Abstract
Previous studies have suggested that there may be species-specific differences in the metabolism of polybrominated diphenyl ethers (PBDEs) among different fish species. In this study, we investigated the in vitro hepatic metabolism of eleven individual PBDE congeners (tri- through decaBDEs) in three different fish species: rainbow trout (Oncorhynchus mykiss), common carp (Cyprinus carpio), and Chinook salmon (O. tschwatcha). In addition, we evaluated the influence of PBDE structural characteristics (i.e., bromine substitution patterns) on metabolism. Six of the eleven congeners we evaluated, BDEs 99, 153, 183, 203, 208, and 209, were metabolically debrominated to lower brominated congeners. All of the congeners that were metabolized contained at least one meta-substituted bromine. Metabolites were not detected for congeners without one meta-substituted bromine (e.g., BDEs 28, 47, and 100). Metabolite formation rates were generally 10–100 times faster in carp than in trout and salmon. BDEs 47, 49, 101, 154, and 183 were the major metabolites observed in all three species with the exception of BDE 47, which was only detected in carp. Carp demonstrated a preference towards meta-debromination, while trout and salmon debrominated meta- and para- bromine atoms to an equal extent. We compared glutathione-S-transferase (GST) and deiodinase (DI) activity among all three species as these enzyme systems have been hypothesized to play a role in PBDE debromination among teleosts. Carp exhibited a preference for meta-deiodination of the thyroid hormone thyroxine, which was consistent with the preference for meta-debromination of PBDEs observed in carp.
Keywords: PBDEs, debromination, carp, trout, salmon, fish, structure-activity, metabolism, deiodinase
Introduction
Polybrominated diphenyl ethers (PBDEs) are flame retardants that are incorporated into consumer products such as textiles, polyurethane foam, and casing for electronics. Two of the commercial PBDE flame retardant mixtures, PentaBDE and OctaBDE, were listed as persistent organic pollutants (POPs) according to the Stockholm Convention on POPs due to their persistence and biomagnification potential (1). Currently, DecaBDE, which consists almost entirely of the fully brominated congener BDE 209, is the only PBDE mixture still used today, and it is scheduled for phase out by the end of 2013 for similar reasons (2). Due to their persistence and ability to bioaccumulate, lower brominated PBDEs are often detected in aquatic organisms, which may be attributable in part to biotransformation of higher brominated congeners (3, 4). The metabolism of PBDEs in humans and other mammals typically occurs via oxidative pathways, producing hydroxylated PBDEs (OH-BDEs) and brominated phenols (5, 6). Contrary to mammals, fish have not been shown to form oxidative metabolites of PBDEs, but have instead demonstrated the ability to reductively debrominate PBDEs both in vivo and in vitro (7, 8).
Studies have shown that PBDE congener profiles in wild fish vary among species. Wild Chinook salmon (Oncorhynchus tshawytscha) typically accumulate higher percentages of BDEs 47, 49, and 99 compared to other congeners (9, 10), while wild common carp (Cyprinus carpio) typically accumulate higher proportions of BDE 47 and 100 and little or no BDE 99 (11, 12). We previously observed that carp could metabolically debrominate BDE 99 to a greater extent than Chinook salmon and that the metabolic products differed between species (BDE 47 was formed in carp and BDE 49 was formed in salmon) (13, 14). Previous studies in carp have shown that the metabolic debromination of BDEs 99, 183, and 209 favored the removal of meta-substituted bromine atoms (7, 8, 15), while in Chinook salmon, the debromination of BDE 99 favored the removal of a para-substituted bromine atom (14). These differences in PBDE metabolism may influence PBDE accumulation patterns observed in wild fish. Little is known about species-specific differences in PBDE metabolism, and previous studies have not investigated species-specific differences in the metabolism of individual congeners other than BDEs 99 and 209 (14, 15).
It has been hypothesized that deiodinase enzymes (DIs) may play a role in the debromination of PBDEs in fish due to the structural similarity of PBDEs to thyroid hormones (7, 15). DIs are responsible for activating and deactivating thyroid hormones by cleaving iodine atoms from thyroxine (T4), 3,3′,5-triiodothyronine (T3), or 3,3′,5′-triiodothyronine (rT3). These dehalogenation reactions occur via the removal of an ortho- or meta-iodine. Definitive evidence establishing the role of DIs in PBDE debromination in fish is still needed (i.e., assessing PBDE debromination with purified DIs), and it is possible that other biotransformation enzyme systems may also be involved, such as glutathione-S-transferases (GSTs). Previous studies have ruled out the likely involvement of cytochrome P450s (16). PBDEs are well known disruptors of thyroid hormone regulation in fish (17), but little is known about the mechanisms of thyroid toxicity. Understanding the metabolism of PBDEs in fish, as well as their potential interactions with DIs, may allow for better understanding of the toxicological mechanisms.
