Abstract
Gliomas are tumors of glial origin formed in the central nervous system and exhibit profound morphological and genetic heterogeneity. The etiology of this heterogeneity involves an interaction between genetic alterations and environmental risk factors. Scientific evidence suggests that certain natural dietary components, such as phytoestrogens, flavonoids, polyunsaturated fatty acids and vitamins may exert a protective effect against gliomas by changing the nature of the interaction between genetics and environment. Similarly, certain anti-inflammatory drugs and dietary modifications, such as methionine restriction and the adoption of low-calorie or ketogenic diets, may take advantage of glioma and normal glial cells’ differential requirements for glucose, methionine, and ketone bodies and may therefore be effective as part of preventive or treatment strategies for gliomas. Treatment trials of glioma patients and chemoprevention trials of individuals with a known genetic predisposition to glioma using the most promising of these agents, such as the anti-inflammatory drugs curcumin and gamma-linolenic acid, are needed to validate or refute these agents’ putative role in gliomas.
Keywords: glioma, glioblastoma, prevention, diet, vitamins
INTRODUCTION
The etiology of human gliomas implicates a complex combination of known and unknown environmental risk factors and cancer-predisposing constitutive genetic alterations that ultimately result in abnormal proliferation and malignant transformation of glial cells (1). Previous exposure to high-dose ionizing radiation is the only proven environmental risk factor for brain tumors (2). There is also experimental evidence that genetically determined increased sensitivity to radiation is an independent risk factor for gliomas (3). The incidence of malignant gliomas could be potentially reduced by either preventing gliomas from forming or preventing low-grade gliomas from developing into high-grade gliomas (4). The hypothesis that genetically determined sensitivity to other environmental carcinogens may contribute to the pathogenesis of these tumors, while logical in its construct, is not yet strongly supported by scientific evidence.
What are the plausible environmental factors that might, under appropriate circumstances, modify the risk of acquiring a glioma or of having a glioma progress from low grade to high grade? Previous studies have indicated that vitamin supplements provide a protective effect against gliomas, with increased protection resulting from increasing frequency of use (5). Dietary factors (6, 7), especially those that add to the total body burden of oxidants (8), may be implicated in the development of cancers such as gliomas. It has been suggested that a glioma’s degree of aggressiveness can be modulated by dietary interventions and that some phytochemicals with antioxidant properties participate in that process (9). In addition, as with other cancers, inflammatory or infective processes may contribute to the development and possible aggressiveness of gliomas (10–12). Thus, it is possible that medications and natural compounds found in spices and herbs that have anti-inflammatory properties may likewise mitigate glioma malignancy. In this review, we profile the most important natural dietary constituents and anti-inflammatorydrugs that have been proposed to either reduce or increase the risk of glioma development and progression and discuss how modification of their intake may affect the prevention or treatment of gliomas (Table 1).
Table 1.
Natural dietary components or derivatives and anti-inflammatory drugs with potential benefit in prevention and treatment of gliomas
| Dietary component or derivative | Foods in which it is found | Mode of action | Potential prevention | Potential treatment | Human studies |
|---|---|---|---|---|---|
| Pro-vitamin A (α-carotene, β- carotene) | Carrots, spinach | Differentiation | Yes | Unknown | Case-control study (75) |
| Retinoic acid and fenretinide | Oxidized form of vitamin A | Differentiation (19), Modification of EGF-receptor (15), apoptosis (19, 22, 23) | Unknown | Yes | Phase 2 trial (104) Case report (105) |
| Vitamin D analogues and metabolites | Fish, mushrooms, milk, cereal | Differentiation (27), apoptosis (30), DNA methylation (33) | Unknown | Yes | Phase 2 trial (34) |
| Vitamin E derivatives | Sunflower seeds, almonds, olives, papaya | Cytostatic effect (35) | Yes | Unknown | No |
| Ascorbyl stearate (lipophilic derivative of vitamin C) | Additive in various foods | Apoptosis (25) | Unknown | Unknown | No |
| Conjugated linoleic acid | Meat, dairy products | Apoptosis (36, 37, 40) | Yes | Yes | Review of 3 open-label clinical studies (39, 106, 107) |
| gamma-linolenic acid | Borage oil | ||||
| Calcium | Dairy products, cabbage, broccoli | Apoptosis, DNA repair | Yes | Unknown | Case-control study (41) |
| Nitrosamine | Cured meats (bacon, ham, pork) | Known carcinogen | Yes, with lower intake | No | Case-control studies (63, 64, 66, 67) |
| Flavonoids: quercetin | Oranges, lemons, grapefruits, onions | Decrease proliferation and viability of glioma cells; protect normal cells (71) | Yes | Unknown | No |
| epicatechin | Dark chocolate | ||||
| Methionine restriction | Sesame seeds, Brazil nuts, meat, fish | Higher requirement for methionine by glioma (78, 108) Decrease in MGMT activity (80) | Unknown | Yes, in combinati on with chemother apy (57, 59) | Phase 1 trial (79) |
| Isothiocyanate iberin | Cabbage, broccoli, cauliflower | Apoptosis | Yes | Unknown | No |
| NSAIDs: indomethacin, aspirin, acetaminophen, sulindac | Upregulation of 15-PGDH and p21 Downregulation of MMP-2, MMP-9 (51) (54) and laminin (55) Inhibition of the MRP (56) | Yes | Suggested | Only epidemiologic studies (11) | |
| Curcumin (diferuloylmethane) | Spice turmeric | Anti- inflammatory action (61) (regulation of transcription factors (109–111), cytokines, redox status (112), enzymes (88, 113)) Apoptosis (84–86, 114–117) Autophagy (84, 118) Anti-invasion (87, 88) Cell cycle arrest (84, 89) | Yes | Unknown | Yes in other cancers (90–97), but not in gliomas |
| Ketone bodies | Ketogenic diets | Differential requirement for energy of glioma and normal glial cells | Unknown | Yes (98–100, 102) | Case report (101) |
| Phytoestrogens (genistein, daidzein, biochanin A, formnonetin, matairesinol, secoisolariciresinol, coumestrol) | Soy, cereal, legumes | Antioxidant | Yes | Suggested (103) | Case-control study (7) |
15-PGDH: 15-hydroxyprostaglandin dehydrogenase; MRP: multidrug resistance-associated protein; EGF: epidermal growth factor; MMP: matrix metalloproteinase
VITAMINS
Vitamins A, C, D, and E and their derivatives may have anti-cancer properties and may modulate gliomagenesis. In this section, we specifically discuss the in vitro and in vivo evidence pointing to these effects.
