Abstract
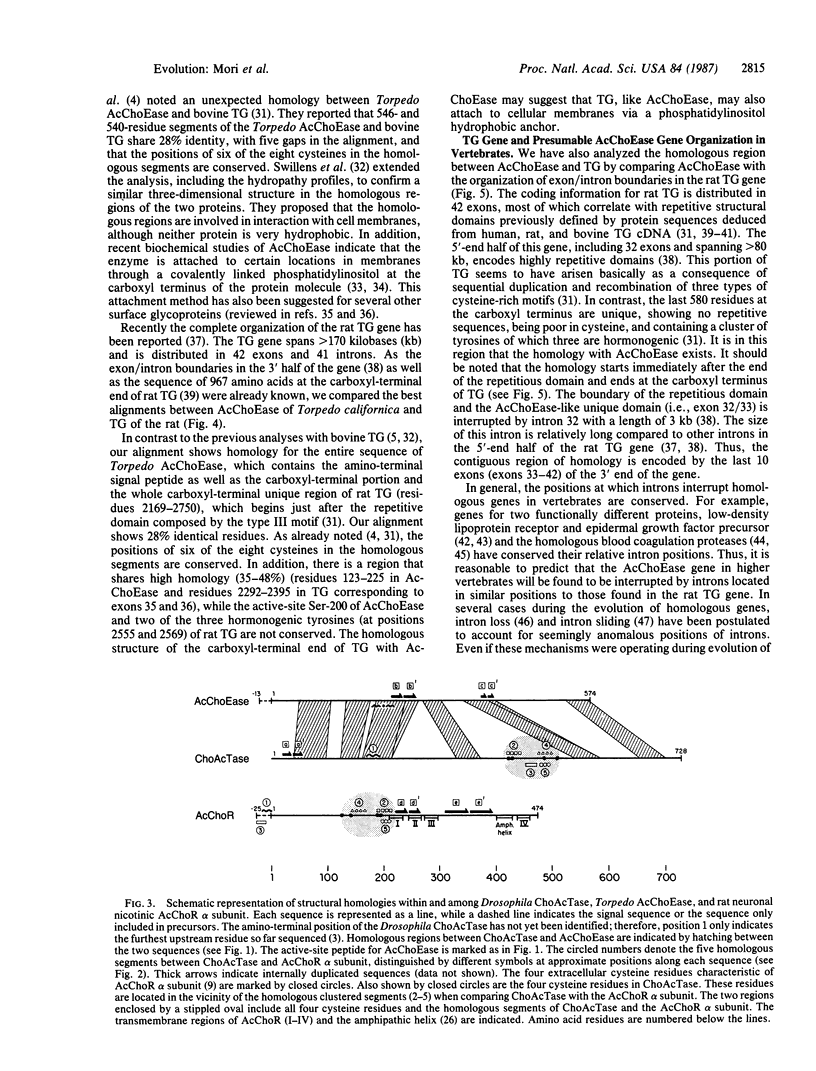
We have compared the amino acid sequences of proteins that are involved in acetylcholine (AcCho) metabolism and cholinergic neurotransmission: choline acetyltransferase (ChoAcTase), acetylcholinesterase (AcChoEase), and a neuronal alpha subunit of nicotinic AcCho receptor (AcChoR). A comparison of Drosophila ChoAcTase and rat neuronal alpha subunit of AcChoR shows a limited segmental type homology, which may suggest a similar acetylcholine binding site in the two proteins evolving by convergence. We note a global homology of 21-44% identity between Drosophila ChoAcTase and Torpedo AcChoEase. Six homologous segments of 40-60 amino acids cover 38% and 54% of the sequences, raising the possibility of a common evolutionary origin. We also note that mammalian thyroglobulin (TG), the precursor for thyroid hormones, contains an AcChoEase-like sequence at its carboxyl end. This homology raises the possibility that the gene for TG has evolved by gene fusion or condensation (i.e., recruiting a preexisting redundant copy of a gene for AcChoEase during vertebrate evolution). Our results demonstrate that the record of evolutionary history for nervous system proteins can be read across the boundaries of separation between vertebrates and invertebrates. They also provide molecular evidence for the common evolutionary origins of the nervous and endocrine systems in vertebrates--both evolving to make intercellular communication possible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Blobel G. Molecular events in the synthesis and assembly of a nicotinic acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):125–134. doi: 10.1101/sqb.1983.048.01.015. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A. M., Obici S., Cocozza S., Di Lauro R. Structural organization of the 3' half of the rat thyroglobulin gene. Nucleic Acids Res. 1984 Apr 25;12(8):3461–3472. doi: 10.1093/nar/12.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boulter J., Luyten W., Evans K., Mason P., Ballivet M., Goldman D., Stengelin S., Martin G., Heinemann S., Patrick J. Isolation of a clone coding for the alpha-subunit of a mouse acetylcholine receptor. J Neurosci. 1985 Sep;5(9):2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikaraishi D. M., Brilliant M. H., Lewis E. J. Cloning and characterization of rat-brain-specific transcripts: rare, brain-specific transcripts and tyrosine hydroxylase. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):309–318. doi: 10.1101/sqb.1983.048.01.034. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Di Lauro R., Obici S., Condliffe D., Ursini V. M., Musti A., Moscatelli C., Avvedimento V. E. The sequence of 967 amino acids at the carboxyl-end of rat thyroglobulin. Location and surroundings of two thyroxine-forming sites. Eur J Biochem. 1985 Apr 1;148(1):7–11. doi: 10.1111/j.1432-1033.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Proteins. Sci Am. 1985 Oct;253(4):88–99. doi: 10.1038/scientificamerican1085-88. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Michaelson D. M., Silman I. Solubilization of membrane-bound acetylcholinesterase by a phosphatidylinositol-specific phospholipase C. J Neurochem. 1985 Nov;45(5):1487–1494. doi: 10.1111/j.1471-4159.1985.tb07217.x. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. Identification of the alpha subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J Biol Chem. 1984 Oct 10;259(19):11662–11665. [PubMed] [Google Scholar]
