Abstract
BACKGROUND
Nonsurgical subxiphoid pericardial access may be useful in ventricular tachycardia ablation and other electrophysiologic procedures but has a risk of right ventricular puncture.
OBJECTIVE
The purpose of this study was to identify a signature pressure frequency that would help identify the pericardial space and guide access.
METHODS
The study consisted of 20 patients (8 women and 12 men; mean age 59.1 ± 14.2 years; left ventricular ejection fraction 25.2% ± 12.2%; failed 1.8 ± 0.5 endocardial ablations; unresponsive to 2.0 ± 1.0 antiarrhythmic drugs; 6 ischemic cardiomyopathy, 12 nonischemic cardiomyopathy, 2 normal heart; 4 previous sternotomy) undergoing epicardial ventricular tachycardia ablation. After pericardial access was obtained, a 10Fr long sheath was used to record pressure inside the pericardium and pleural space. Pressures were analyzed using a fast Fourier transform to identify dominant frequencies in each chamber.
RESULTS
Mean pressures in the pleural space and the pericardium were not different (7.7 ± 1.9 mmHg vs 7.8 ± 0.9 mmHg, respectively). However, the pericardial space in each patient demonstrated two frequency peaks that correlated with heart rate (1.16 ± 0.21 Hz) and respiratory rate (0.20 ± 0.01 Hz), whereas the pleural space in each patient had a single peak correlating with respiratory rate (0.20 ± 0.01 Hz).
CONCLUSION
The pericardial space demonstrates a signature pressure frequency that is significantly different from the surrounding space. This difference may make minimally invasive subxiphoid pericardial access safer for nonsurgeons and may have important implications for electrophysiologic procedures.
Keywords: Catheter ablation, Epicardial ablation, Pressure frequency, Subxiphoid access, Ventricular tachycardia
Introduction
Access to the pericardial space may allow for multiple innovative electrophysiologic procedures, including ventricular tachycardia (VT) ablation, atrial fibrillation ablation, left atrial appendage removal, and left ventricular (LV) pacing for cardiac resynchronization.1–8 In particular, epicardial VT ablation may better target VT substrate compared with endocardial ablation alone. In a series of 231 patients undergoing endocardial VT, only 53% of patients were free of VT shocks at 6 months.9 Although no series has compared the long-term success rates of endocardial to epicardial ablation, one reason for the failure of endocardial ablation is the significant portion of VT circuits that can be epicardial. For example, Sacher et al10 found that 72% of patients with ischemic or nonischemic VT had at least some epicardial substrate.
Subxiphoid access allows for minimally invasive access to the pericardial space and the epicardium of the heart but is fraught with a 1% to 20% right ventricular (RV) perforation rate.11–13 Although most of these inadvertent RV punctures do not require surgical repair or abortion of the ablation procedure, this risk can be a barrier to broader adoption of epicardial electrophysiologic techniques, particularly in centers that have not performed many epicardial procedures.
When accessing the subxiphoid space using a nonsurgical technique, the needle goes through the diaphragm, briefly into the thorax, and then into the pericardial space.14 However, if the needle advances beyond the 1-mm-thick pericardial space, it can perforate the RV. One way to increase the safety of subxiphoid epicardial access would be to define a physiologic signature of the pericardial space that clearly differentiates it from the thorax. In transseptal access, for example, the pressure differences between the right and left atria and aorta are used to increase safety.