Retinoids
Retinoids are chemical compounds related to vitamin A. They strongly inhibit the proliferation and migration of cells in primary cultures of human glioblastoma multiforme but not in established cell lines, probably because the two types of cells have different retinoid receptor expression patterns (13). Retinoic acid (RA) up-regulated RA receptor (RAR)-alpha and RAR-beta mRNAs, but only RAR-beta proteins, in glioma cell lines, suggesting that RA conferred its effects at a post-transcriptional level (14). The RA-induced growth inhibition in glioma cells may arise, at least in part, through alterations in the epidermal growth factor (EGF) receptor and its structure (15). Clinical phase 2 trials of isotretinoin (13-cis-retinoic acid) have shown that RA has activity against glioblastoma (16, 17). However, results with all-trans retinoic acid (ATRA) were not as successful in clinical phase 2 trials (18). Nonetheless, a combination of ATRA and interferon-gamma could control the growth of both PTEN-proficient and PTEN-deficient glioblastoma cells by arresting cell division and inducing differentiation and apoptosis (19).
In addition, glioblastoma cell lines and fresh glioblastoma tissue samples, but not normal human glial tissue, expressed high levels of peroxisome proliferator-activated receptor-gamma (PPARγ). In addition, the PPARγ ligand pioglitazone, induced apoptosis and inhibited proliferation of glioblastoma cells; these effects were associated with downregulation of bcl-2 and upregulation of bax proteins (20). An enhanced effect was observed when pioglitazone and ATRA were combined, suggesting that these ligands may possess an additive therapeutic effect for patients with glioblastoma (20).
The synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR or fenretinide) is in clinical trials for the treatment of several malignancies. It was shown that fenretinide induces apoptotic cell death in human glioma and medulloblastoma cells in vitro, in part by activating caspase-3-dependent cell death (21); this effect could be partially blocked by the antioxidant l-ascorbic acid, suggesting that free-radical intermediates might be involved in fenretinide’s effects (22). Similar results were reported for ATRA (23). Unfortunately, fenretinide was not active in a phase 2 study of patients with recurrent high-grade gliomas (24).
Vitamin C
In one study, ascorbyl stearate, a lipophilic derivative of ascorbic acid (or vitamin C), had antiproliferative and apoptotic effects on T98G glioma cells, probably through modulation of IGF-IR expression and consequent facilitation of programmed cell death (25). Further studies, both in vitro and in vivo are needed to further examine if vitamin C has any role in glioma prevention or treatment.
Vitamin D
Vitamin D receptor has important effects not only on physiological processes related to Ca2+ metabolism but also on cell growth and differentiation. Vitamin D receptor mRNA levels have been reported to be significantly higher in glioblastomas than in both low-grade and anaplastic astrocytomas (26), and there is in vitro evidence that vitamin D metabolites alone or in combination with retinoids may be potentially useful agents in the differentiation therapy of human malignant gliomas (27). The effects of vitamin D metabolites on brain tumor cells may be at least partially independent from the activation of the classic nuclear receptor pathway and are possibly mediated through the sphingomyelin pathway (28).
The secosteroid 1,25-dihydroxyvitamin D3 1,25(OH)2D3 is the major biologically active metabolite of vitamin D. Evidence suggests that 1,25(OH)2D3 has a cytotoxic effect on rat and human glioma cells (29). In vitro studies of the effect of several vitamin D3 analogues on glioma cell growth have suggested that 1,25(OH)2D3 analogues such as KH 1060, EB 1089, or CB 1093, alone or in combination with other therapeutic approaches, could have the potential to treat brain glial tumors. The vitamin D3 effect seems to be mediated by apoptosis (30). In vitro evaluation of the apoptotic potential of a representative set of vitamin D analogues, each of them in the 3alpha and 3beta conformations, and of natural vitamin D metabolites in the rat C6 glioma cell line, demonstrated that the 3alpha epimers showed equivalent or even higher activity than their respective 3beta forms, suggesting that these 3alpha epimers would have advantages over the 3beta epimers (31). However, further studies in the rat glioma cell line C6.9 showed that noradrenaline and the beta-adrenoceptor agonist isoproterenol inhibited 1,25(OH)2D3-induced apoptosis and that the beta-adrenoceptor antagonist propanolol reversed this inhibition. These findings suggest that the efficiency of antiproliferative vitamin D-related therapies could be influenced by endogenous levels of noradrenaline (32). Furthermore, changes in the DNA methylation pattern could suppress 1,25(OH)2D3-mediated apoptosis via expression of hypermethylated genes, such as proto- oncogenes, with death-repressor activity (33).
Alfacalcidol, a vitamin D analogue able to bind to nuclear receptors regulating mitotic activity, was tested in 11 patients in a phase 2 trial involving surgery or biopsy, radiotherapy, chemotherapy with teniposide-lomustine or fotemustine, and alfacalcidol at a daily dose of 0.04 mcg/kg for the treatment of malignant gliomas. Three of the 11 patients (27%), 2 with glioblastomas and 1 with a grade III astrocytoma, exhibited a response, consisting of progressive regression of the lesion on radiographic imaging, with a decrease of the gadolinium-enhanced area, and complete clinical remission, observed for 7, 5 and 4 years, respectively. These results suggest that alfacalcidol may be able to induce in some patients with malignant gliomas durable remissions when combined with classical surgery-radiotherapy-chemotherapy regimens (34).
Vitamin E
Studies on the effects of several tocopherols (the most abundant/common of which is vitamin E) on the proliferation and death of murine glioma C6 cells demonstrated that gamma-tocopherol was an effective inhibitor of cell cycle progression, leading to lowered expression of cyclin E and cyclin-dependent kinases 2 and 4 and overexpression of p27. This cytostatic effect suggests a possible chemopreventive role for vitamin E in gliomas (35). No in vivo studies or clinical trials have expanded on this premise, however.
FATTY ACIDS
Both n-6 and n-3 polyunsaturated fatty acids are dietary fats important for cell function and possibly supportive of tumorigenesis. One study found that levels of the polyunsaturated fatty acid docosahexaenoic acid were significantly reduced and of the n-6 polyunsaturated fatty acid linoleic acid were significantly increased in glioma tissue compared to control samples, indicating that the fatty acid composition of human gliomas differs from that of nonmalignant brain tissue (36). In addition, conjugated linoleic acid, found in ruminant meat (beef and lamb) and dairy products, was shown to exert antineoplastic activity in vitro (37). Further in vitro studies showed that gamma-linolenic acid (GLA), a major ingredient in borage oil, induced apoptosis of tumor cells without harming normal cells by increasing levels of free radicals and lipid peroxides, decreasing the expression of the oncogenes Ras and Bcl-2, and enhancing the activity of p53 (38). Unexpectedly, the anti-oxidant vitamin E blocked the tumoricidal action of GLA. In three open-label clinical studies, intra-tumoral injection of GLA significantly reduced the size and number of glioma lesions without causing any significant side effects (39). Furthermore, GLA enhanced the radiosensitivity of astrocytoma tumor cells but not normal astrocytes in vivo (40), suggesting that it may be suitable to consider using GLA for adjunctive therapy of gliomas and/or to prevent glioma recurrence in high-risk patients whose cancer is in remission. To date, however, neither use of GLA has been tested.
