Abstract
We have previously isolated several cDNA clones of mRNAs that have the unusual property of being localized to either the animal pole or the vegetal pole of frog eggs. To gain insight into the function of these maternal mRNAs we have determined their DNA sequence and deduced the sequence of the proteins they encode. Here we report that An2, an mRNA localized to the animal pole of Xenopus oocytes and eggs, codes for the alpha chain of mitochondrial ATPase. Furthermore, we compare the intracellular localization of the An2 mRNA and mitochondria in oocytes and eggs and find that they do not have the same degree of localization. In the light of these results we discuss possible reasons for the maternal localization of the An2 mRNA.
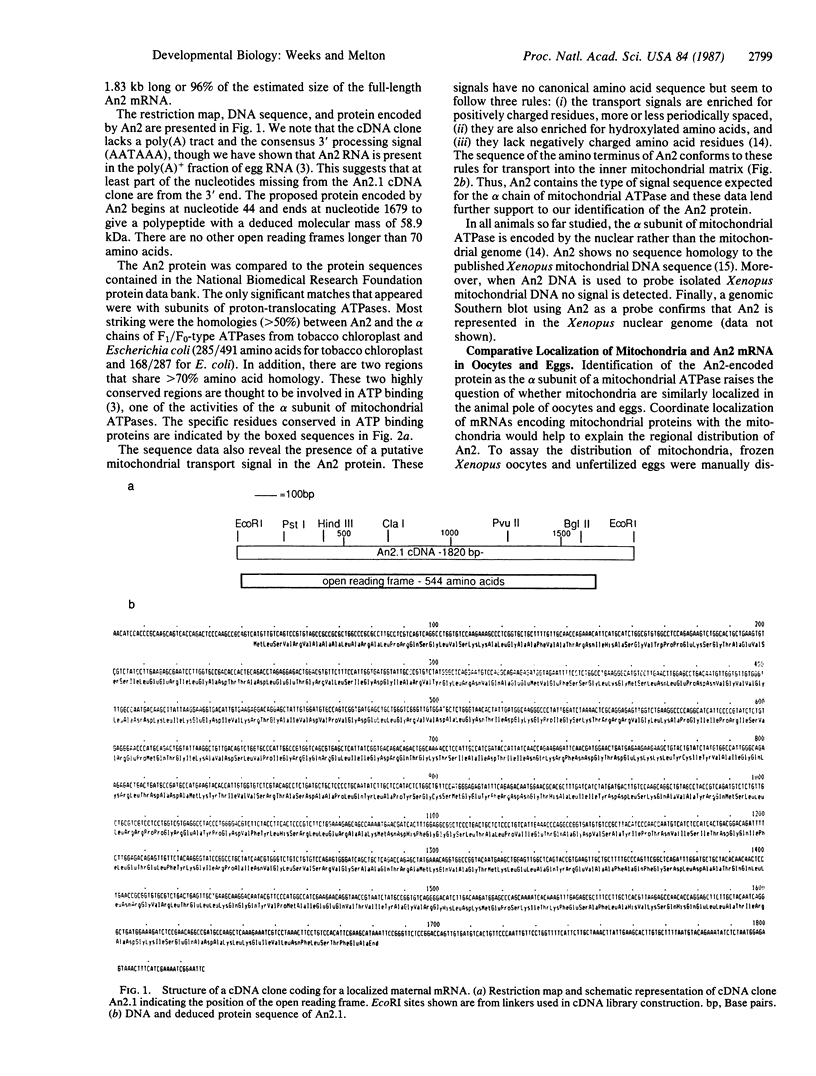
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase J. W., Dawid I. B. Biogenesis of mitochondria during Xenopus laevis development. Dev Biol. 1972 Apr;27(4):504–518. doi: 10.1016/0012-1606(72)90189-3. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Gall J. G. Giant readthrough transcription units at the histone loci on lampbrush chromosomes of the newt Notophthalmus. Chromosoma. 1985;92(4):243–253. doi: 10.1007/BF00329807. [DOI] [PubMed] [Google Scholar]
- Heasman J., Quarmby J., Wylie C. C. The mitochondrial cloud of Xenopus oocytes: the source of germinal granule material. Dev Biol. 1984 Oct;105(2):458–469. doi: 10.1016/0012-1606(84)90303-8. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Mahon K. A., Gavis E. R., Gall J. G. Histone RNA in amphibian oocytes visualized by in situ hybridization to methacrylate-embedded tissue sections. EMBO J. 1984 Sep;3(9):1939–1943. doi: 10.1002/j.1460-2075.1984.tb02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Developmental regulation of a gastrula-specific gene injected into fertilized Xenopus eggs. EMBO J. 1985 Dec 16;4(13A):3463–3471. doi: 10.1002/j.1460-2075.1985.tb04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Cortese R. Transcription of cloned tRNA genes and the nuclear partitioning of a tRNA precursor. Cell. 1979 Dec;18(4):1165–1172. doi: 10.1016/0092-8674(79)90229-0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moen T. L., Namenwirth M. The distribution of soluble proteins along the animal-vegetal axis of frog eggs. Dev Biol. 1977 Jul 1;58(1):1–10. doi: 10.1016/0012-1606(77)90070-7. [DOI] [PubMed] [Google Scholar]
- Nelson L., Lorentzon R., Boquist L., Løvtrup S. Morphological differentiation of mitochondria in the early amphibian embryo. Exp Cell Res. 1982 Jan;137(1):25–29. doi: 10.1016/0014-4827(82)90003-9. [DOI] [PubMed] [Google Scholar]
- Phillips C. R. The regional distribution of poly (A) and total RNA concentrations during early Xenopus development. J Exp Zool. 1982 Nov 1;223(3):265–275. doi: 10.1002/jez.1402230308. [DOI] [PubMed] [Google Scholar]
- Rastl E., Dawid I. B. Expression of the mitochondrial genome in Xenopus laevis: a map of transcripts. Cell. 1979 Oct;18(2):501–510. doi: 10.1016/0092-8674(79)90067-9. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough G. A. A chemically explicit model for the molecular mechanism of the F1F0 H+-ATPase/ATP synthases. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3688–3692. doi: 10.1073/pnas.83.11.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showman R. M., Wells D. E., Anstrom J., Hursh D. A., Raff R. A. Message-specific sequestration of maternal histone mRNA in the sea urchin egg. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5944–5947. doi: 10.1073/pnas.79.19.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A. C., Smith L. D. Accumulation of mitochondrial DNA during oogenesis in Xenopus laevis. Dev Biol. 1977 Mar;56(1):219–225. doi: 10.1016/0012-1606(77)90166-x. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Rebagliati M. R., Harvey R. P., Melton D. A. Localized maternal mRNAs in Xenopus laevis eggs. Cold Spring Harb Symp Quant Biol. 1985;50:21–30. doi: 10.1101/sqb.1985.050.01.005. [DOI] [PubMed] [Google Scholar]







