0 pups: P1 pups without prior odor exposure showed odor-evoked motor activity at test but similar activity was not seen in P1 pups that had prior odor exposure. P0 pups on the other hand only show odor-evoked motor activity after prior odor exposure.
Heightened odor-induced motor activity in P0 pups with previous odor exposure compared to non-exposed pups may indicate recognition of that odor. Nevertheless, sensitization is a possible alternative explanation. Sensitization, or increased responsiveness after successive exposures, is unlikely however since previous experiments have found that animals given two odor exposures prior to activity testing did not exhibit levels of activity higher than pups with only one odor exposure (Miller & Spear, 2009). Given the apparent lack of odor specificity in the effect of such neonatal odor exposure (Miller & Spear, 2009), it is also possible that such responding could indicate odor-induced neurophysiological changes in the olfactory bulb. Yet, odor recognition and neural changes in the olfactory bulb are not mutually exclusive explanations. Beyond recognition, however, it is impossible to know for certain the affective state, if any, of the pup. Motor activity may signify either an appetitive response (an attempt to move toward the odor) or an aversive response (an attempt to move away from the odor). It is interesting to note, however, that when odor exposure on P0 was paired with pulses of appetitively reinforcing milk, motor activity was increased beyond that of pups given exposure to the odor alone (Miller & Spear, 2009). Furthermore, several studies have documented odor preferences resulting from mere odor exposure in rat pups during their second postnatal week (e.g., Alberts & May, 1984; Caza & Spear, 1984).
In addition to the effects of prior odor exposure on odor-evoked activity, there were also differences in baseline activity in P0 pups based on the presence of the dam during the retention interval (Exp 3). P0 pups that spent the retention interval with the dam had much lower baseline levels of activity than those spending it without the dam. This effect may be due to maternal regulation of infant behavior and physiology (Johanson & Hall, 1982; Levine, 1968; Stanton, Gutierrez, & Levine, 1988). No age-related differences in baseline activity were seen in Experiment 1, although dam presence was not manipulated.
Regulation of offspring behavior can also be seen when novel environments cause locomotor activation in the pups that is mitigated by familiar odors (e.g., anesthetized dam or littermates; Hofer & Shair, 1987). With previous innocuous or perhaps appetitive exposure to the odor, pups may learn that the odor is not related to an aversive consequence (Hill, 1978; Zajonc, 2001; Spear & Rudy, 1991). Alternatively, initial motor activity to odors could reflect neophobia and hence aversion that can be decreased by mere exposure to the novel stimulus (Domjan, 1976). Given the absence of prior experience with the dam, it was not surprising that P0 pups responded differently than P1 pups in the presence of an odor not previously experienced. It may be advantageous for a pup to respond uniquely to the first odor it is exposed to because the first odors are likely to be the dam or nest odors (Miller & Spear, 2009; Romantshik, Porter, Tillmann, & Varendi, 2007). If prior dam exposure were the driving force behind the current age-related differences we would expect that giving P0 rats exposure to a dam prior to odor exposure should result in a pattern of results more similar to what was seen in P1 rats. Alternatively, prior dam exposure might not be necessary; exposure to any prior odor before test might be sufficient.
Prior exposure to the dam may explain why P0 and P1 pups differ in their response to a previously experienced odor. Neurochemical changes surrounding the birthing process may also mechanistically underlie the unique properties of early olfactory learning. For example, norepinephrine levels increase dramatically after birth (Herlineus & Lagercrantz, 2001). Norepinephrine has not only been implicated in learning and memory in both infant and adult animals, but studies have correlated NE levels at birth with later olfactory preference test scores subsequent to a mere odor exposure procedure occurring shortly after birth in human neonates (Varendi, Porter, & Winberg, 2002). An age-related difference in olfactory learning was detected when comparing associative interference between rat pups taught on the day of birth and one day later (Cheslock et al., 2004). Furthermore, there is some evidence with human infants that mere odor exposure learning within half an hour after birth is more effective than 12 hours later (Romantshik, Porter, Tillman, Varendi, 2007). Mere odor exposure occurring immediately after birth decreased the latency to attach to a similarly-scented surrogate nipple more effectively than if odor exposure had occurred hours later (Miller & Spear, 2009). In addition to norepinephrine, P0 pups have had more recent exposure to amniotic fluid than P1 pups. Since amniotic fluid promotes endogenous opioid release, P0 pups’ odor exposure may have occurred under heightened opioid levels. Like NE, opioids are also involved in learning as well as responsiveness to sensory input (Robinson & Méndez-Gallardo, 2009).