CALCIUM, COPPER, AND ZINC
Calcium may exert a protective effect against gliomas through induction of apoptosis, DNA repair, or other mechanisms. A comparison of the consumption of calcium and other dairy components and dairy foods (cholesterol, fat, protein, calories, milk, and cheese) between 337 astrocytic glioma case patients and 450 healthy controls in the San Francisco Bay Area Adult Glioma Study, 1991–1995, showed a statistically significant inverse association between dietary calcium intake and glioma incidence for women but not men. Since increased levels of circulating estradiol in women stimulate intestinal absorption of calcium, this may account for the lower incidence of gliomas in women consuming greater amounts of calcium in foods and supplements (41).
Penicillamine is an oral agent used to treat intracerebral copper overload in Wilson’s disease. The copper ion, a co-factor of angiogenesis, is sequestered in human brain tumors and the adjacent normal brain tissue and may be involved in glioma invasion. Animal experiments indicated that copper depletion inhibited the infiltrative spread of the normally invasive 9L gliosarcoma (42–44). However, a clinical study in 40 patients with newly diagnosed glioblastoma multiforme who received a low-copper diet and escalating doses of penicillamine along with radiation therapy failed to demonstrate that a reduction of serum copper levels improved survival rates (45).
Zinc is a trace metal important to the function of many enzymes in the body, some of which have a role in DNA and RNA synthesis (46–48). As a result, body levels of zinc might be expected to have a role on glioma and other cancer cells. In one population-based case-control study of 637 patients diagnosed with a glioma or meningioma and 876 controls in the UK, zinc intake was found to have no significant effect on the risk of glioma or meningioma (49).
ANTI-INFLAMMATORY DRUGS
Nonsteroidal anti-inflammatory drugs (NSAIDs) can suppress the growth of various malignancies by inhibiting cyclooxygenase-2 (COX-2) enzyme activity. In vitro studies have revealed that aspirin strongly inhibits the growth of rat glioma (RG 2) cells in concentrations used in medicine for rheumatic diseases (50). NSAIDs may also inhibit the growth of glioblastoma multiforme cells through COX-2-independent mechanisms, such as up-regulation of the key prostaglandin catabolic enzyme 15-hydroxyprostaglandin dehydrogenase (15-PGDH) and the cell cycle inhibitor p21 (51). NSAIDs such as indomethacin, acetaminophen, and sulindac sulfide inhibited C6 rat glioma cell proliferation (52). Moreover, the effect was more potent when indomethacin-loaded nanocapsules and indomethacin ethyl ester-loaded nanocapsules were used (53). In addition, indomethacin inhibited the invasion of gliomas by downregulating the expression of matrix metalloproteinase (MMP)-2, MMP-9 (54), and laminin (55). Furthermore, indomethacin enhanced the cytotoxic effects of doxorubicin and vincristine in T98G human malignant glioma cells; this effect was associated with inhibition of the multidrug resistance-associated protein (MRP) (56). Epidemiologic studies have reported that the regular use of NSAIDs is associated with a 33% reduction in the risk of glioma, suggesting possible antitumor activity (11).
While one cannot always draw inferences about one cancer from observations in another, in light of NSAIDs’ effects on glioma cells, it is worth considering the impressive reported effects of sulindac and alpha-difluoromethylorinithine (DFMO) in the treatment of malignant colon polyps (57). In this randomized trial, the combination of sulindac and DFMO reduced the number of malignant colon polyps formed by 92%? Or please explain nature of the improvement] in a high-risk population. Since DFMO has shown activity against gliomas as a single agent and in combination with cytotoxic drugs (58, 59), it is not unreasonable to expect that DFMO and sulindac might help to maintain remissions in glioma patients. On the other hand, since DFMO does not cross the intact blood-brain barrier well, its use will require that gliomas treated have permeability greater than normal brain (60–62).
DIET AND LIFE STYLE FACTORS INCREASING RISK
In addition to the dietary factors discussed earlier, some effort has been made to understand other dietary factors and the total body burden of oxidants (6, 7) on the development of brain tumors. A population-based study of 94 women with intracranial gliomas and 94 individually matched controls in Los Angeles County found that dietary sources of nitroso compounds, such as cured meats like bacon, may be important in the development of gliomas (63). Similarly, another population-based case-control study in southwest Germany in 1987–88 involving 115 glioma patients and 418 randomly selected controls found that a higher than average intake of processed meat, especially cooked ham, processed pork, and fried bacon, was significantly associated with an increased risk of glioma (64). Although tobacco products are major contributors of exogenous N-nitroso compounds, there is consistent evidence that cigarette smoking is not associated with an appreciably elevated risk of adult glioma (65).
In a small prospective study to determine the relationship between processed meat consumption and risk of primary malignant brain tumors there was a suggestion of a dose-response trend, with increasing consumption of processed meat leading to increased risk in men. Specifically, consuming three or four servings per week of processed meat was associated with increased glioma risk compared to no or low consumption for men but not for women (66). Another population-based case-control study of 416 adults with gliomas and 409 age- and sex-matched controls conducted in Melbourne, Australia, between 1987 and 1991 reported an increased odds ratio (OR) for glioma in males who consumed high levels of bacon, corned meats, apples, melons, and oil and decreased ORs in men consuming cabbage and cola drinks and women consuming whole-grain bread, pasta, corned meat, bananas, cauliflower, broccoli, cola drinks and nuts. Elevated ORs for glioma in men, but not women, were associated with the intake of N-nitroso dimethylamine, retinol, and vitamin E (67). A hospital-based case-control study in northeast China showed that consumption of fresh vegetables such as cabbage and onions, fruits, fresh fish, and poultry was inversely related to the risk of developing brain cancer (68). While vitamin E and calcium exerted a protective effect, the study reported, the consumption of salted vegetables and salted fish increased risk (68).
A population-based case-control study of adults with glioma in Nebraska evaluated the effects of exposure to nitrates in utility-supplied drinking water over a 20-year period and found that the nitrate levels present did not significantly increase glioma risk (69). A meta-analysis of nine epidemiological studies also showed that the available data do not provide clear support for the suspected causal association between ingestion of N-nitroso compounds from cured meat in adults and subsequent brain tumor risk, suggesting that uncontrolled confounding may have accounted for the previously noted positive association seen in some epidemiological studies (70). Thus, there is no conclusive evidence that consumption of N-nitroso compounds from cured meats increases the risk of glioma development.
DIET AND LIFESTYLE FACTORS MITIGATING RISK
Epidemiological studies have suggested that dietary flavonoids found in oranges, tangerines, lemons, and grapefruit--in particular, quercetin--may play a beneficial role by preventing or inhibiting tumorigenesis. In vitro studies showed that quercetin decreased the proliferation and viability of U138MG human glioma cells while exerting a cytoprotective effect in normal cell cultures (71). In addition, grapefruit is very high in levels of exogenous polyamines that are important polycations required for DNA and RNA function (72–74).
