Abstract
Clinical trials are evaluating the effect of neoadjuvant chemotherapy on men with high risk prostate cancer. Little is known about the clinical significance of post-chemotherapy tumor histopathology. We assessed the prognostic and predictive value of histological features (intraductal carcinoma, vacuolated cell morphology, inconspicuous glands, cribriform architecture, and inconspicuous cancer cells) observed in 50 high-risk prostate cancers treated with pre-prostatectomy docetaxel and mitoxantrone. At a median follow-up of 65 months, the overall relapse-free survival (RFS) at 2 and 5 years was 65% and 49%, respectively. In univariate analyses (using Kaplan-Meier method and log-rank tests) intraductal (p=0.001) and cribriform (p=0.014) histologies were associated with shorter RFS. In multivariate analyses, using Cox’s proportional hazards regression, baseline PSA (p=0.004), lymph node metastases (p<0.001), and cribriform histology (p=0.007) were associated with shorter RFS. In multivariable logistic regression analysis, only intraductal pattern (p=0.007) predicted lymph node metastases. Intraductal and cribriform histologies apparently predict post-chemotherapy outcome.
Keywords: Neoadjuvant, Chemotherapy, Prostate, Carcinoma, Intraductal, cribriform
INTRODUCTION
Prostate cancer is the most common malignancy (excluding skin cancer) diagnosed in American men. In 2008, an estimated 186,320 men were diagnosed with prostate cancer and 28,660 have died of their disease.1
The risk of relapse and death of disease after prostatectomy is significant in prostate cancer patients who are at high-risk of progression, i.e. >60% of men who underwent prostatectomy for Gleason score ≥ 4+3 carcinoma died of disease at 10 years follow-up in one series.2 Accordingly, there is a need to develop more effective treatment of prostate adenocarcinoma. Although the negative surgical margin rate was improved with neoadjuvant hormonal therapy,3–5 randomized clinical trials have failed to demonstrate a relapse-free or overall survival benefit with this approach.3, 6–8
Neoadjuvant cytotoxic chemotherapy, capable of targeting androgen-independent clones, is therefore under investigation in high risk prostate cancer.9–13 While in most cases, hormonal therapy has been combined with chemotherapy in neoadjuvant studies, several investigators have examined chemotherapy in isolation permitting a more careful assessment of the impact of cytotoxic chemotherapy on untreated prostate cancer.10, 11, 14, 15
Some types of systemic therapy result in characteristic histologic effects on benign and cancerous prostate tissue. For example, androgen deprivation therapy has a predictable effect on both non-neoplastic and malignant prostate glands. Non-neoplastic glands are characterized by a reduced gland diameter, a flattened epithelium with loss of micropapillary architecture, and prominence of the basal cell layer which is hyperplastic. Carcinoma glands collapse and shrink. Cancer cells are characterized by pyknotic nuclei and voluminous vacuolated cytoplasm.16–22
Histological changes in prostate tissue treated with chemotherapy have been previously described, though only in a limited fashion.23 We report histological patterns observed in radical prostatectomy specimens obtained from patients at high-risk for prostate cancer progression who were treated with neoadjuvant docetaxel and mitoxantrone. Furthermore, we discuss the predictive power of two histological patterns, intraductal carcinoma (IDC-P) and cribriform architecture, for nodal metastases and relapse-free survival.
MATERIALS AND METHODS
Patients
Between January 2001 and November 2004, 57 patients with localized prostate cancer at high-risk for progression were recruited for a phase II trial clinical trial of neoadjuvant chemotherapy. To be categorized as high-risk, patients had to meet at least one of three criteria – the tumor was stage cT2b or cT3a, the serum PSA was ≥ 15 ng/ml, or the Gleason score was ≥ 4+3. The design of the clinical trial has been previously reported.24 The study was approved by the Institutional Review Boards of all participating institutions. All patients provided signed informed consent.
Selection of Radical Prostatectomy Slides for Histological Evaluation
Samples from radical prostatectomy tissue from the first 50 patients were used to construct a tissue microarray (TMA) that was assembled to identify prognostic biomarkers. In selecting the tissue blocks from which to obtain cores of tissue for the TMA, the slides that had the greatest amount of prostate carcinoma were identified and reviewed by the pathologist (LT) for this study.
Selection of Core Biopsy Slides for Histological Evaluation
Pre-treatment core biopsy tissue slides were retrieved for 22 of the 50 patients whose post-neoadjuvant therapy cancers were evaluated. As the pre-treatment biopsies were not systematically collected as part of the study, the remaining 28 could not be retrieved for analysis.