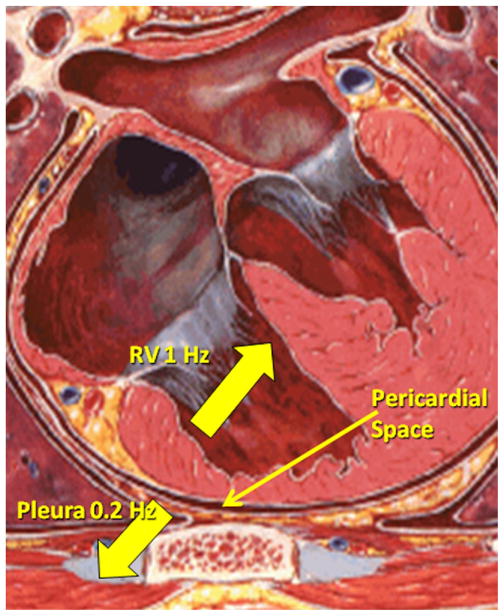
Because multiple studies have shown that thorax and pericardial space mean pressures are identical, pressure itself cannot be used to differentiate thorax from pericardial space.15 Although noting the difference of pressures between the RV and pericardial space can help identify perforations, finding a signature of the pericardial space to inform clinicians when they are safely in the pericardium and when further needle advancement is dangerous would be more helpful. The pericardial space is actually bordered by two structures: the heart and the lung field. The inferior pericardial sac is also attached to the diaphragm. The heart typically moves at 60 to 90 bpm. The lung field and diaphragm moves at 12 cycles per minute in intubated patients (Figure 1). Thus, although the pressure is the same in the thorax and pericardial space, the pressure frequency signatures should be different. In particular, we expected that the thorax frequency would be 0.2 Hz (12 breaths per minute). Inside the heart, the frequency peak should be 0.8 to 2 Hz (50–120 bpm), whereas a double frequency peak would be seen in the pericardial space at both approximately 0.2 Hz and 1 Hz. Initial studies in three patients as well as computer simulations suggest this hypothesis is accurate.16 Accordingly, we sought to determine if these signature pressure frequencies existed in 20 human patients undergoing subxiphoid epicardial access for VT ablation.
Figure 1.
Graphic illustration suggesting that pericardial sac frequency is influenced by respiration and heart rate. RV = right ventricle.
Methods
Patients
Twenty sequential patients underwent epicardial ablation for drug-refractory VT that had failed previous endocardial ablation (mean 1.8 ± 0.5, range 1–3). The 20 patients (12 male and 8 female) had a mean age of 59.1 ± 14.2 years and mean LV ejection fraction of 25.2% ± 12.2%. Four patients had undergone previous cardiac surgery. Twelve patients had nonischemic cardiomyopathy, 6 ischemic cardiomyopathy, and 2 idiopathic ventricular arrhythmia in the setting of preserved ejection fraction. All patients had a history of documented symptomatic VT. Patients had failed a mean of 2.0 ± 1.0 antiarrhythmic drugs, including amiodarone in 16 (80%). Eighteen patients had an implantable cardioverter-defibrillator and had received at least one appropriate shock (mean 11 ± 7 in the last 6 months). Eighteen patients were taking beta-adrenergic blockers, 14 angiotensin-converting enzyme inhibitors, and 15 HMG-CoA reductase inhibitors. Prior sternotomy was not an exclusion criterion, but patients who required a pericardial window for access were excluded from the study. Approval was obtained for all patients in accordance with the policy of the University of Virginia Institutional Review Board.
Intubation
All patients were intubated and mechanically ventilated at 12 breaths per minute using a Fabius GS anesthesia machine (ARYEO119) to achieve an end-tidal CO2 of 35 to 45 mmHg. Tidal volumes and positive end-expiratory pressure were adjusted by the operator. Positive end-expiratory pressure was always set between 2 and 5 cmH2O. Anesthesia was induced with propofol or etomidate as well as a short-acting muscle paralytic at the discretion of the attending anesthesiologist but was stopped 30 minutes prior to epicardial ablation. Deep general anesthesia was maintained with either propofol or volatile anesthetics (sevoflurane or desflurane). Fentanyl was administered at the discretion of the anesthesia care provider.
Access
The pericardial space was accessed using a 17-gauge epidural (Tuohy) needle as described by Sosa et al.14 Intracardiac echocardiography (ICE) in the RV and fluoroscopy with contrast were used to guide access. An anterior approach was used initially in patients without prior sternotomy. In patients with a prior sternotomy or a failed anterior approach, a posterior approach was used.
An entrance site was identified approximately 10 to 30 mm below the xiphoid, and the needle was angled toward the left shoulder and anterior wall of the RV under left anterior oblique fluoroscopic guidance. ICE in the RV was used to detect inadvertent RV entry. The needle was slowly advanced with periodic contrast injections through the diaphragm and into the pleural space. The needle then was slowly twisted and inserted into the pericardial space. Ventilation was not arrested in these patients. Once the pericardial space was accessed, the position was confirmed with contrast and by the lack of RV bubbles on ICE. Next, a 0.038-inch guidewire was placed over the needle in the left anterior oblique view to further confirm a pericardial position. The needle then was removed. A 5Fr sheath was placed over the wire, contrast dye injected in the pericardial space to confirm pericardial access, fluid removed to confirm the absence of blood, and the sheath upsized to a 10Fr sheath.