Studies examining fruit and vegetable consumption and risk of glioma have reported inconsistent results. The incidence of glioma was inversely related to the intake of dark yellow vegetables and beans, but no association was seen between glioma risk and dietary sources of nitrosamines or high-nitrate vegetables (75). Overall, there was a significant inverse association between the risk of adult glioma and dietary intake of pro-vitamin A carotenoids, alpha-carotene, beta-carotene, and fiber from beans. However, there was no significant association between risk of adult glioma and intake of nitrate, nitrite, vitamin C, vitamin E, saturated fat, cholesterol, dietary fiber from grain products, or fiber from fruit and vegetables (75). A prospective study that examined the relationship between consumption of fruit and carotenoids and the risk of glioma among men and women in 3 large US cohort studies--the Health Professionals Follow-Up Study and the Nurses’ Health Studies I and II--found that fruit, vegetable, and carotenoid consumption is not likely associated strongly with the risk of adult glioma (76).
In rodent models of glioma, methionine restriction in combination with chloroethylnitrosoureas resulted in lengthened survival. Chronic depletion of plasma methionine in experimental animals could result in regression of high-grade gliomas, probablybecause these tumors require high amounts of methionine to maintain a state of active proliferation (77). Similar results were obtained by other investigators: methionine deprivation and methionine analogues inhibited cell proliferation and growth of human xenografted gliomas (78). A phase 1 clinical trial of dietary methionine restriction in association with chloroethylnitrosourea (cystemustine) treatment for patients with recurrent glioma or metastatic melanoma demonstrated the feasibility and tolerability of the association of a methionine-free diet and cystemustine treatment, and a phase 2 clinical trial has been initiated to test the activity of this regimen (79). Methionine depletion resulted in a decrease of O6- methylguanine-DNA methyltransferase (MGMT) in the peripheral blood mononuclear cells of patients treated with cystemustine, suggesting that methionine-induced regulation of MGMT activity may serve as a mechanism for an anti-glioma effect (80). A later report indicated that methionine depletion had the potential to enhance the antitumor effects of chemotherapeutic agents in chemotherapy-resistant tumors such as gliomas (81).
The diet-derived isothiocyanate iberin, a bioactive agent in Brassicaceae species, inhibited growth of glioma cells in proliferation assays, enhanced cytotoxicity, and induced apoptosis by activation of caspase-3 and caspase-9, indicating potential usefulness in the prevention of brain tumors (82).
Curcumin (diferuloylmethane), is found in the spice turmeric and has been known for its anti-inflammatory activity for hundreds of years. Extensive research has indicated that curcumin can regulate numerous transcription factors, cytokines, protein kinases, adhesion molecules, reactive oxygen species, and enzymes that have been linked to inflammation (83). Since the process of inflammation may play a role in neoplastic disease, curcumin it may have a role in the prevention of proinflammatory states that precede cancer. Curcumin has been shown to have direct effects on glioma cells by affecting apoptosis (84–86), the MMPs and invasion (87, 88), and cell cycle arrest (84, 89). While curcumin has limited use in gliomas, it has generated much interest in prevention trials for several other cancers (90–97).
OXIDATIVE STRESS
In contrast to brain tumor cells, which lack metabolic flexibility and are largely dependent on glucose for growth and survival, normal brain cells can metabolize both glucose and ketone bodies for energyModerate dietary restriction of total food and caloric intake without causing nutritional deficiencies may shift the tumor microenvironment from proangiogenic to antiangiogenic (98). In addition, the effect of restricting dietary caloric intake by 40% in experimental animals resulted in decreased vascularity(as determined by testing for factor VIII) and increased apoptosis (as determined by terminal deoxynucleotidyl transferase-mediated nick end labeling) in all tumors (99). Further studies by the same group of investigators indicated that caloric restriction may activate AMP-activated protein kinase (AMPK), a known physiological cellular energy sensor, which becomes phosphorylated in response to changes in cellular ATP levels. Thus, energy stress induced by glucose withdrawal caused more ATP depletion, AMPK phosphorylation, and apoptosis in glioma cells than in normal astrocytes. These results suggest that use of an AMPK activator in combination with a glycolysis inhibitor may be an effective therapy for treating malignant astrocytoma (100).
Studies of two children with gliomas who were placed on a ketogenic diet showed that blood glucose levels declined to low-normal levels, blood ketones were elevated 20- to 30-fold, and there was a mean 22% decrease in glucose uptake at the tumor site, as shown on PET. One patient exhibited significant clinical improvements in mood and new skill development and stabilization of her disease for 12 months (101). A study demonstrated that KetoCal, a nutritionally balanced high-fat/low-carbohydrate ketogenic diet, has antitumor and antiangiogenic effects in experimental mouse tumors (99). The therapeutic effect of KetoCal for brain cancer management was due largely to the reduction of total caloric content, which reduced the circulating glucose required for rapid tumor growth. This preclinical study thus indicates that restricted KetoCal is a safe and effective diet therapy, but a large randomized study showing benefit in malignant brain cancer is needed before it can be considered an alternative therapeutic option (102).
Studies to date suggest that a starvation-based differential stress resistance strategy has the potential to maximize chemotherapy’s differential toxicity to normal and cancer cells and enhance its efficacy against gliomas (103). The antioxidant and weak estrogenic properties of dietary phytoestrogens may attenuate oxidative stress, which mayplay a role in adult glioma formation. A study that evaluated the long-term consumption of dietary antioxidants and phytoestrogens, such as genistein, daidzein, biochanin A, formononetin, matairesinol, secoisolariciresinol and coumestrol, yielded data supporting an inverse association between glioma occurrence and a higher dietary antioxidant index and a higher intake of certain phytoestrogens, especially daidzein (7).
CLOSING REMARKS
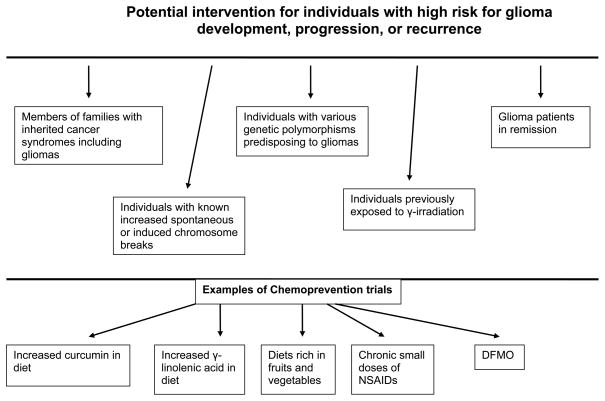
In this paper we highlight potential research areas of various dietary and nutraceutical approaches that might prevent gliomas or reduce their rate of transformation to a higher grade (figure 1). Certain natural dietary components - such as phytoestrogens found in soy and flavonoids found in oranges, lemons, grapefruits, and dark yellow vegetables--may exert a protective effect against gliomas. Similar effects may result from pro-vitamin A carotenoids and beans. Methionine restriction may modulate MGMT activity and polyamine activity and may be useful for the therapy of gliomas when combined with chemotherapy. Since glioma cells depend mainly on glucose for survival, in contrast to normal glial cells, which can also utilize ketone bodies, ketogenic diets and overall calorie restriction may be an adjunct to other treatment modalities for gliomas. Anti-inflammatory drugs, curcumin, retinoids, and certain polyunsaturated fatty acids such as GLA (found in borage oil) may exert antineoplastic activity against gliomas without harming normal cells. Certain vitamin D3 analogues and calcium may be proven either therapeutic or preventive agents for gliomas. These agents could be tested in individuals or families with a genetic predisposition to glioma and patients with gliomas as adjuncts to standard therapies (figure 1).