The objective of this study was to compare the metabolism of eleven individual PBDE congeners among carp (Cyprinus carpio), rainbow trout (Oncorhynchus mykiss), and Chinook salmon (O. tshawytscha). We used environmentally relevant PBDE congeners from seven homologue groups (tri- through deca-) to investigate the structural characteristics and bromine substitution patterns that influence debromination. We also compared the activities of two classes of enzymes, GSTs and DIs, among the three fish species to determine if their relative activities were reflective of the differences in PBDE metabolism.
Methods and Materials
Materials
Individual PBDE congeners used for dosing, BDEs 28, 47, 49, 99, 100, 153, 154, 183, 203, 208, and 209, were purchased as neat standards from AccuStandard, Inc. (New Haven, CT, USA). Quantification standards for the previously mentioned congeners and BDEs 101, 144, 149, 180, and 187 were also purchased from AccuStandard, Inc. Internal and surrogate standards 13C-2,2′,3,4,5,5′-hexachlorodiphenylether (CDE 141) and 13C-decabromodiphenylether (13C-BDE 209) were purchased from Wellington Labs (Guelph, Canada), 4′-fluoro-2,3′,4,6-tetrabromodiphenylether (FBDE 69) was purchased from Chiron (Trondheim, Norway), and 13C-3,3′ diiodithyronine (13C-3,3′ T2), 13C-T3, 13C-rT3, and 13C-T4 were purchased from Isotec (Miamisburg, OH). Unlabeled thyroid hormones (T4, T3, rT3, 3,3′ T2 and 3,5 T2), reduced glutathione, 1-chloro-2,4-dinitrobenzene (CDNB), and dithiothreitol (DTT) were purchased from Sigma Aldrich (St. Louis, MO). All solvents and other materials were HPLC grade.
Animals
Adult common carp livers were donated by Dr. Carys Mitchelmore from the Chesapeake Biological Laboratory (Solomons, MD) and were originally purchased as juveniles from Hunting Creek Fisheries in Thurmont, MD. Adult rainbow trout livers were donated by the Armstrong State Fish Hatchery in Marion, NC. Juvenile Chinook salmon livers were donated by Dr. Evan Gallagher from the University of Washington in Seattle, WA.
Preparation of Hepatic Sub-Cellular Fractions
Five grams of liver tissue pooled from five individual fish (1 g per fish) of each species was homogenized for five minutes using a Bullet Blender with 0.5 mm zirconium oxide beads (Next Advance, Averill Park, NY). Using previously published methods, microsomal suspensions were prepared from homogenized liver tissues with 10 mM dithiothreitol (DTT) in all buffers (18). Protein concentrations were determined using a bicinchoninic acid assay (Pierce, Rockford, IL). Heat-inactivated microsomes (submersed in boiling water for 15 min) spiked with individual PBDEs were used as negative controls.
Incubations
Incubations were performed in glass test tubes at 25°C in a shaking water bath at 140 rpm using our published method (13). The 1-mL incubation mixtures contained 0.1 M potassium phosphate buffer (pH 7.4) with 10 mM DTT, 100 μM NADPH, microsomes at a concentration of 1 mg protein mL−1, and approximately 1 nmol of a PBDE congener delivered in 5 μL of acetone. Previous studies have shown that higher substrate concentrations (>50 μM) result in a wider variety and higher abundance of metabolites (13). However, for this study, we chose a lower substrate concentration of 1 μM to more closely reflect environmentally relevant concentrations, while still producing detectable levels of metabolites. All rainbow trout and salmon incubations, as well as carp microsomal incubations with higher brominated PBDEs (i.e., heptaBDEs and higher) were conducted for 24 hours. Incubations containing lower brominated PBDE congeners (i.e., hexaBDEs and lower) with carp microsomes were conducted for one hour due to their higher metabolic activity. Previous studies in carp and salmon have shown that debromination can continue for up to 24 hours during in vitro incubations (13, 14).
Extraction and Analysis
Incubations were stopped with 1 mL of ice-cold methanol, extracted with hexane, and cleaned with sulfuric acid using our previously published method (13). FBDE 69 and 13C-BDE 209 were added prior to extraction as surrogate internal standards, and CDE 141 was added prior to GC/MS analysis to measure their recovery. Samples were analyzed using a GC/MS (Agilent Model numbers 6890N and 5975, respectively) operated in electron capture negative ionization (ECNI) mode using published run parameters (19). A calibration mixture containing 43 PBDE congeners was used for identification and quantification of metabolites (less brominated congeners) and parent compounds. Analytes were confirmed by homologue group using GC/MS operated in electron ionization mode (GC/EI-MS). Samples were also analyzed using a liquid chromatography tandem mass spectrometer (LC-MS/MS) (Agilent 1200 SL binary pump and an Agilent 6410 MS/MS) operated in multiple reaction monitoring (MRM) mode (monitoring MRM transitions for tri- through nona-OH-BDEs) with a Hypersil C-18 column following a previously published method (20) to determine if OH-BDEs or 2,4,5 tribromophenol were formed as metabolites
DI Assay
To measure the activity of endogenous DI enzymes in microsomal fractions, approximately 500 pmol of T4 was used as a substrate instead of PBDE congeners in the previously described incubations. Incubations were performed for 90 minutes and were stopped with 1 mL of ice-cold acetone, deproteinated, and extracted using our lab’s published method for thyroid hormones (21). The formation rates of T3, rT3, 3,5 T2 and 3,3′ T2 were determined using LC/MS/MS in positive-ESI mode by monitoring MRM transitions using 13C-3,3′ T2, 13C-T3, and 13C-T4 as surrogate internal standards. We injected 20 μL of sample onto a Synergi 2.5 μm Polar-RP 100A column (2.5 μm, 50 mm × 2.0 mm) (Phenomenex, Torrance, CA) with water (A) and acetonitrile (B) mobile phases with 0.01 M formic acid. A gradient method at a flow rate of 0.40 mL/min was used with the following parameters: 30% B from 0–2 min, 90% B from 2–5 min, and 30% B from 5–10 min.