An age-related pattern of responding dependent on preexposure was also documented in perinatal rats by Mickley et al (2000). After preexposure to saccharin infusions on embryonic day 19 (E19), P3 pups displayed increased responding to saccharin whereas decreased responding to saccharin was seen in cesarean sectioned pups tested on E21 pups, compared to water pretreated neonates. These differences were discussed by Mickley et al as possibly being related to nursing history since E21 rats had not yet nursed whereas P3 rats had. However, pups with previous nursing experience in our experiment (P1s) responded with less activity to a preexposed odor than those without such experience (P0s). Differences between our studies and those of Mickley and colleagues include age, stimuli used (taste versus odor), test procedures, and retention interval. These differences may account for the different pattern of results.
Learning about odors soon after birth is fundamental to forming familial attachments in most mammals, including humans (Leon, 1992). Studying such processes will provide a window into how these attachments are created and maintained. For instance, the current study found that the presence of the dam during a retention interval affects learning and/or memory in an age-dependent manner. Future studies can dissect this effect and study the underlying mechanisms. Possible mechanisms include but are certainly not limited to prior experience with the dam or other olfactory stimuli, norepinephrine levels during odor exposure (Ronca et al., 2006), and recent amniotic fluid exposure (Robinson & Méndez-Gallardo, 2009) all of which presumably differ from the day of birth (P0) to P1.
Acknowledgments
The research presented in this article was supported by grants from National Institute of Mental Health (RO1MH035219) and the National Institute on Alcohol Abuse and Alcoholism (RO1AA013098, R01AA015992 and R01AA011960) to Norman E. Spear. We express our appreciation to Teri Tanenhaus for assistance with the manuscript.
References
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental Psychobiology. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Spear NE. Isolation disrupts retention in preweanling rat pups. Behavioral Neuroscience. 1995;109:744–758. doi: 10.1037//0735-7044.109.4.744. [DOI] [PubMed] [Google Scholar]
- Balogh RD, Porter RH. Olfactory preferences resulting from mere exposure in human neonates. Infant Behavior and Development. 1986;9:395–401. [Google Scholar]
- Bordner KA, Spear NE. Olfactory learning in the one-day old rat: Reinforcing effects of isoproterenol. Neurobiology of Learning and Memory. 2006;86:19–27. doi: 10.1016/j.nlm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Caza PA, Spear NE. Short-term exposure to an odor increases its subsequent preference in preweanling rats: a descriptive profile of the phenomenon. Developmental Psychobiology. 1984;17:407–422. doi: 10.1002/dev.420170407. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Sanders SK, Spear NE. Learning during the newborn’s first meal: special resistance to retroactive interference. Developmental Science. 2004;7:581–598. doi: 10.1111/j.1467-7687.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behavioral Neuroscience. 2000;114:484–495. [PubMed] [Google Scholar]
- Coureaud G, Schaal B, Hudson R, Orgeur P, Coudert P. Transnatal olfactory continuity in the rabbit: behavioral evidence and short-term consequence of its disruption. Developmental Psychobiology. 2002;40:372–390. doi: 10.1002/dev.10038. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Brain Research. 1986;392:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Domjan M. Determinants of the enhancement of flavored-water intake by prior exposure. Journal of Experimental Psychology. Animal Behavior Processes. 1976;2:17–27. doi: 10.1037//0097-7403.2.1.17. [DOI] [PubMed] [Google Scholar]
- Eilam D, Smotherman WP. How the neonatal rat gets to the nipple: common motor modules and their involvement in the expression of early motor behavior. Developmental Psychobiology. 1998;32:57–66. doi: 10.1002/(sici)1098-2302(199801)32:1<57::aid-dev7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hepper PG. The amniotic fluid: an important priming role in kin recognition. Animal Behavior. 1987;35:1343–1346. [Google Scholar]
- Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Human Development. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- Hill WF. Effects of mere exposure on preferences in nonhuman mammals. Psychological Bulletin. 1978;85:1177–1198. [PubMed] [Google Scholar]
