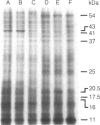
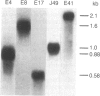
Abstract
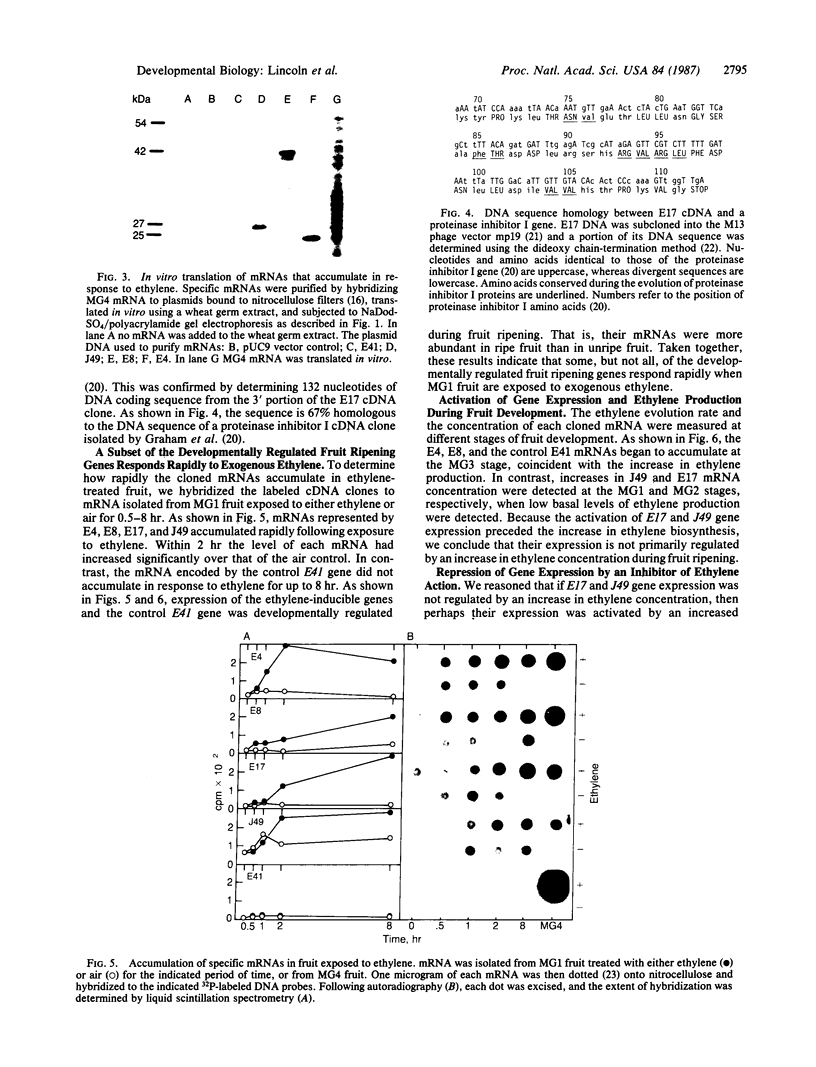
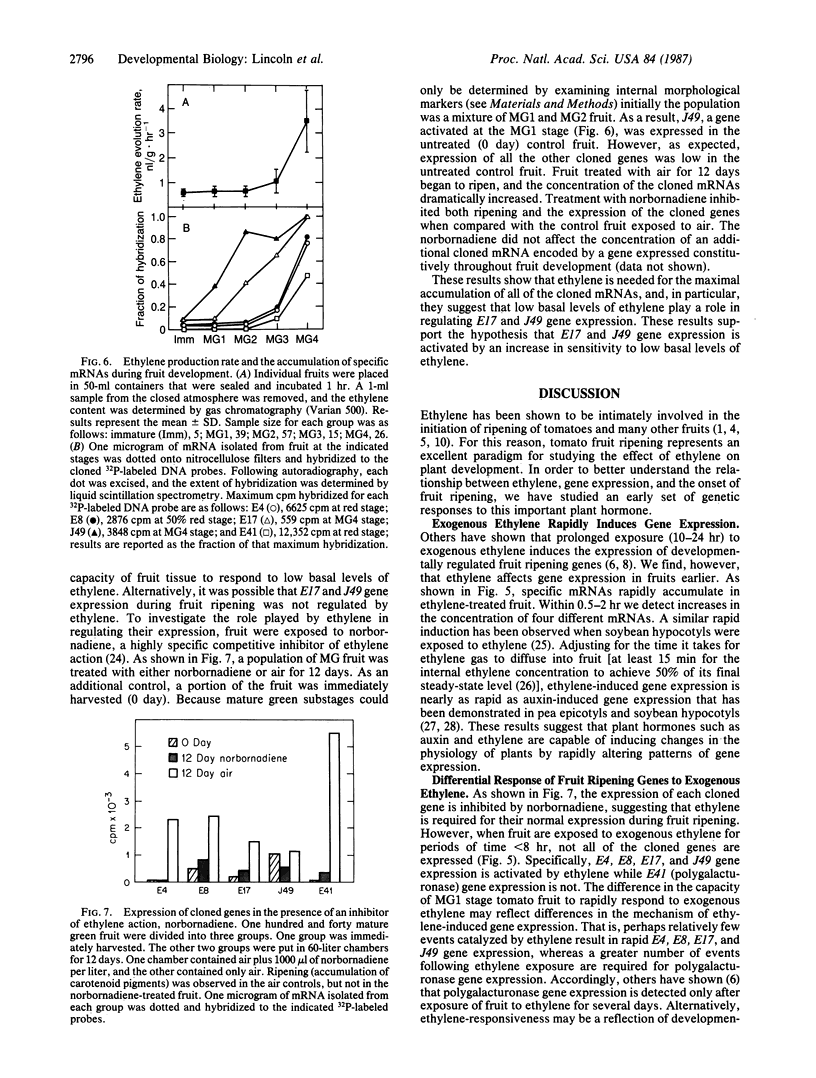
We have investigated the regulation of gene expression by the plant hormone ethylene by cloning mRNAs that accumulate in unripe tomato fruit (Lycopersicon esculentum) exposed to exogenous ethylene. The response to exogenous ethylene is rapid; within 30-120 min we detect an increase in the cloned mRNA concentrations. DNA sequence analysis indicates that one of the ethylene-inducible genes is related to a gene encoding wound-inducible proteinase inhibitor I. We have measured ethylene production during fruit development and detect low basal levels in unripe fruit and much higher levels in ripening fruit. Blot hybridization experiments show that expression of the cloned genes is developmentally regulated by ethylene during fruit ripening: the mRNAs produced by these genes are more abundant in ripe fruit than in unripe fruit, and this mRNA accumulation is repressed by a competitive inhibitor of ethylene action, norbornadiene. However, during fruit development some of the cloned mRNAs begin to accumulate when ethylene production is at a basal level, whereas other mRNAs begin to accumulate later when the endogenous ethylene concentration increases, suggesting that gene expression during fruit development can be activated by ethylene in two ways. In some cases gene expression is primarily activated by an increase in sensitivity to basal ethylene levels, whereas in other cases it may be regulated by an increase in ethylene concentration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. C., Yang S. F. A simple method for the determination of resistance to gas diffusion in plant organs. Plant Physiol. 1982 Jul;70(1):21–23. doi: 10.1104/pp.70.