Cytochrome C Reductase Assay
The integrity of the microsomal fractions was assessed using an NADPH-cytochrome c reductase assay (Thermo-Pierce, Rockford, IL). NADPH-cytochrome c reductase is an enzyme involved in the hepatic mixed-function monooxygenase system that transfers electrons to cytochrome P-450 and other oxygenases in the endoplasmic reticulum (22). Thus, NADPH-cytochrome c reductase activity is a reliable indicator of microsomal integrity. The values obtained for NADPH-cytochrome c reductase activity were 9 ± 1, 11 ± 1, and 10 ± 1 nmol min−1 mg protein−1 for carp, rainbow trout, and salmon, respectively.
mGST Assay
GSTs are a multigenic family of Phase II metabolizing enzymes that include mitochondrial, cytosolic, and microsomal isoforms with the ability to conjugate glutathione to xenobiotics. The GST activity was assessed in each pool of microsomes using a published method, which measured the conjugation of glutathione to 1-chloro-2,4-dinitrobenzene (CDNB) to form glutathione-2,4-dinitrobenzene (13).
QA
The average recovery of FBDE 69 in the microsomal extracts was 103 ± 12% and the average 13C-BDE 209 recovery was 107 ± 7%. The average recoveries of 13C-T2, 13C-T3, 13C-rT3, and 13C-T4 were 103 ± 15%, 100 ± 16%, 102 ± 14%, and 80 ± 10%, respectively. Limits of detection were defined as 3 times the standard deviation of heat-inactivated and buffer controls. Samples with active microsomes were blank corrected for low concentrations of impurities detected in spiking solutions observed in incubations with heat-inactivated microsomes. Metabolite formation is reported as pmol or fmol hr−1 mg protein−1. Statistical analyses were performed using a Student’s t-test in Sigma Plot 9.0 with statistical significance was defined as p<0.05.
Results and Discussion
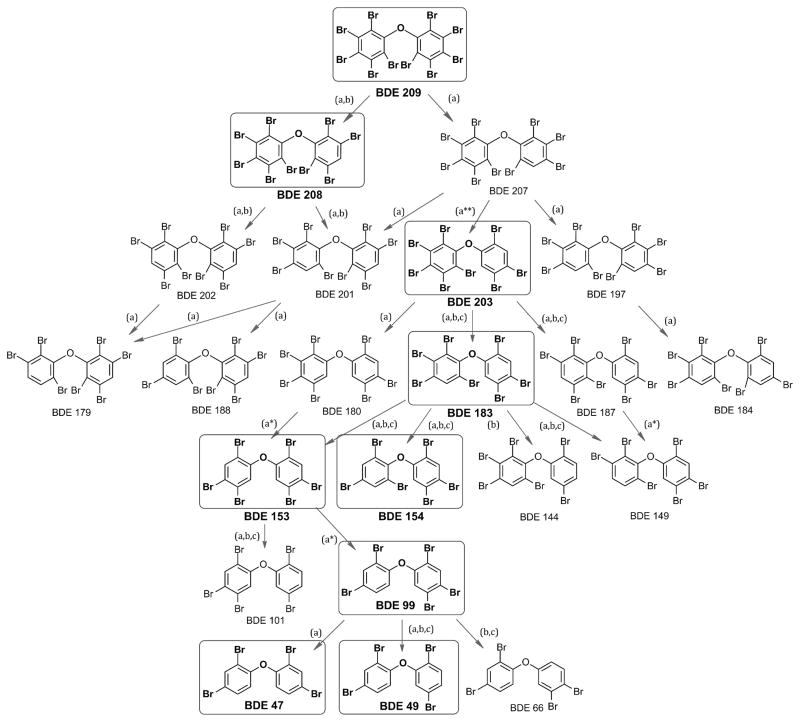
Extracts from the microsomal incubations were analyzed using GC/MS to identify and quantify the debrominated metabolites of eleven environmentally relevant PBDE congeners (BDEs 28, 47, 49, 99, 100, 153, 154, 183, 203, 208, and 209) (23). Metabolic debromination was observed in incubations with six of eleven congeners tested (BDEs 99, 153, 183, 203, 208, and 209), while no metabolism was observed in incubations with the remaining five (BDEs 28, 47, 49, 100, and 154). The most rapidly metabolized congeners were BDEs 99 and 183, and the slowest metabolized congeners were BDEs 208 and 209. Up to six metabolites were observed for each metabolized congener. Some PBDE congeners detected as metabolites were used as substrates in our experiments. For example, BDE 203 was metabolically debrominated to form BDE 183, and in a separate incubation, BDE 183 was debrominated to form several hexaBDEs. The results from incubations with individual congeners were combined to create a metabolic pathway that characterized the potential debromination of BDE 209 to tetra- and pentaBDEs (Fig. 1).