In patients who failed two anterior access attempts, a posterior approach was attempted by placing the needle next to the xiphoid and aiming for the RV posterior wall. In patients with prior sternotomy, only posterior access was attempted. If the four attempts at access failed, a surgical pericardial window was created, and the patient excluded from the study. Of note, in this study pressure frequency was not used to guide access but was measured later.
Mapping and ablation
The CARTO three-dimensional mapping system (Biosense-Webster, Diamond Bar, CA, USA) with ICE guidance (AcuNav Siemens AG, Malvern, PA, USA) was used to generate voltage and activation maps of the RV, LV, and epicardium as well as to guide ablation.
Mapping and ablation were performed using an externally irrigated catheter with a 3.5-mm tip (Biosense-Webster). In patients with hemodynamically unstable VT, RV, LV, and epicardial substrate mapping was performed. In the other patients, entrainment and activation mapping was used. Ablation was performed using up to 50 W and up to 30 mL of irrigation using ICE and three-dimensional mapping guidance.
Pressure frequency measurements
At the end of the procedure, as much fluid as possible was withdrawn from the pericardial sac using ICE guidance. A 120-second pressure tracing was recorded inside the pericardial space via the 10Fr sheath used for ablation. The sheath was attached to 45-cm pressure tubing, which in turn was attached to an electrophysiologic recording system (EP Medical Systems, St. Jude Medical, West Berlin, NJ, USA). This was the same setup used to obtain arterial pressures. Sampling rate was 2,000 Hz, and bandpass filter was set for 0.05 to 50 Hz with no rectification. The sheath was slowly withdrawn from the pericardial space with contrast injected every 2 mm. Once out of the pericardial space as judged by fluoroscopy and ICE during contrast injections, pressure tracings were recorded for 120 seconds. If contrast was seen below the diaphragm, the sheath was reinserted using a dilator and guidewire prior to recording. The 10Fr sheath then was reinserted over the wire (using a dilator if needed), a 7Fr Jackson Pratt drain was placed inside the sheath, and the sheath was removed.
Heart rate and respiratory rate were recorded continuously via an A-line and the ventilator, respectively, during withdrawal of the 10Fr sheath.
Data analysis
Data were visually analyzed to find the best representative 60-second tracing in both the pericardial space and the thorax. These two tracings were transferred to an analysis routine created in MATLAB 7.7.0 (The MathWorks, Natick, MA, USA) for postprocedure analysis. Sampling rate was reduced to 20 Hz. No window or additional filters were added. A fast Fourier transform was performed using a standard MATLAB routine, and the magnitude of the fast Fourier transform was graphed against the representative frequencies in the signal in order to obtain the frequency characteristics in the pericardial space and thorax for all 20 patients. The dominant peaks were compared to the breathing and heart rates of the patient at the time of data acquisition.
Statistical analysis
Primary measures of interest included thoracic and pericardial pressures, pericardial frequency, and pericardial frequency magnitude. Continuous variables are reported as mean ± SD. Analysis of variance was used for comparison of all continuous variables. P <.05 was considered significant. Data manipulation and analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC, USA).
Results
Twenty-four patients underwent epicardial VT ablation between November 2007 and March 2008; however, four required a subxiphoid window (three with prior coronary artery bypass graft surgery, one with large body habitus). These four patients were excluded from subsequent description and analysis.
Procedure complications
No complications occurred due to pressure frequency measurements. Total time for measurement in each patient was 7.8 ± 2.1 minutes. No deaths or strokes occurred during the procedure or postoperative hospital stay. However, during the procedure, one patient had ventricular fibrillation that did not respond to 15 external shocks. An internal implantable cardioverter-defibrillator shock returned the patient to sinus rhythm. This patient suffered no neurologic sequelae and underwent a substrate-based ablation. In addition, three patients suffered hematomas requiring a vascular surgery consult, but none required intervention.