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Cohen D. I., Nielsen E. A., Steinmetz M., Paul W. E., Hood L. Cell-type-specific cDNA probes and the murine I region: the localization and orientation of Ad alpha. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2194–2198. doi: 10.1073/pnas.81.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Tam S. H., Ditta G. S., Breidenbach R. W. Abundance, diversity, and regulation of mRNA sequence sets in soybean embryogenesis. Dev Biol. 1981 Apr 30;83(2):201–217. doi: 10.1016/0012-1606(81)90467-x. [DOI] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of pre-inhibitor I and its post-translational processing. J Biol Chem. 1985 Jun 10;260(11):6555–6560. [PubMed] [Google Scholar]
- Grierson D., Tucker G. A., Keen J., Ray J., Bird C. R., Schuch W. Sequencing and identification of a cDNA clone for tomato polygalacturonase. Nucleic Acids Res. 1986 Nov 11;14(21):8595–8603. doi: 10.1093/nar/14.21.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Hoffman N. E., Yang S. F. Promotion by Ethylene of the Capability to Convert 1-Aminocyclopropane-1-carboxylic Acid to Ethylene in Preclimacteric Tomato and Cantaloupe Fruits. Plant Physiol. 1985 Feb;77(2):407–411. doi: 10.1104/pp.77.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Su L. Y., Yang S. F. Ethylene Promotes the Capability To Malonylate 1-Aminocyclopropane-1-carboxylic Acid and d-Amino Acids in Preclimacteric Tomato Fruits. Plant Physiol. 1985 Apr;77(4):891–895. doi: 10.1104/pp.77.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Van Ness J., Hahn W. E. Physical parameters affecting the rate and completion of RNA driven hybridization of DNA: new measurements relevant to quantitation based on kinetics. Nucleic Acids Res. 1982 Dec 20;10(24):8061–8077. doi: 10.1093/nar/10.24.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]