Figure 1.
Hepatic microsomal biotransformation pathway for (a) common carp, (b) rainbow trout, and (c) Chinook salmon. Boxes indicate congeners used as substrates in incubations. (*) Steps in which two bromine atoms were removed in one incubation were hypothesized based on the structure of the metabolite assuming no rearrangement of bromine atoms. (**) BDE 203 coelutes with BDE 200, which is also a potential metabolite not shown here.
Samples were also screened for oxidative metabolites using LC/MS/MS. Hydroxylated BDEs and 2,4,5 tribromophenol have been observed as BDE 99 metabolites in mammalian studies (5, 6). However, OH-BDEs (i.e., tri- through nona-OH-BDEs) or brominated phenols were not detected in any samples in this study, which is consistent with previous fish studies (7). Therefore, reductive debromination appears to be the primary pathway of PBDE metabolism in fish.
Debromination in Common Carp
In carp, in vitro incubations with BDE 209 generated small masses (<1% of the substrate) of a variety of metabolites from hepta- through nonaBDEs. A previous study detected hexaBDEs as metabolites of BDE 209 in juvenile carp hepatic microsomal incubations and pentaBDEs after in vivo exposure to BDE 209 (7, 15). The more limited debromination observed in this study may have been due to differences in the expression and activity of the enzyme(s) systems responsible for the metabolism of PBDEs as a result of different aged fish used in each study. The major metabolites of BDE 209 were octaBDE 197 and nonaBDEs 207 and 208. The lowest brominated metabolite of BDE 209 detected in our samples was heptaBDE 184 (40 ± 5 fmol hr−1 mg protein−1). The lowest brominated metabolites of BDE 208 were heptaBDEs 179 and 188 (39 ± 22 and 68 ± 19 fmol hr−1 mg protein−1). The only two metabolites of BDE 209 that were evaluated separately as substrates in this study were BDEs 203 and 208.
BDE 203 was debrominated to form several hepta- and hexaBDEs, including BDEs 183 (1,190 ± 190 fmol hr−1 mg protein−1) and 154 (230 ± 30 fmol hr−1 mg protein−1) and to a lesser extent, BDE 153 (44 ± 12 fmol hr−1 mg protein−1). The formation of BDE 154 as a metabolite of both BDEs 183 and 203 occurred fairly rapidly in comparison to many of the other reactions observed (Table 1). Furthermore, BDE 154 was resistant to metabolic debromination in our experiments. These results suggest that BDE 154 is likely to accumulate to a greater extent than other higher brominated congeners in vivo as a result of metabolic debromination. Several studies examining PBDEs in marine mammals have observed higher concentrations of BDE 154 relative to BDE 153, even though commercial PBDE formulations contain higher concentrations of BDE 153 (3, 23). This inconsistency may be explained in part by the debromination of higher brominated PBDEs in fish, resulting in accumulation of BDE 154 in higher trophic level marine mammals.
Table 1.
Hepatic microsomal formation rates of debrominated metabolites in incubations containing 1 nmol of the parent congener expressed as fmol hr−1 mg protein−1 with standard error.