Pressure tracing results
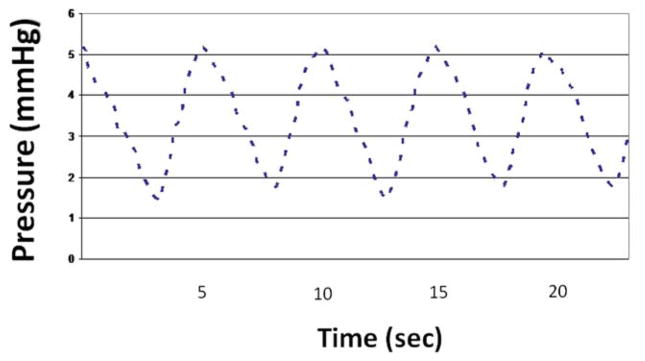
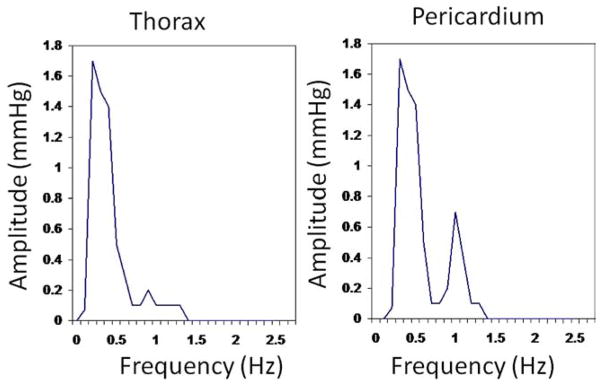
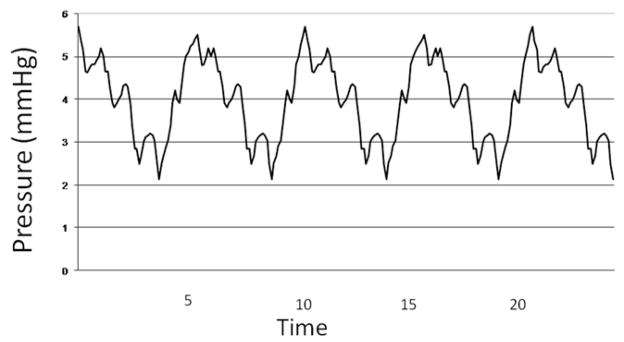
Mean pressures in the 20 patients were not different in the thorax and pericardial space (7.7 ± 1.9 vs 7.8 ± 0.9 mmHg, respectively, P = .45). Furthermore, in each of the 20 patients, the individual mean pressure in the thorax and in the pericardium were not different (Table 1). However, the pressure frequencies in the 20 thoraxes contained a single peak at 0.20 Hz ± 0.01 Hz with a mean amplitude of 1.1 ± 0.4 mmHg, whereas the pressure frequencies in 20 pericardia contained two peaks reflecting the breathing rate (0.20 ± 0.01 Hz) with a mean amplitude of 1.2 ± 0.3 mmHg and the heart rate (1.16 ± 0.21 Hz) with a mean amplitude of 0.6 ± 0.2 mmHg. Moreover, in each patient the peak frequency characteristics were different in the thorax and the pericardium (Table 1). A representative set of pressure tracings in one patient, from the thorax, pericardium, and the corresponding FFT are included as Figures 2–4. No patient had a frequency peak greater than 0.22 Hz in the thorax, and no patient had a second frequency peak less than 0.8 Hz in the pericardium (Figure 5).
Table 1.
Pressure and pressure frequencies from a 10Fr sheath in the thorax and pericardial space after epicardial ventricular tachycardia ablation in 20 patients
| Patient no. | Prior sternotomy | Thorax pressure (mmHg) | Thorax pressure frequency peak (Hz) | Pericardial pressure (mmHg) | Heart rate (bpm) | Pericardial pressure frequency peaks (Hz) |
|---|---|---|---|---|---|---|
| 1 | No | 8.2 | 0.21 | 8 | 60 | 0.20, 1.0 |
| 2 | Yes | 7.7 | 0.22 | 8 | 65 | 0.20, 1.1 |
| 3 | No | 8.1 | 0.21 | 8 | 90 | 0.21, 1.5 |
| 4 | No | 11.0 | 0.18 | 8 | 60 | 0.18, 1.0 |
| 5 | No | 8.1 | 0.19 | 7 | 90 | 0.19, 1.4 |
| 6 | No | 8.0 | 0.20 | 7 | 60 | 0.20, 1.0 |
| 7 | No | 4.1 | 0.21 | 7 | 60 | 0.21, 1.0 |
| 8 | No | 7.2 | 0.20 | 8 | 70 | 0.20, 1.2 |
| 9 | No | 8.2 | 0.20 | 7 | 65 | 0.22, 1.1 |
| 10 | No | 8.1 | 0.21 | 8 | 60 | 0.21, 1.0 |
| 11 | No | 6.3 | 0.22 | 8 | 60 | 0.22, 1.0 |
| 12 | Yes | 7.1 | 0.18 | 5 | 60 | 0.18, 1.0 |
| 13 | No | 11.0 | 0.20 | 10 | 50 | 0.20, 0.8 |
| 14 | No | 8.0 | 0.19 | 8 | 65 | 0.19, 1.1 |
| 15 | No | 8.1 | 0.18 | 8 | 70 | 0.18, 1.2 |
| 16 | No | 4.0 | 0.18 | 8 | 90 | 0.18, 1.4 |
| 17 | No | 8.0 | 0.20 | 8 | 60 | 0.20, 1.0 |
| 18 | Yes | 5.2 | 0.20 | 8 | 90 | 0.20, 1.4 |
| 19 | Yes | 7.2 | 0.18 | 8 | 90 | 0.18, 1.5 |
| 20 | No | 10.1 | 0.20 | 8 | 90 | 0.20, 1.5 |
Figure 2.