Figure 1.
Potential chemoprevention for gliomas. Inherited cancer syndromes with gliomas include the Li-Fraumeni syndrome (119), melanoma-astrocytoma syndrome (120, 121), neurofibromatosis 1 (122) and 2 (123), Turcot’s syndrome (124, 125) and BRCA syndrome (126). The various studies describing the individuals with increased chromosome breaks and genetic polymorphisms predisposing to gliomas have been recently reviewed (1). NSAIDs: non steroidal anti-inflammatory drugs; DFMO: alpha-difluoromethylorinithine.
References
- 1.Kyritsis AP, Bondy ML, Rao JS, Sioka C. Inherited predisposition to glioma. Neuro Oncol. 2009 doi: 10.1093/neuonc/nop011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin VA, Leibel SA, Gutin PH. Neoplasms of the central nervous system. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Lippincott-Raven; Philadelphia: 2001. pp. 2100–2160. [Google Scholar]
- 3.Bondy ML, Kyritsis AP, Gu J, et al. Mutagen sensitivity and risk of gliomas: a case-control analysis. Cancer Res. 1996;56:1484–1486. [PubMed] [Google Scholar]
- 4.Levin VA. Are gliomas preventable? Recent Results. Cancer Res. 2007;174:205–215. doi: 10.1007/978-3-540-37696-5_18. [DOI] [PubMed] [Google Scholar]
- 5.Preston-Martin S, Mack W. Gliomas and meningiomas in men in Los Angeles County: investigation of exposures to N-nitroso compounds. IARC Sci.Publ; 1991. pp. 197–203. [PubMed] [Google Scholar]
- 6.Kaplan S, Novikov I, Modan B. Nutritional factors in the etiology of brain tumors: potential role of nitrosamines, fat, and cholesterol. Am J Epidemiol. 1997;146:832–841. doi: 10.1093/oxfordjournals.aje.a009201. [DOI] [PubMed] [Google Scholar]
- 7.Tedeschi-Blok N, Lee M, Sison JD, Miike R, Wrensch M. Inverse association of antioxidant and phytoestrogen nutrient intake with adult glioma in the San Francisco Bay Area: a case-control study. BMC Cancer. 2006;6:148. doi: 10.1186/1471-2407-6-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA) Cancer Causes Control. 1997;8:13–24. doi: 10.1023/a:1018470802969. [DOI] [PubMed] [Google Scholar]
- 9.Pouliquen D, Olivier C, Hervouet E, et al. Dietary prevention of malignant glioma aggressiveness, implications in oxidant stress and apoptosis. Int J Cancer. 2008;123:288–295. doi: 10.1002/ijc.23513. [DOI] [PubMed] [Google Scholar]
- 10.Scheurer ME, Amirian E, Cao Y, et al. Polymorphisms in the interleukin-4 receptor gene are associated with better survival in patients with glioblastoma. Clin Cancer Res. 2008;14:6640–6646. doi: 10.1158/1078-0432.CCR-07-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheurer ME, El-Zein R, Thompson PA, et al. Long-term anti-inflammatory and antihistamine medication use and adult glioma risk. Cancer Epidemiol Biomarkers Prev. 2008;17:1277–1281. doi: 10.1158/1055-9965.EPI-07-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouterfa H, Picht T, Kess D, et al. Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery. 2000;46:419–430. doi: 10.1097/00006123-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Carpentier AF, Leonard N, Lacombe J, et al. Retinoic acid modulates RAR alpha and RAR beta receptors in human glioma cell lines. Anticancer Res. 1999;19:3189–3192. [PubMed] [Google Scholar]
- 15.Steck PA, Hadi A, Lotan R, Yung WK. Inhibition of epidermal growth factor receptor activity by retinoic acid in glioma cells. J Cell Biochem. 1990;42:83–94. doi: 10.1002/jcb.240420204. [DOI] [PubMed] [Google Scholar]
- 16.Yung WK, Kyritsis AP, Gleason MJ, Levin VA. Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid. Clin Cancer Res. 1996;2:1931–1935. [PubMed] [Google Scholar]
- 17.See SJ, Levin VA, Yung WK, Hess KR, Groves MD. 13-cis-Retinoic acid in the treatment of recurrent glioblastoma multiforme. Neuro-Oncol. 2004;6:253–258. doi: 10.1215/S1152851703000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaba SE, Kyritsis AP, Conrad C, et al. The treatment of recurrent cerebral gliomas with all-trans-retinoic acid (tretinoin) J Neurooncol. 1997;34:145–151. doi: 10.1023/a:1005743707803. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Banik NL, Ray SK. Combination of all-trans retinoic acid and interferon- gamma upregulated p27(kip1) and down regulated CDK2 to cause cell cycle arrest leading to differentiation and apoptosis in human glioblastoma LN18 (PTEN-proficient) and U87MG (PTEN-deficient) cells. Cancer Chemother Pharmacol. 2008;62:407–416. doi: 10.1007/s00280-007-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zang C, Wachter M, Liu H, et al. Ligands for PPARgamma and RAR cause induction of growth inhibition and apoptosis in human glioblastomas. J Neurooncol. 2003;65:107–118. doi: 10.1023/b:neon.0000003728.80052.a8. [DOI] [PubMed] [Google Scholar]
- 21.Puduvalli VK, Saito Y, Xu R, Kouraklis GP, Levin VA, Kyritsis AP. Fenretinide activates caspases and induces apoptosis in gliomas. Clin Cancer Res. 1999;5:2230–2235. [PMC free article] [PubMed] [Google Scholar]
- 22.Damodar RC, Guttapalli A, Adamson PC, et al. Anticancer effects of fenretinide in human medulloblastoma. Cancer Lett. 2006;231:262–269. doi: 10.1016/j.canlet.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Gumireddy K, Sutton LN, Phillips PC, Reddy CD. All-trans-retinoic acid-induced apoptosis in human medulloblastoma: activation of caspase-3/poly(ADP-ribose) polymerase 1 pathway. Clin Cancer Res. 2003;9:4052–4059. [PubMed] [Google Scholar]
- 24.Puduvalli VK, Yung WK, Hess KR, et al. Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: A North American Brain Tumor Consortium study. J Clin Oncol. 2004;22:4282–4289. doi: 10.1200/JCO.2004.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidu KA, Tang JL, Prockop LD, Nicosia SV, Coppola D. Antiproliferative and apoptotic effect of ascorbyl stearate in human glioblastoma multiforme cells: modulation of insulin-like growth factor-I receptor (IGF-IR) expression. J Neurooncol. 2001;54:15–22. doi: 10.1023/a:1012545311054. [DOI] [PubMed] [Google Scholar]
- 26.Magrassi L, Bono F, Milanesi G, Butti G. Vitamin D receptor expression in human brain tumors. J Neurosurg Sci. 1992;36:27–30. [PubMed] [Google Scholar]
- 27.Magrassi L, Butti G, Pezzotta S, Infuso L, Milanesi G. Effects of vitamin D and retinoic acid on human glioblastoma cell lines. Acta Neurochir(Wien) 1995;133:184–190. doi: 10.1007/BF01420072. [DOI] [PubMed] [Google Scholar]