| Parent | Metabolite | [Br] Position | Formation Rates by Species |
||
|---|---|---|---|---|---|
| Common Carp | Rainbow Trout | Chinook Salmon | |||
| pentaBDE 99 | |||||
| 47 | m | 237,980 ± 22,600 | ND | ND | |
| 49 | p | 1,750 ± 250 | 140 ± 10 | 210 ± 10 | |
| 66 | o | ND | 8.2 ± 4 | 17 ± 2 | |
| hexaBDE 153 | |||||
| 47 | m,m | 1,290 ± 300 | ND | ND | |
| 101 | p | 990 ± 130 | 6.6 ± 1.3 | 87 ± 8 | |
| heptaBDE 183 | |||||
| 144 | p | ND | 9 ± 1 | ND | |
| 149 | p | 530 ± 60 | 37 ± 3 | 14 ± 1 | |
| 153 | o | 320 ± 40 | 37 ± 1 | 16 ± 7 | |
| 154 | m | 4,840 ± 490 | 1,500 ± 70 | 470 ± 3 | |
| octaBDE 203 | |||||
| 149 | m,p | 42 ± 4 | ND | ND | |
| 153 | m,o | 44 ± 12 | ND | 12 ± 1 | |
| 154 | m,m | 230 ± 30 | 10 ± 2 | 61 ± 6 | |
| 180 | o | 28 ± 7 | ND | ND | |
| 183 | m | 1,190 ± 190 | 150 ± 10 | 340 ± 60 | |
| 187 | p | 640 ± 90 | 150 ± 10 | 440 ± 70 | |
| nonaBDE 208 | |||||
| 179 | m,p | 39 ± 22 | ND | ND | |
| 188 | m,m | 68 ± 19 | ND | ND | |
| 201 | m | 760 ± 310 | 110 ± 20 | ND | |
| 202 | p | 690 ± 250 | 200 ± 50 | ND | |
| decaBDE 209 | |||||
| 184 | m,m,m | 40 ± 5 | ND | ND | |
| 197 | m,m | 100 ± 10 | ND | ND | |
| 201 | m,p | 58 ± 8 | ND | ND | |
| 202 | p,p | 45 ± 10 | 16 ± 2 | ND | |
| 203/200 | m,o/m,p | 45 ± 3 | ND | ND | |
| 207 | m | 250 ± 80 | ND | ND | |
| 208 | p | 130 ± 40 | ND | ND | |
BDE 153 was observed as a metabolite of BDEs 183 and 203. However, unlike BDE 154, BDE 153 was metabolized to form pentaBDE 101 and tetraBDE 47. The formation of BDE 47 from BDE 153 was the only transformation in which a metabolic product with two fewer bromine atoms than the parent was detected without detecting the intermediate (in this case, BDE 99). This was probably due to the rapid metabolism of BDE 99 to 47 (238 ± 23 pmol hr−1 mg protein−1), which was by far the most rapid transformation observed in this study. Furthermore, similar to BDE 154, BDE 47 was resistant to metabolism, which further increases its potential to bioaccumulate in organisms such as carp.
Debromination in Rainbow Trout and Chinook Salmon
In general, the metabolic products and rates were similar for trout and salmon. Unlike carp, which debrominated BDE 209 to form hepta- through nonaBDEs, very limited metabolism of BDE 209 occurred in rainbow trout and salmon. The only significant metabolites of BDEs 209 and 208 in rainbow trout were BDEs 202 and 201. Due to the slow biotransformation of BDEs 208 and 209 in salmon, quantification of metabolites was not possible. However, other congeners that were metabolized in carp (i.e., BDEs 99, 153, 183, and 203) were also metabolized in trout and salmon, albeit at much slower rates. The biotransformation rates for trout and salmon were typically 10–100 times slower than those observed in carp. This indicated a greater ability of carp to metabolically debrominate PBDEs, which was consistent with previous studies (14, 15).
The only congener that was debrominated to similar products in all three fish species was BDE 183. BDE 154 was the dominant metabolite and BDEs 153 and 149 were minor metabolites of BDE 183 in all species, even though the metabolite formation rates were more rapid in carp. For the other congeners, there were differences in both the products and rates of PBDE debromination between carp and the salmonids (trout and salmon). In incubations with BDEs 153 and 99, there were major differences in the metabolic products and formation rates. BDE 47 was not detected as a metabolite in trout or salmon. The absence of this metabolic pathway resulted in much slower metabolism of BDEs 99 and 153 in trout and salmon. Unlike carp, which readily debrominated BDE 153 to form BDE 47, only BDE 101 was observed as a metabolite of BDE 153 in trout and salmon.
Structure-Activity Relationships
As mentioned previously, the slowest metabolite formation rates were observed for BDEs 208 and 209 in all three species. In carp, there was a general trend towards higher biotransformation rates in congeners with fewer than six bromine atoms, which was clearly evidenced by the rapid biotransformation of BDE 99 in carp. For trout and salmon, heptaBDE 183 and octaBDE 203 were most rapidly metabolized with slower biotransformation of BDEs 99 and 153.
In all three species, only congeners with at least one meta-substituted bromine atom were debrominated. Furthermore, congeners without meta-substituted bromines, BDEs 28, 47, and 100, did not undergo metabolism to any extent. Thus, it appeared that the presence of a meta-substituted bromine atom increased the potential for metabolic debromination of the congener. Because all of the debrominated metabolites observed in this study, except BDE 47, possessed at least one meta-substituted bromine, it is possible that many of these metabolites will be further debrominated in vivo based on this structure-activity relationship. However, because BDEs 154 and 49 both contain one meta-substituted bromine and were not metabolized, there are likely other structural factors affecting PBDE metabolism in fish.