Histological Evaluation of Radical Prostatectomy Specimens
Sections that contained the largest fraction of tumor in each case were reviewed to select blocks for constructing tissue microarrays. Based upon the observation that many of these tumors exhibited histological patterns that are infrequent to rare in prostate cancers that had not been exposed to neoadjuvant chemotherapy, all sections of each radical prostatectomy sample were systematically re-reviewed to tabulate the relative frequency of each of these patterns. The following patterns were tabulated: (a) intraductal growth pattern (intraductal carcinoma), (b) prominent vacuolization of tumor cells, (c) an inconspicuous appearance of collapsed tumor glands (d) cribriform architecture, and (e) inconspicuous appearance of cancer cells (cancer cells that if viewed at relatively low magnification, i.e. 40x final magnification, might be easily overlooked). We also assessed the sections for features that characterize both benign and malignant prostate consequent to other modalities of non-surgical therapy, such as androgen deprivation therapy. These features included atrophy and shrinkage of non-neoplastic and/or cancer glands, basal cell hyperplasia and prominence and squamous metaplasia of benign glands, and stromal changes, e.g. stromal cell atypia, focal necrosis, foci of hypocellular scarring, vascular ectasia, and prominent stromal inflammation. Finally, two tumors had features that are quite rare in conventional prostate adenocarcinomas. The majority of carcinoma cells in one cancer was large and had abundant eosinophilic cytoplasm with markedly pleomorphic nuclei. Most cells in a second tumor had a nested, carcinoid-like growth pattern. The fact that there were only single examples of each of these histological patterns precluded assessing the predictive power of these patterns.
Based on these publications we used the following criteria for IDC in the present study: (1) two-fold expansion of prostate gland lumina, (2) neoplastic cells span, in a solid or cribriform architecture, the lumen of glands in which a basal cell layer is retained, and (3) nuclear atypia.25, 26, 28 This histological lesion is different than it’s near namesake - prostatic duct adenocarcinoma – which is invasive and has a tubulovillous architecture resembling endometrioid adenocarcinoma of the gynecologic tract.29 None of the cases in this series was a prostatic duct adenocarcinoma. Descriptively, the distinction between intraductal and ductal adenocarcinoma is based on, first, the fact that ductal adenocarcinoma most frequently is in a central location and has a tubulovillous pattern although one of the patterns is that of a solid intraductal type growth pattern, and, second, that foci of ductal adenocarcinoma having a cribriform pattern the are a lot larger than any of the foci of intraductal carcinoma seen in our cases.
Histological Evaluation of Core Biopsy Sections
Since virtually all of the histological patterns that we found distinctive of prostate cancer treated with neoadjuvant chemotherapy occur in other settings - both treatment-naive and following other neoadjuvant regimens - we sought to tabulate the frequency of each of the six patterns in the pre-treatment biopsies. The retrieved slides were anonymized so that the pathology review was blinded as to the patient outcome and the pathology of the prostatectomy sample. Histological criteria applied to reviewing the Gleason grade of cancer in the pre-neoadjuvant biopsies were those of the 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading.30
RESULTS
Patient Characteristics and Sample Collection
The characteristics of the 50 patients whose tissue was evaluated are outlined in Table 1. The median serum PSA was 12.0 ng/mL (range 1.4 – 58.6 ng/mL). Forty-four of the patients (88%) had Gleason score 7, 8, or 9 cancer in their biopsy specimens. Five patients had Gleason score 6 cancers and 1 patient had a Gleason score 10 cancer. The clinical stage of most patients was at least T2a, reflecting the pre-specified entry criteria for the trial.
Table 1.
Patient Characteristics
| Number | 50 |
| Age (in years) | |
| Median | 63 |
| Range | 52–74 |
| ECOG Performance Status | |
| 0 | 47 |
| 1 | 3 |
| Serum PSA (as ng/ml) | |
| Median | 14.6 |
| Range | 1.3–58.6 |
| Clinical Stage (2002 criteria) | |
| T1c | 7 |
| T2a | 7 |