- 28.Magrassi L, Adorni L, Montorfano G, et al. Vitamin D metabolites activate the sphingomyelin pathway and induce death of glioblastoma cells. Acta Neurochir(Wien) 1998;140:707–713. doi: 10.1007/s007010050166. [DOI] [PubMed] [Google Scholar]
- 29.Naveilhan P, Berger F, Haddad K, et al. Induction of glioma cell death by 1,25(OH)2 vitamin D3: towards an endocrine therapy of brain tumors? J Neurosci Res. 1994;37:271–277. doi: 10.1002/jnr.490370212. [DOI] [PubMed] [Google Scholar]
- 30.Baudet C, Chevalier G, Naveilhan P, Binderup L, Brachet P, Wion D. Cytotoxic effects of 1 alpha,25-dihydroxyvitamin D3 and synthetic vitamin D3 analogues on a glioma cell line. Cancer Lett. 1996;100:3–10. doi: 10.1016/0304-3835(95)04054-4. [DOI] [PubMed] [Google Scholar]
- 31.Elias J, Marian B, Edling C, et al. Induction of apoptosis by vitamin D metabolites and analogs in a glioma cell line. Recent Results Cancer Res. 2003;164:319–332. doi: 10.1007/978-3-642-55580-0_22. [DOI] [PubMed] [Google Scholar]
- 32.Canova C, Baudet C, Chevalier G, Brachet P, Wion D. Noradrenaline inhibits the programmed cell death induced by 1,25-dihydroxyvitamin D3 in glioma. Eur J Pharmacol. 1997;319:365–368. doi: 10.1016/s0014-2999(96)00942-9. [DOI] [PubMed] [Google Scholar]
- 33.Canova C, Chevalier G, Remy S, Brachet P, Wion D. Epigenetic control of programmed cell death: inhibition by 5-azacytidine of 1,25-dihydroxyvitamin D3-induced programmed cell death in C6.9 glioma cells. Mech Ageing Dev. 1998;101:153–166. doi: 10.1016/s0047-6374(97)00172-3. [DOI] [PubMed] [Google Scholar]
- 34.Trouillas P, Honnorat J, Bret P, Jouvet A, Gerard JP. Redifferentiation therapy in brain tumors: long-lasting complete regression of glioblastomas and an anaplastic astrocytoma under long term 1-alpha-hydroxycholecalciferol. J Neurooncol. 2001;51:57–66. doi: 10.1023/a:1006437003352. [DOI] [PubMed] [Google Scholar]
- 35.Betti M, Minelli A, Canonico B, et al. Antiproliferative effects of tocopherols (vitamin E) on murine glioma C6 cells: homologue-specific control of PKC/ERK and cyclin signaling. Free Radic Biol Med. 2006;41:464–472. doi: 10.1016/j.freeradbiomed.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38:1096–1103. [PubMed] [Google Scholar]
- 37.Maggiora M, Bologna M, Ceru MP, et al. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer. 2004;112:909–919. doi: 10.1002/ijc.20519. [DOI] [PubMed] [Google Scholar]
- 38.Das UN. Essential fatty acids, lipid peroxidation and apoptosis. Prostaglandins Leukot Essent Fatty Acids. 1999;61:157–163. doi: 10.1054/plef.1999.0085. [DOI] [PubMed] [Google Scholar]
- 39.Das UN. Gamma-linolenic acid therapy of human glioma-a review of in vitro, in vivo, and clinical studies. Med Sci Monit. 2007;13:RA119–RA131. [PubMed] [Google Scholar]
- 40.Vartak S, McCaw R, Davis CS, Robbins ME, Spector AA. Gamma-linolenic acid (GLA) is cytotoxic to 36B10 malignant rat astrocytoma cells but not to ‘normal’ rat astrocytes. Br J Cancer. 1998;77:1612–1620. doi: 10.1038/bjc.1998.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tedeschi-Blok N, Schwartzbaum J, Lee M, Miike R, Wrensch M. Dietary calcium consumption and astrocytic glioma: the San Francisco Bay Area Adult Glioma Study, 1991–1995. Nutr Cancer. 2001;39:196–203. doi: 10.1207/S15327914nc392_6. [DOI] [PubMed] [Google Scholar]
- 42.Brem S, Tsanaclis AM, Zagzag D. Anticopper treatment inhibits pseudopodial protrusion and the invasive spread of 9L gliosarcoma cells in the rat brain. Neurosurgery. 1990;26:391–396. doi: 10.1097/00006123-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Brem SS, Zagzag D, Tsanaclis AM, Gately S, Elkouby MP, Brien SE. Inhibition of angiogenesis and tumor growth in the brain Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am J Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida D, Ikeda Y, Nakazawa S. Copper chelation inhibits tumor angiogenesis in the experimental 9L gliosarcoma model. Neurosurgery. 1995;37:287–292. doi: 10.1227/00006123-199508000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7:246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130:1500S– 1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 47.Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009;106:750–757. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreosti IE. Zinc and the gene. Mutat Res. 2001;475:161–167. doi: 10.1016/s0027-5107(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 49.Dimitropoulou P, Nayee S, Liu JF, et al. Dietary zinc intake and brain cancer in adults: a case-control study. Br J Nutr. 2008;99:667–673. doi: 10.1017/S0007114507831692. [DOI] [PubMed] [Google Scholar]
- 50.Aas AT, Tonnessen TI, Brun A, Salford LG. Growth inhibition of rat glioma cells in vitro and in vivo by aspirin. J Neurooncol. 1995;24:171–180. doi: 10.1007/BF01078487. [DOI] [PubMed] [Google Scholar]
- 51.Wakimoto N, Wolf I, Yin D, et al. Nonsteroidal anti-inflammatory drugs suppress glioma via 15-hydroxyprostaglandin dehydrogenase. Cancer Res. 2008;68:6978–6986. doi: 10.1158/0008-5472.CAN-07-5675. [DOI] [PubMed] [Google Scholar]
- 52.Bernardi A, Jacques-Silva MC, Delgado-Canedo A, Lenz G, Battastini AM. Nonsteroidal anti-inflammatory drugs inhibit the growth of C6 and U138-MG glioma cell lines. Eur J Pharmacol. 2006;532:214–222. doi: 10.1016/j.ejphar.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Bernardi A, Frozza RL, Jager E, et al. Selective cytotoxicity of indomethacin and indomethacin ethyl ester-loaded nanocapsules against glioma cell lines: an in vitro study. Eur J Pharmacol. 2008;586:24–34. doi: 10.1016/j.ejphar.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 54.Wang M, Yoshida D, Liu S, Teramoto A. Inhibition of cell invasion by indomethacin on glioma cell lines: in vitro study. J Neurooncol. 2005;72:1–9. doi: 10.1007/s11060-004-1392-0. [DOI] [PubMed] [Google Scholar]
- 55.Ishibashi M, Bottone FG, Jr, Taniura S, Kamitani H, Watanabe T, Eling TE. The cyclooxygenase inhibitor indomethacin modulates gene expression and represses the extracellular matrix protein laminin gamma1 in human glioblastoma cells. Exp Cell Res. 2005;302:244–252. doi: 10.1016/j.yexcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 56.Roller A, Bahr OR, Streffer J, et al. Selective potentiation of drug cytotoxicity by NSAID in human glioma cells: the role of COX-1 and MRP. Biochem Biophys Res Commun. 1999;259:600–605. doi: 10.1006/bbrc.1999.0825. [DOI] [PubMed] [Google Scholar]