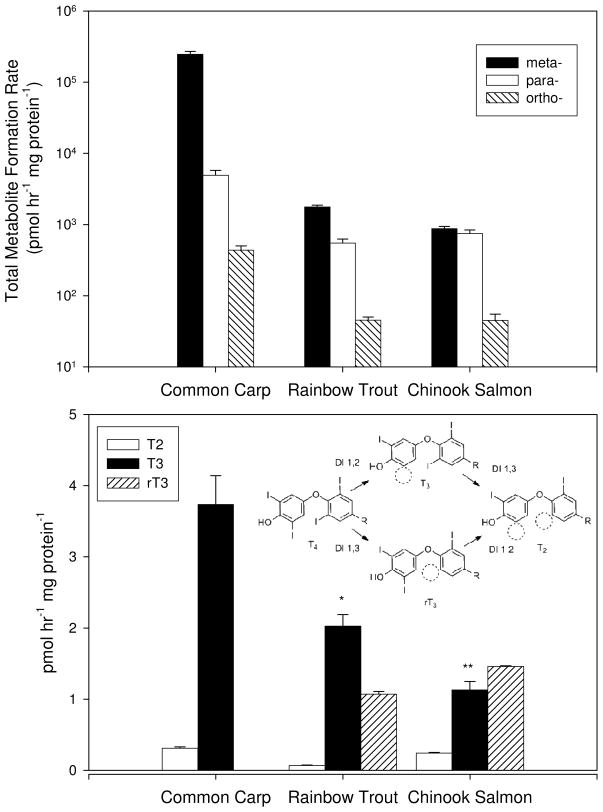
Although the substrate preference for PBDE congeners with at least one meta-substituted bromine constituent was conserved across all three species, there were other differences among species in the site of debromination. Fig. 2 presents a summation of all of the metabolite formation rates on a logarathmic scale categorized by the position of the bromine atom cleaved during metabolism. Carp demonstrated a clear preference for meta-debromination. However, in trout and salmon, there appeared to be no clear preference between meta- or para-debromination. The metabolism of BDE 183 to 154 was the only case in which meta-debromination was predominant in trout or salmon. In every other incubation, para-debromination was predominant. Fig. 2 shows the absence of any preference between para- or meta-debromination in salmon. This was a signficant difference between carp and salmonids in their biotransformation of PBDEs.
Figure 2.
Summed formation rates (fmol hr−1 mg protein−1) for all metabolites formed from BDEs 99, 153, 183, 203, 208, and 209 based on the position of debromination (top). Formation rates for 3,3′ T2, T3 and rT3 in hepatic microsomes incubated with 500 pmol of T4 and the thyroid hormone deiodination pathway (bottom). * denotes a significant difference from carp (p<0.05). ** denotes a significant difference from carp (p<0.01).
In carp, every debromination involved the removal of a bromine atom that was adjacent to at least one other bromine. For example, out of the three ortho-substituted bromines on BDE 183, only the ortho-bromine adjacent to a meta-substituted bromine underwent debromination to form BDE 153, while the other two ortho-substituted bromines were unmodified, as shown in Fig. 1. In trout and salmon, a small amount of BDE 66 was formed via the removal of an isolated ortho-substituted bromine, but this was only a minor metabolite.
Biotransformation Enzyme Activity
Previous work has investigated the involvement of DIs and GSTs in the debromination of PBDEs in fish (13, 14, 16). GSTs are responsible for Phase II conjugation of many xenobiotics with glutathione, and are capable of catalyzing reductive dehalogenation reactions, as evidenced by the reductive dechlorination of DDT by Drosophila GST (24). Although most GST activity is present in the cytosol, about 3% of microsomal protein is GST (mGST) (25). We compared mGST activity towards the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione among the three species. The mGST activities for carp, trout, and salmon microsomes were 38 ± 2, 65 ± 7, and 14 ± 3 nmol min−1 mg protein−1, respectively. Trout had higher mGST activity, which was not reflective of the PBDE debromination rates among species but was similar to values observed in a previous study (26). Although this does not definitively rule out the involvement of GSTs in PBDE debromination, it provides further indication, along with a previous study (14), that GSTs are not involved in PBDE debromination in fish.
PBDEs are structurally similar to the thyroid hormones T4, T3, and rT3, which are substrates in deiodination reactions mediated by DIs. The earliest evidence for the involvement of DIs was demonstrated when in vivo debromination of PBDEs in carp generally favored the removal of meta-substituted bromine atoms, which was similar to the removal of a meta-iodine atom from thyroid hormones by DIs (7). Later studies showed that debromination of BDE 99 by salmon microsomes decreased when DTT, which is a required co-substrate for in vitro activity of DI enzymes, was omitted from incubation buffers (14, 16). Other studies showed that a DI inhibitor, iodoacetate, inhibited PBDE debormination, and rT3 and to a lesser extent T4 also inhibited PBDE debromination at various substrate concentrations, which implied that PBDEs and thyroid hormones competed as substrates (13, 16).