Patient with no prior surgery. Pressure tracing in thorax after withdrawal of sheath from pericardial space.
Figure 4.
Fast Fourier transform of pressure tracings shown in Figures 2 and 3 of patient without prior cardiac surgery.
Figure 5.
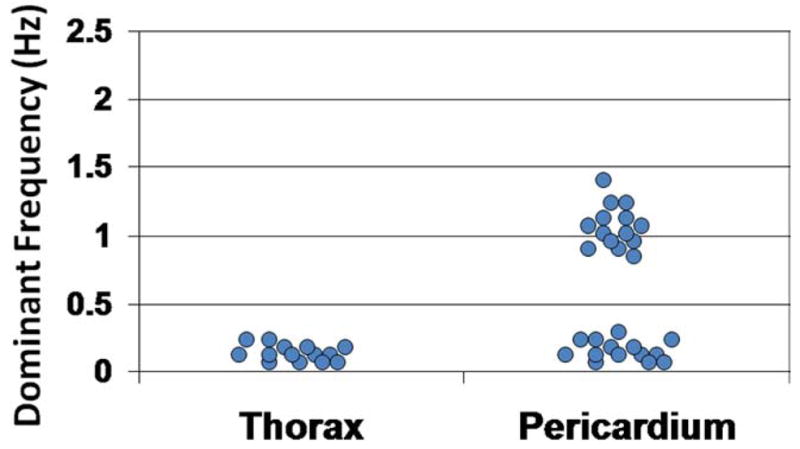
Dominant frequencies of thorax and pericardial sac pressure tracings in 20 patients.
Four patients had previous sternotomy, making comparisons difficult. Nonetheless, no difference in thoracic mean pressure frequency was seen in patients with and those without prior sternotomy (0.19 ± 0.01 Hz vs 0.20 ± 0.01 Hz, respectively, P = .20). In addition, no difference in the second pressure frequency peak (heart rate) in the pericardium was seen between patients with and those without prior surgery (1.25 ± 0.24 Hz vs 1.14 ± 0.21 Hz, respectively, P = .36). Furthermore, in all patients, the second frequency was separate from the first frequency by at least 0.82 Hz. However, the amplitude of the second peak frequency tended to be lower in patients with than in those without prior surgery (0.33 ± 0.05 mmHg vs 0.7 ± 0.13 mmHg, respectively, P <.001; Table 1).
Discussion
One of the barriers to epicardial VT ablation is the high risk of RV perforation during access. In the initial 240 patients reported by Sosa et al,12 the RV puncture rate was 5%. This complication rate decreases with experience, but a persistent high complication rate in general is likely as less experienced centers begin performing epicardial procedures. This can be a barrier to attempting nonsurgical subxiphoid epicardial access for VT ablation and other procedures.
When accessing one chamber from another, multiple techniques are available for reducing complications, including ICE and fluoroscopy. However, with ICE we are unaware of a method to consistently visualize the needle as it enters the pericardial space. Fluoroscopy with contrast is routinely used and can identify the pericardial space. However, the frequent contrast injections can obscure fluoroscopic guidance during mapping and ablation because the contrast does not easily dissipate. Furthermore, use of contrast for identifying the pericardial space can be difficult in poststernotomy patients. Consequently, pressure frequency measurements have the potential to minimize contrast injections and to be used in poststernotomy patients along with fluoroscopy and ICE.