- Kao P. N., Karlin A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J Biol Chem. 1986 Jun 25;261(18):8085–8088. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Taylor P., Taylor S. Primary structures of the catalytic subunits from two molecular forms of acetylcholinesterase. A comparison of NH2-terminal and active center sequences. J Biol Chem. 1985 Oct 5;260(22):12185–12189. [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Sequence of the 5'-end quarter of the human-thyroglobulin messenger ribonucleic acid and of its deduced amino-acid sequence. Eur J Biochem. 1985 Feb 15;147(1):53–58. doi: 10.1111/j.1432-1033.1985.tb08717.x. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., De Martynoff G., Swillens S., Vassart G. Presence of hormonogenic and repetitive domains in the first 930 amino acids of bovine thyroglobulin as deduced from the cDNA sequence. Eur J Biochem. 1985 Feb 15;147(1):59–64. doi: 10.1111/j.1432-1033.1985.tb08718.x. [DOI] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Musti A. M., Avvedimento E. V., Polistina C., Ursini V. M., Obici S., Nitsch L., Cocozza S., Di Lauro R. The complete structure of the rat thyroglobulin gene. Proc Natl Acad Sci U S A. 1986 Jan;83(2):323–327. doi: 10.1073/pnas.83.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine Y., Pearson D., Altus M. S., Reich E. cDNA and gene nucleotide sequence of porcine plasminogen activator. Nucleic Acids Res. 1984 Dec 21;12(24):9525–9541. doi: 10.1093/nar/12.24.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Numa S., Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Kikyotani S. Molecular structure of the nicotinic acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):57–69. doi: 10.1101/sqb.1983.048.01.008. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Prothero D. R. Mid-oligocene extinction event in north american land mammals. Science. 1985 Aug 9;229(4713):550–551. doi: 10.1126/science.229.4713.550. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Scott J., Urdea M., Quiroga M., Sanchez-Pescador R., Fong N., Selby M., Rutter W. J., Bell G. I. Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science. 1983 Jul 15;221(4607):236–240. doi: 10.1126/science.6602382. [DOI] [PubMed] [Google Scholar]
- Shepherd J. C., McGinnis W., Carrasco A. E., De Robertis E. M., Gehring W. J. Fly and frog homoeo domains show homologies with yeast mating type regulatory proteins. Nature. 1984 Jul 5;310(5972):70–71. doi: 10.1038/310070a0. [DOI] [PubMed] [Google Scholar]
- Slemmon J. R., Salvaterra P. M., Crawford G. D., Roberts E. Purification of choline acetyltransferase from Drosophila melanogaster. J Biol Chem. 1982 Apr 10;257(7):3847–3852. [PubMed] [Google Scholar]
- Stroud R. M., Finer-Moore J. Acetylcholine receptor structure, function, and evolution. Annu Rev Cell Biol. 1985;1:317–351. doi: 10.1146/annurev.cb.01.110185.001533. [DOI] [PubMed] [Google Scholar]
- Swillens S., Ludgate M., Mercken L., Dumont J. E., Vassart G. Analysis of sequence and structure homologies between thyroglobulin and acetylcholinesterase: possible functional and clinical significance. Biochem Biophys Res Commun. 1986 May 29;137(1):142–148. doi: 10.1016/0006-291x(86)91187-3. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Goldstein J. L., Brown M. S., Sanchez-Pescador R., Bell G. I. Cassette of eight exons shared by genes for LDL receptor and EGF precursor. Science. 1985 May 17;228(4701):893–895. doi: 10.1126/science.3873704. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]