In this study, we attempted to further investigate the involvement of DIs in PBDE metabolism by assessing DI activity in pooled microsomes from each species. We hypothesized that the species-specific differences we observed in PBDE debromination would correspond with species-specific differences in DI activity. The conversion of T4 to its deiodinated metabolites T3 and rT3 was monitored using LC/MS/MS in microsomal incubations similar to the PBDE incubations, but using T4 instead of PBDEs as the substrate. Distributions of deiodinated metabolites of T4 varied among all three species, as shown in Figure 2. Carp demonstrated faster formation of T3 from T4 than rainbow trout (p<0.05) or salmon (p<0.01) via a meta-deiodination reaction. Trout and salmon demonstrated ortho-deiodination of T4 to form rT3, which was not observed in carp. These differences in DI activity between carp and salmonids may be responsible for the species differences observed in PBDE debromination (Fig. 2), but further studies using purified DIs are needed to confirm the involvement of DIs in PBDE debromination.
Implications
Metabolism likely plays an important role in influencing congener distributions of PBDEs in wild fish. Differences in metabolite formation rates observed in this study corresponded with PBDE congener distributions in wild fish. In wild salmon, PBDE congener distributions included higher proportions of BDEs 47 and 99 than other congeners (27). In wild common carp, BDEs 47, 100, and 154 dominated the congener distribution, with little or no detectable BDE 99 (11). According to the results of this study, the enrichment of BDE 47 in wild carp may attributable in part to the rapid metabolism of BDEs 153 and 99 to BDE 47. Salmon demonstrated much slower debromination rates of BDE 99 than carp, which may allow BDE 99 to accumulate in salmon to a greater extent.
While understanding characteristics of biotransformation of PBDEs is important in understanding their environmental fate, it may also be important from a toxicological perspective. Because lower brominated congeners are often regarded as more toxic and have a higher biomagnification potential than higher brominated congeners, debromination may enhance the toxicity of PBDEs in fish and may increase the effects on higher trophic level organisms (7, 17). Furthermore, the potential involvement of DIs in PBDE metabolism may result in altered DI activity, thereby disrupting thyroid regulation. In lake trout and fathead minnows, PBDEs caused decreases in circulating T4 and T3, which may be indicative of altered thyroid hormone homeostasis (17, 28). Furthermore, Szabo et al. (29) observed decreases in DI activity following exposure to PBDEs in rats. Future studies should consider examining the effects of PBDEs on thyroid hormone metabolism to determine whether PBDEs may interfere with basal DI activity.
In conclusion, we observed debromination in six of eleven PBDE congeners analyzed in this study, including penta- through decaBDEs, in carp and rainbow trout. This provides further evidence that BDE 209 may degrade in the environment to form more toxic, lower brominated congeners. Species-specific differences in metabolic rates and products were observed for each substrate. Meta-debromination was predominant in carp, while there was no preference between meta- or para-debromination in rainbow trout and salmon. These species-specific structure-activity relationships may be important in modeling the distribution of PBDE congeners in the environment. It is likely that carp will accumulate BDEs 47 and 154 as a result of metabolism, while trout and salmon will likely accumulate BDEs 99 and 154 to a greater extent. Species-specific differences were also observed in DI activity; carp microsomes demonstrated a strong preference for meta-deiodination of T4, while trout and salmon demonstrated higher rates of ortho-deiodination. These species-specific differences in DI activity corresponded to the species-specific differences observed in PBDE debromination and provided further indication of the involvement of DIs in the metabolic debromination of PBDEs.
Acknowledgments
This study was supported by a grant from the National Institute of Environmental Health Sciences, R01ES016099. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- 1.EU. Stockholm Convention on Persistent Organic Pollutants. 2009. Adoption of Amendments to Annexes A, B, and C. Decisions SC-4/13, 14, 18. Available at chm.pops.int.
- 2.USEPA. DecaBDE phase-out initiative. 2010 Available at http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html.