- 57.Meyskens FL., Jr McLaren CE, Pelot D, et al Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin VA, Prados MD, Yung WK, Gleason MJ, Ictech S, Malec M. Treatment of recurrent gliomas with eflornithine. J Natl Cancer Inst. 1992;84:1432–1437. doi: 10.1093/jnci/84.18.1432. [DOI] [PubMed] [Google Scholar]
- 59.Levin VA, Hess KR, Choucair A, et al. Phase III randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–990. [PubMed] [Google Scholar]
- 60.Levin VA, Landahl HD, Freeman-Dove MA. The application of brain capillary permeability coefficient measurements to pathological conditions and the selection of agents which cross the blood-brain barrier. J Pharmacokinet Biopharm. 1976;4:499–519. doi: 10.1007/BF01064555. [DOI] [PubMed] [Google Scholar]
- 61.Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem. 1980;23:682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- 62.Levin VA, Csejtey J, Byrd DJ. Brain, CSF, and tumor pharmacokinetics of alpha-difluoromethylornithine in rats and dogs. Cancer Chemother Pharmacol. 1983;10:196–199. doi: 10.1007/BF00255762. [DOI] [PubMed] [Google Scholar]
- 63.Blowers L, Preston-Martin S, Mack WJ. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA) Cancer Causes Control. 1997;8:5–12. doi: 10.1023/a:1018437031987. [DOI] [PubMed] [Google Scholar]
- 64.Boeing H, Schlehofer B, Blettner M, Wahrendorf J. Dietary carcinogens and the risk for glioma and meningioma in Germany. Int J Cancer. 1993;53:561–565. doi: 10.1002/ijc.2910530406. [DOI] [PubMed] [Google Scholar]
- 65.Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of cigarette smoking and adult glioma: dosage, duration, and latency. Neuro Oncol. 2007;9:326–334. doi: 10.1215/15228517-2007-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rollison DE, Helzlsouer KJ. Processed meat consumption and adult gliomas in a Maryland cohort. Cancer Causes Control. 2004;15:99–100. doi: 10.1023/b:caco.0000016675.85386.54. [DOI] [PubMed] [Google Scholar]
- 67.Giles GG, McNeil JJ, Donnan G, et al. Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer. 1994;59:357–362. doi: 10.1002/ijc.2910590311. [DOI] [PubMed] [Google Scholar]
- 68.Hu J, La VC, Negri E, et al. Diet and brain cancer in adults: a case-control study in northeast. China Int J Cancer. 1999;81:20–23. doi: 10.1002/(sici)1097-0215(19990331)81:1<20::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 69.Ward MH, Heineman EF, McComb RD, Weisenburger DD. Drinking water and dietary sources of nitrate and nitrite and risk of glioma. J Occup Environ Med. 2005;47:1260–1267. doi: 10.1097/01.jom.0000184879.67736.ae. [DOI] [PubMed] [Google Scholar]
- 70.Huncharek M, Kupelnick B, Wheeler L. Dietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studies. J Environ Pathol Toxicol Oncol. 2003;22:129–137. doi: 10.1615/jenvpathtoxoncol.v22.i2.60. [DOI] [PubMed] [Google Scholar]
- 71.Braganhol E, Zamin LL, Canedo AD, et al. Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs. 2006;17:663–671. doi: 10.1097/01.cad.0000215063.23932.02. [DOI] [PubMed] [Google Scholar]
- 72.Zoumas-Morse C, Rock CL, Quintana EL, Neuhouser ML, Gerner EW, Meyskens FL., Jr Development of a polyamine database for assessing dietary intake. J Am Diet Assoc. 2007;107:1024–1027. doi: 10.1016/j.jada.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cipolla BG, Havouis R, Moulinoux JP. Polyamine contents in current foods: a basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids. 2007;33:203–212. doi: 10.1007/s00726-007-0524-1. [DOI] [PubMed] [Google Scholar]
- 74.Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: mechanisms and therapeutic potential. Amino Acids. 2007;33:213–223. doi: 10.1007/s00726-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 75.Chen H, Ward MH, Tucker KL, et al. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes Control. 2002;13:647–655. doi: 10.1023/a:1019527225197. [DOI] [PubMed] [Google Scholar]
- 76.Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr. 2007;85:877–886. doi: 10.1093/ajcn/85.3.877. [DOI] [PubMed] [Google Scholar]
- 77.Kokkinakis DM, Wick JB, Zhou QX. Metabolic response of normal and malignant tissue to acute and chronic methionine stress in athymic mice bearing human glial tumor xenografts. Chem Res Toxicol. 2002;15:1472–1479. doi: 10.1021/tx020033n. [DOI] [PubMed] [Google Scholar]
- 78.Poirson-Bichat F, Lopez R, Bras Goncalves RA, et al. Methionine deprivation and methionine analogs inhibit cell proliferation and growth of human xenografted gliomas. Life Sci. 1997;60:919–931. doi: 10.1016/s0024-3205(96)00672-8. [DOI] [PubMed] [Google Scholar]
- 79.Durando X, Thivat E, Farges MC, et al. Optimal methionine-free diet duration for nitrourea treatment: a Phase I clinical trial. Nutr Cancer. 2008;60:23–30. doi: 10.1080/01635580701525877. [DOI] [PubMed] [Google Scholar]
- 80.Thivat E, Durando X, Demidem A, et al. A methionine-free diet associated with nitrosourea treatment down-regulates methylguanine-DNA methyl transferase activity in patients with metastatic cancer. Anticancer Res. 2007;27:2779–2783. [PubMed] [Google Scholar]
- 81.Poirson-Bichat F, Goncalves RA, Miccoli L, Dutrillaux B, Poupon MF. Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clin Cancer Res. 2000;6:643–653. [PubMed] [Google Scholar]
- 82.Jadhav U, Ezhilarasan R, Vaughn SF, Berhow MA, Mohanam S. Dietary isothiocyanate iberin inhibits growth and induces apoptosis in human glioblastoma cells. J Pharmacol Sci. 2007;103:247–251. doi: 10.1254/jphs.sc0060148. [DOI] [PubMed] [Google Scholar]
- 83.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 85.Gao X, Deeb D, Jiang H, Liu YB, Dulchavsky SA, Gautam SC. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol. 2005;5:39–48. [PubMed] [Google Scholar]