In this study cohort, we showed that a signature pressure frequency exists in the pericardial space that differs from the pressure in thorax adjacent to it. Thus, it is conceivable that pressure frequency at the tip of an access needle could aid an operator in accessing the pericardial space and confirming proper location. This confirmation could be made immediately upon entering the pericardial space while minimizing the risk of advancing the needle too far and perforating the RV. Pressure frequency then could be used to supplement fluoroscopy and ICE.
For the four patients with previous sternotomy, there appeared to be no difference in the pressure frequency separation among patients with and those without prior sternotomy. Although prior cardiac surgery may alter the pericardium and result in regional areas of adherence of visceral and parietal pericardium, we have found that using a posterior approach often allowed access to the pericardial space in these patients. Prior cardiac surgery alone does not necessarily disqualify an epicardial approach, and pressure frequency measurement may assist during access in this patient population.
Our findings are not surprising given what is known about thorax physiology. However, to our knowledge, this pressure frequency difference has not been investigated or described previously. More importantly, it has not been shown during epicardial procedures. Thus, our results suggest that pressure frequency measurements on the needle tip during access could potentially improve safety for subxiphoid epicardial access. This approach can be used safely in patients with and those without prior cardiac surgery, thereby increasing its potential utility.
Study limitations
This study has limitations. First, we did not measure the pressure frequency on access and did not use it to guide access. Instead, we measured pressure frequency at the end of the procedure during sheath withdrawal because we had no way both to measure pressure frequency with the single-lumen Tuohy needle (standard for epicardial access). However, we expected little to no variation in pressure frequency from the beginning to the end of the procedure because we aspirated all pericardial fluid prior to measurements. Nevertheless, future testing should measure pressure frequency on entry in a large cohort of patients.
Second, we measured the pressure off the sheath side arm and not the needle tip. This could lead to damping due to the compressible nature of the sheath. However, we suspected that measurements obtained at the tip would be more accurate and that this technique would accentuate any difference in pressure frequency. Furthermore, more precise measurement may be obtained from a high-fidelity pressure frequency tool at the tip of the access needle. The findings in this study may justify those future studies.
An additional limitation was the offline processing of all of our data. However, it should be possible to produce a fast Fourier transform rapidly during the access procedure in order to help identify the pericardial space during access.
Small number of poststernotomy patients
The study included only a small number of patients with prior sternotomy because the threshold for performing an epicardial ablation in these patients is higher. Nonetheless, we saw no difference in pressure frequency morphology or measurements in patients with and those without prior surgery, although the magnitude of the dominant frequency was smaller in patients with prior surgery.
Holding ventilation
Some centers that perform epicardial access hold ventilation during access. We did not measure pressure frequency during a ventilation hold. We expect that a ventilation hold would remove the thorax (0.2 Hz) component in either the pericardial space or the thorax. However, we also expect the cardiac component to be preserved in the pericardial space. In this case, the pressure frequency in the pleura and pericardial space would be different. This still should help differentiate the pericardial space from the thorax and inform the operator to not advance the needle.
Spontaneous ventilation
Some patients may breathe spontaneously, especially if they are not given paralytics. However, with good pain control, patients generally do not breathe more than 20 breathes per minute. That rate is distinct from the heart rate, allowing the frequencies to be different enough for separation.
Positive end-expiratory pressure
We expect positive end-expiratory pressure settings to affect pressure readings but not pressure frequency measurements, although this was not studied in this study. We expect most centers to use similar positive end-expiratory pressure settings as in our study, however.
RV pressure frequency characteristics
The main concern of epicardial access is RV puncture. We suspect that the pressure frequency of the RV would be 1 Hz (reflecting heart rate). However, we have no RV pressure data in this study because we did not place our sheath in the RV. However, animal studies have clearly documented that RV pressure is higher than pericardial pressure.15 Thus, we can envision using a combination of a “heart rate” pressure frequency of 1 Hz and a pressure of 10 mmHg as a sign that the needle has perforated RV. Although we did not measure RV pressures in this study, future animal studies may more clearly elucidate this approach.