- Hofer MA, Shair HN. Isolation distress in two-week-old rats: influence of home cage, social companions, and prior experience with littermates. Developmental Psychobiology. 1987;20:465–476. doi: 10.1002/dev.420200410. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive conditioning in neonatal rats: conditioned orientation to a novel odor. Developmental Psychobiology. 1982;15:379–397. doi: 10.1002/dev.420150410. [DOI] [PubMed] [Google Scholar]
- Leon M. Neuroethology of olfactory preference development. Journal of Neurobiology. 1992;23:1557–1573. doi: 10.1002/neu.480231012. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of infantile stimulation on the response to stress during preweanling development. Developmental Psychobiology. 1968;1:67–70. [Google Scholar]
- Mickley GA, Remmers-Roeber DR, Crouse C, Walker C, Dengler C. Detection of novelty by perinatal rats. Physiology & Behavior. 2000;70:217–225. doi: 10.1016/s0031-9384(00)00229-8. [DOI] [PubMed] [Google Scholar]
- Miller SS, Spear NE. Olfactory learning in the rat neonate soon after birth. Developmental Psychobiology. 2008;50:554–565. doi: 10.1002/dev.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Spear NE. Olfactory learning in the rat immediately after birth: unique salience of first odors. Developmental Psychobiology. 2009;51:488–504. doi: 10.1002/dev.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. Journal of Neuroscience. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. DHEW Publication No 86–23. Washington, DC: U.S. Government Printing Office; 1986. Guide for the care and use of laboratory animals. [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: Effects on suckling plasticity. Behavioral Neuroscience. 2001;115:1318–1331. [PubMed] [Google Scholar]
- Petrov ES, Nizhnikov ME, Varlinskaya EI, Spear NE. Dynorphin A (1–13) and responsiveness of the newborn rat to a surrogate nipple: Immediate behavioral consequences and reinforcement effects in conditioning. Behavioral Brain Research. 2006;170:1–14. doi: 10.1016/j.bbr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Méndez-Gallardo V. Amniotic fluid as an extended milieu interieur. In: Hood KE, Halpern CT, Greenberg G, Lerner RM, editors. Handbook of Developmental Science, Behavior, and Genetics. Malden, MA: Wiley Blackwell; 2009. [Google Scholar]
- Romantshik O, Porter RH, Tillmann V, Varendi H. Preliminary evidence of a sensitive period for olfactory learning by human newborns. Acta Paediatrica. 2007;96:372–376. doi: 10.1111/j.1651-2227.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Abel RA, Ronan PJ, Renner KJ, Alberts JR. Effects of labor contractions on catecholamine release and breathing frequency in newborn rats. Behavioral Neuroscience. 2006;120:1308–1314. doi: 10.1037/0735-7044.120.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Spear NE. Effects of the home environment on withholding behaviors and conditioning in infant and neonatal rats. Science. 1978;202:217–225. doi: 10.1126/science.694538. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Spear NE. Home environmental stimuli facilitate learning of shock escape spatial discrimination in rats 7–11 days of age. Behavioral and Neural Biology. 1981;31:360–365. doi: 10.1016/s0163-1047(81)91439-4. [DOI] [PubMed] [Google Scholar]
- Spear NE. Ecologically determined dispositions control the ontogeny of learning and memory. In: Kail R, Spear NE, editors. Comparative Perspectives on the Development of Memory. Hillsdale, NJ: Erlbaum; 1984. [Google Scholar]
- Spear NE, Kucharski D, Hoffmann H. Contextual influences on conditioned taste aversions in the developing rat. Annals of the New York Academy of Sciences. 1985;443:42–53. doi: 10.1111/j.1749-6632.1985.tb27062.x. [DOI] [PubMed] [Google Scholar]
- Spear NE, Rudy JW. Tests of the ontogeny of learning and memory: Issues, methods, and results. In: Shair H, Barr G, Hofer MA, editors. Developmental Psychobiology: New methods and changing concepts. New York: Oxford Press; 1991. [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Developing a sense of safety: the neurobiology of neonatal attachment. Annals of the New York Academy of Science. 2003;1008:122–131. doi: 10.1196/annals.1301.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Research Bulletin. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Varendi H, Porter RH, Winberg J. The effect of labor on olfactory exposure learning within the first postnatal hour. Behavioral Neuroscience. 2002;116:206–211. doi: 10.1037//0735-7044.116.2.206. [DOI] [PubMed] [Google Scholar]
- Zajonc RB. Mere Exposure: A Gateway to the Subliminal. Current Directions in Psychological Science. 2001;10:224–228. [Google Scholar]