- 3.Hites RA. Polybrominated diphenyl ethers in the environment and in people:3 a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 4.Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, Sonne C, Verreault J, Vijayan MM, Gabrielsen GW. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ. 2010;408(15):2995–3043. doi: 10.1016/j.scitotenv.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytesin vitro. Environ Health Perspect. 2008;117(2) doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L-J, Lebetkin EH, Sanders JM, Burka LT. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica. 2006;36(6):515–534. doi: 10.1080/00498250600674477. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol. 2004;38(1):112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton HM, Letcher RJ, Baker JE. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio) Environ Sci Technol. 2004;38(4):1054–1061. doi: 10.1021/es0348804. [DOI] [PubMed] [Google Scholar]
- 9.Sloan C, Anulacion B, Bolton J, Boyd D, Olson O, Sol S, Ylitalo G, Johnson L. Polybrominated diphenyl ethers in outmigrant juvenile Chinook salmon from the Lower Columbia River and Estuary and Puget Sound, Washington. Arch Environ Contam Toxicol. 2010;58(2):403–414. doi: 10.1007/s00244-009-9391-y. [DOI] [PubMed] [Google Scholar]
- 10.Montory M, Habit E, Fernandez P, Grimalt JO, Barra R. PCBs and PBDEs in wild Chinook salmon (Oncorhynchus tshawytscha) in the Northern Patagonia, Chile. Chemosphere. 2010;78(10):1193–1199. doi: 10.1016/j.chemosphere.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Fuentetaja A, Lupton S, Clapsadl M, Samara F, Gatto L, Biniakewitz R, Aga DS. PCB and PBDE levels in wild common carp (Cyprinus carpio) from eastern Lake Erie. Chemosphere. 2010;81(4):541–547. doi: 10.1016/j.chemosphere.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Xia K, Luo MB, Lusk C, Armbrust K, Skinner L, Sloan R. Polybrominated diphenyl ethers (PBDEs) in biota representing different trophic levels of the Hudson River, New York: from 1999 to 2005. Environ Sci Technol. 2008;42(12):4331–4337. doi: 10.1021/es703049g. [DOI] [PubMed] [Google Scholar]
- 13.Noyes PD, Kelly SM, Mitchelmore CL, Stapleton HM. Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquat Toxicol. 2010;97(2):142–150. doi: 10.1016/j.aquatox.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browne EP, Stapleton HM, Kelly SM, Tilton SC, Gallagher EP. In vitro hepatic metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE 99) in Chinook salmon (Onchorhynchus tshawytscha) Aquat Toxicol. 2009;92(4):281–287. doi: 10.1016/j.aquatox.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stapleton HM, Brazil B, Holbrook RD, Mitchelmore CL, Benedict R, Konstantinov A, Potter D. In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol. 2006;40(15):4653–4658. doi: 10.1021/es060573x. [DOI] [PubMed] [Google Scholar]
- 16.Benedict RT, Stapleton HM, Letcher RJ, Mitchelmore CL. Debromination of polybrominated diphenyl ether-99 (BDE-99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere. 2007;69(6):987–993. doi: 10.1016/j.chemosphere.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environ Sci Technol. 2004;38(5):1496–1504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- 18.McKinney MA, Arukwe A, De Guise S, Martineau D, Béland P, Dallaire A, Lair S, Lebeuf M, Letcher RJ. Characterization and profiling of hepatic cytochromes P450 and phase II xenobiotic-metabolizing enzymes in beluga whales (Delphinapterus leucas) from the St. Lawrence River Estuary and the Canadian Arctic. Aquat Toxicol. 2004;69(1):35–49. doi: 10.1016/j.aquatox.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42(18):6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 20.Mas S, Jauregui O, Rubio F, de Juan A, Tauler R, Lacorte S. Comprehensive liquid chromatography-ion-spray tandem mass spectrometry method for the identification and quantification of eight hydroxylated brominated diphenyl ethers in environmental matrices. J Mass Spectrom. 2007;42(7):890–899. doi: 10.1002/jms.1224. [DOI] [PubMed] [Google Scholar]
- 21.Wang DL, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2010;397(5):1831–1839. doi: 10.1007/s00216-010-3705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shephard EA, Pike SF, Rabin BR, Phillips IR. A rapid one-step purification of NADPH-cytochrome c (P-450) reductase from rat liver microsomes. Anal Biochem. 1983;129(2):430–433. doi: 10.1016/0003-2697(83)90573-0. [DOI] [PubMed] [Google Scholar]
- 23.La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used Penta-, Octa-, and Deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 24.Tang AH, Tu CP. Biochemical characterization of Drosophila glutathione S-transferases D1 and D21. J Biol Chem. 1994;269(45):27876–27884. [PubMed] [Google Scholar]
- 25.Morgenstern R, Lundqvist G, Andersson G, Balk L, Depierre JW. The distribution of microsomal glutathione transferase among different organelles, different organs, and different organisms. Biochem Pharmacol. 1984;33(22):3609–3614. doi: 10.1016/0006-2952(84)90145-x. [DOI] [PubMed] [Google Scholar]
- 26.Laurén DJ, Halarnkar PP, Hammock BD, Hinton DE. Microsomal and cytosolic epoxide hydrolase and glutathione S-transferase activities in the gill, liver, and kidney of the rainbow trout, Salmo gairdneri: baseline levels and optimization of assay conditions. Biochem Pharmacol. 1989;38(6):881–887. doi: 10.1016/0006-2952(89)90275-x. [DOI] [PubMed] [Google Scholar]
- 27.Hites RA, Foran JA, Schwager SJ, Knuth BA, Hamilton MC, Carpenter DO. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol. 2004;38(19):4945–4949. doi: 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- 28.Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (PBDE-47) Alters Thyroid Status and Thyroid Hormone–Regulated Gene Transcription in the Pituitary and Brain. Environ Health Perspect. 2008;116(12) doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic Phase I, Phase II, Phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107(1):27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]