Future studies
This study highlights important areas for future investigation. One potential area is measurement of pressure and pressure frequency at the needle tip during subxiphoid access from below the diaphragm, through the thorax, and into the pericardial space. In addition, pressure measurements during purposeful RV perforation in animal models appear warranted to further identifying noninvasive indicators of RV perforation during subxiphoid pericardial access.
Conclusion
In this study, we demonstrated a significant difference in pressure frequency between the pericardial space and the thorax. This result suggests that needle-tip pressure frequency measurement during access may add safety to subxiphoid access by helping an electrophysiologist identify the pericardial space before hitting the RV. This pressure frequency difference may allow safer access to the pericardial space.
Figure 3.
Pressure tracing from pericardial sac from same patient as in Figure 2.
Acknowledgments
This project was supported by NIH Award Number T32HL007849 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. This study was supported by a grant from the University of Virginia. Dr. Mahapatra, J. Tucker-Schwartz, Dr. Gillies, and Dr. Ailawadi may receive patent royalties related to epicardial access and ablation through their employment by, and relationship to, the University of Virginia.
ABBREVIATIONS
- ICE
intracardiac echocardiography
- LV
left ventricle
- RV
right ventricle
- VT
ventricular tachycardia
References
- 1.Friedman PA, Asirvatham S, Dalegrave C, et al. Percutaneous epicardial left atrial appendage closure: preliminary results of electrogram guided approach. J Cardiovasc Electrophysiol. 2009;20:908–915. doi: 10.1111/j.1540-8167.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- 2.Garcia FC, Bazman V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 3.Hsai TY, Bradley S, LaPage MJ, et al. Novel minimally invasive intrapericardial implantable cardiac defibrillator coil system: a useful approach to arrhythmia therapy in children. Ann Thorac Surg. 2009;87:1234–1238. doi: 10.1016/j.athoracsur.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Ota T, Degani A, Schwartzman D, et al. A highly articulated robotic surgical system for minimally invasive surgery. Ann Thorac Surg. 2009;87:1253–1256. doi: 10.1016/j.athoracsur.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ota T, Degani A, Zubiate B, et al. Epicardial atrial ablation using a novel articulated robotic medical probe via a percutaneous subxiphoid approach. Innovations Phil PA. 2009;1:335–340. doi: 10.1097/imi.0b013e31802f43b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scanavacca M, Pisani CF, Hachul D, et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 7.Schweikert RA, Saliba WI, Tomassoni G, et al. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation. 2003;108:1329–1335. doi: 10.1161/01.CIR.0000087407.53326.31. [DOI] [PubMed] [Google Scholar]
- 8.van Brakel TJ, Herman J, Accord RE, et al. Effects of intrapericardial sotalol and flecainide on transmural atrial electrophysiology and atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:207–215. doi: 10.1111/j.1540-8167.2008.01318.x. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H, Epstein A, Packer DL, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. J Am Coll Cardiol. 2000;35:1905–1914. doi: 10.1016/s0735-1097(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 10.Sacher F, Maury P, Nault I, et al. Prevalence of epicardial scar in patients referred for ventricular tachycardia ablation. Heart Rhythm. 2009;6(5S):S175. [Google Scholar]
- 11.Sosa E, Scanavacca M, d’Avilla A, et al. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998;9:229 –239. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 12.Sosa E, Scanavacca M, d’Avilla A. Transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia. Curr Cardiol Rep. 2001;3:451–458. doi: 10.1007/s11886-001-0066-1. [DOI] [PubMed] [Google Scholar]
- 13.Tedrow U, Stevenson W. Strategies for epicardial mapping and ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2009;20:710–713. doi: 10.1111/j.1540-8167.2008.01427.x. [DOI] [PubMed] [Google Scholar]
- 14.Sosa E, Scanavacca M, d’Avilla A, Plleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 15.Beverly C, Morgan W, Gunderthon G, Dillard D. Relationship of pericardial pressure to pleural pressure during quiet respiration and cardiac tamponade. Circ Res. 1965;16:493–498. doi: 10.1161/01.res.16.6.493. [DOI] [PubMed] [Google Scholar]
- 16.Tucker-Schwartz JM, Gillies G, Scanavacca M, Sosa E, Mahapatra S. Pressure frequency sensing subxiphoid access system for use in percutaneous cardiac electrophysiology: prototype design and pilot study results. IEEE Trans Biomed Eng. 2009;56:1160–1168. doi: 10.1109/TBME.2008.2009527. [DOI] [PubMed] [Google Scholar]