- 86.Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–174. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- 87.Kim SY, Jung SH, Kim HS. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun. 2005;337:510–516. doi: 10.1016/j.bbrc.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 88.Woo MS, Jung SH, Kim SY, et al. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 89.Liu E, Wu J, Cao W, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol. 2007;85:263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 90.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 91.Calabrese V, Bates TE, Mancuso C, et al. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 92.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res. 2008;52 (Suppl 1):S103–127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 95.Lynch PM. Chemoprevention with special reference to inherited colorectal cancer. Fam Cancer. 2008;7:59–64. doi: 10.1007/s10689-007-9158-4. [DOI] [PubMed] [Google Scholar]
- 96.Reddy BS. Strategies for colon cancer prevention: combination of chemopreventive agents. Subcell Biochem. 2007;42:213–225. doi: 10.1007/1-4020-5688-5_10. [DOI] [PubMed] [Google Scholar]
- 97.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 98.Mukherjee P, El-Abbadi MM, Kasperzyk JL, Ranes MK, Seyfried TN. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer. 2002;86:1615–1621. doi: 10.1038/sj.bjc.6600298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res. 2004;10:5622–5629. doi: 10.1158/1078-0432.CCR-04-0308. [DOI] [PubMed] [Google Scholar]
- 100.Mukherjee P, Mulrooney TJ, Marsh J, Blair D, Chiles TC, Seyfried TN. Differential effects of energy stress on AMPK phosphorylation and apoptosis in experimental brain tumor and normal brain. Mol Cancer. 2008;7:37. doi: 10.1186/1476-4598-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14:202–208. doi: 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- 102.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yung WK, Kyritsis AP, Gleason MJ, Levin VA. Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid Y1996. Clin Cancer Res. 1996;2:1931–1935. [PubMed] [Google Scholar]
- 105.Kaba SE, Langford LA, Yung WK, Kyritsis AP. Resolution of recurrent malignant ganglioglioma after treatment with cis-retinoic acid. J Neurooncol. 1996;30:55–60. doi: 10.1007/BF00177443. [DOI] [PubMed] [Google Scholar]
- 106.Bakshi A, Mukherjee D, Banerji AK, Das UN. Gamma-linolenic acid therapy of human gliomas. Nutrition. 2003;19:305–309. doi: 10.1016/s0899-9007(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 107.Naidu MR, Das UN, Kishan A. Intratumoral gamma-linoleic acid therapy of human gliomas. Prostaglandins Leukot Essent Fatty Acids. 1992;45:181–184. doi: 10.1016/0952-3278(92)90110-5. [DOI] [PubMed] [Google Scholar]
- 108.Kokkinakis DM, Schold SC, Jr, Hori H, Nobori T. Effect of long-term depletion of plasma methionine on the growth and survival of human brain tumor xenografts in athymic mice. Nutr Cancer. 1997;29:195–204. doi: 10.1080/01635589709514624. [DOI] [PubMed] [Google Scholar]
- 109.Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 110.Tsunoda K, Kitange G, Anda T, et al. Expression of the constitutively activated RelA/NF-kappaB in human astrocytic tumors and the in vitro implication in the regulation of urokinase-type plasminogen activator, migration, and invasion. Brain Tumor Pathol. 2005;22:79–87. doi: 10.1007/s10014-005-0186-1. [DOI] [PubMed] [Google Scholar]
- 111.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 112.Panchal HD, Vranizan K, Lee CY, Ho J, Ngai J, Timiras PS. Early anti-oxidative and anti-proliferative curcumin effects on neuroglioma cells suggest therapeutic targets. Neurochem Res. 2008;33:1701–1710. doi: 10.1007/s11064-008-9608-x. [DOI] [PubMed] [Google Scholar]
- 113.Belkaid A, Copland IB, Massillon D, Annabi B. Silencing of the human microsomal glucose-6-phosphate translocase induces glioma cell death: potential new anticancer target for curcumin. FEBS Lett. 2006;580:3746–3752. doi: 10.1016/j.febslet.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 114.Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384:420–425. doi: 10.1016/j.bbrc.2009.04.149. [DOI] [PubMed] [Google Scholar]
- 115.Karmakar S, Banik NL, Ray SK. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32:2103–2113. doi: 10.1007/s11064-007-9376-z. [DOI] [PubMed] [Google Scholar]
- 116.Karmakar S, Banik NL, Patel SJ, Ray SK. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci Lett. 2006;407:53–58. doi: 10.1016/j.neulet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 117.Nagai S, Kurimoto M, Washiyama K, Hirashima Y, Kumanishi T, Endo S. Inhibition of cellular proliferation and induction of apoptosis by curcumin in human malignant astrocytoma cell lines. J Neurooncol. 2005;74:105–111. doi: 10.1007/s11060-004-5757-1. [DOI] [PubMed] [Google Scholar]
- 118.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 119.Li FP, Fraumeni JF, Jr, Mulvihill JJ, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988 Sep 15;48:5358–5362. [PubMed] [Google Scholar]
- 120.Randerson-Moor JA, Harland M, Williams S, et al. A germline deletion of p14(ARF) but not CDKN2A in a melanoma-neural system tumour syndrome family. Hum Mol Genet. 2001 Jan 1;10:55–62. doi: 10.1093/hmg/10.1.55. [DOI] [PubMed] [Google Scholar]
- 121.Tachibana I, Smith JS, Sato K, Hosek SM, Kimmel DW, Jenkins RB. Investigation of germline PTEN, p53, p16(INK4A)/p14(ARF), and CDK4 alterations in familial glioma. Am J Med Genet. 2000 May 15;92:136–141. doi: 10.1002/(sici)1096-8628(20000515)92:2<136::aid-ajmg11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 122.Gutmann DH, James CD, Poyhonen M, et al. Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology. 2003 Nov 25;61:1397–1400. doi: 10.1212/wnl.61.10.1397. [DOI] [PubMed] [Google Scholar]
- 123.Evans DG, Sainio M, Baser ME. Neurofibromatosis type 2. J Med Genet. 2000;37:897–904. doi: 10.1136/jmg.37.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995 Mar 30;332:39–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 125.Poley JW, Wagner A, Hoogmans MM, et al. Biallelic germline mutations of mismatch-repair genes: a possible cause for multiple pediatric malignancies. Cancer. 2007 Jun 1;109:2349–2356. doi: 10.1002/cncr.22697. [DOI] [PubMed] [Google Scholar]
- 126.Elmariah SB, Huse J, Mason B, Leroux P, Lustig RA. Multicentric glioblastoma multiforme in a patient with BRCA-1 invasive breast cancer. Breast J. 2006 Sep;12:470–474. doi: 10.1111/j.1075-122X.2006.00307.x. [DOI] [PubMed] [Google Scholar]