| T2b | 8 |
| T2c | 17 |
| T3a | 9 |
| T3b | 2 |
| Biopsy Gleason Score | |
| 6 | 5 |
| 7 | 19 |
| 8 | 13 |
| 9 | 12 |
| 10 | 1 |
| Chemotherapy Doses Delivered | |
| Median | 11 |
| Range | 1–12 |
| Pathologic Stage (2002 criteria) | |
| T2a | 4 |
| T2b | 5 |
| T2c | 15 |
| T3a | 8 |
| T3b | 16 |
| T4 | 2 |
Histological Patterns Identified in Radical Prostatectomy Slides
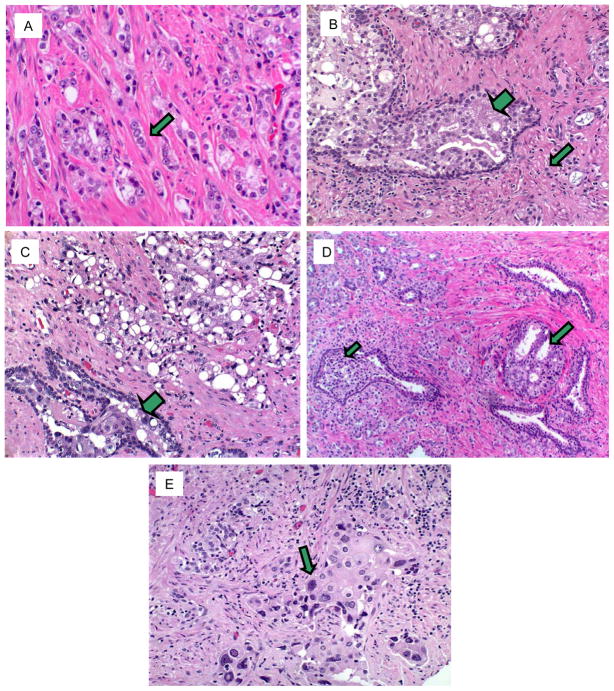
Most of the carcinomas in the prostatectomy specimens had at least one of the distinctive histological patterns, which, in decreasing frequency of occurrence, were: (a) relatively inconspicuous, collapsed glands (44% of cases) (Fig. 1A), (b) small, inconspicuous tumor cells (29% of cases) (Fig. 1B), (c) prominently vacuolated tumor cell cytoplasm (23% of cases) (Fig. 1C), (d) intraductal growth pattern (20% of cases) (Figs. 1B–C), (e) cribriform architecture (14% of cases) (Fig. 1D), (f) tumor-associated, basophilic, mucin-like material (2 cases) (not illustrated), (g) nested, growth pattern (1 case) (not illustrated), and (h) individual, large, pleomorphic, eosinophilic tumor cells (1 case) (Fig. 1E). Tumors in thirty-six (72%) of the 50 patients exhibited at least one pattern, while 17 (34%) had at least 2 patterns (Table 2, Table 3).
Fig. 1.
A. Relatively inconspicuous glands. Depicted (arrow) are cancer glands that we recorded as “inconspicuous”. Note the collapsed nature of the glands without lumina. Some of these tumor cell collections are so small and tend to blend with adjacent stroma to a degree that they might be overlooked if the microscopic review of the slide was cursory. B. Inconspicuous tumor cells. Depicted (arrow) are cancer cells (as individual cells or small clusters of cells) with small, pyknotic, basophilic nuclei lacking observable nucleoli and having scanty cytoplasm. These features make these cells relatively inconspicuous. Also note (large arrow) a duct peripherally lined by prominent basal cells and expanded by a population of dysplastic neoplastic cells having a cribriform architecture. This pattern meets current histological criteria for intraductal carcinoma. C. Vacuolated tumor cells. Sheets of carcinoma are composed of cells, many having prominent, large, clear cytoplasmic vacuoles. Also illustrated (large arrow) is a focus of intraductal carcinoma. D. Cribriform architecture. Foci of cancer (arrows) have a cribriform architecture; This pattern, which is indistinguishable from one form of Gleason pattern 4 prostate carcinoma, was exhibited by many of the cancers in their post-chemotherapy manifestation. E. Pleomorphic tumor cells. One case was characterized by individual, large, pleomorphic, tumor cells with abundant cytoplasm (arrow).
Table 2.
Frequency of Specific Histologic Patterns in Tissue Specimens
| Pattern | Radical Prostatectomy Specimens (n=50): Frequency | Biopsy Specimens (n=22): Frequency |
|---|---|---|
| Intraductal Carcinoma | 20 % | 0 % |
| Cribriform architecture | 14 % | 18 % |
| Prominent vacuolated cytoplasm | 26 % | 5 % |
| Inconspicuous, collapsed glands | 46 % | 18 % |
| Small, Inconspicuous tumor cells | 28 % | 0 % |
| Patients with Any Pattern | 68 % | 36 % |
| Patients with > 1 Pattern | 32 % | 5 % |
Table 3.
Histologic features of cancer in the pre- and post-chemotherapy tissue from the 8 patients who exhibited at least one of the chemotherapy-associated patterns in the pre-chemotherapy biopsy.
| Patient ID | Pattern(s) Identified in Biopsy Slides | Pattern(s) Identified in prostatectomy Slides |
|---|---|---|
| 6082-01 | Cribriform | None |
| 6082-03 | Vacuolated | None |
| 6082-05 | Cribriform Inconspicuous glands |
Cribriform Intraductal |
| 6082-19 | Cribriform | Inconspicuous glands |
| 6082-20 | Inconspicuous glands | Inconspicuous glands Cribriform |
| 6082-35 | Cribriform | Inconspicuous glands |
| 6082-36 | Inconspicuous glands | None |
| 6082-37 | Inconspicuous glands | Inconspicuous glands |
Other histological features were either so common - atrophy and shrinkage of non-neoplastic and/or cancer glands, and basal cell hyperplasia – or so rare - squamous metaplasia of benign glands, and stromal changes, e.g. stromal cell atypia, focal necrosis, foci of hypocellular scarring, vascular ectasia, and prominent stromal inflammation – as to not warrant statistical analysis.
Histologic Patterns Identified in Core Biopsy Slides/Specimens
Of the 22 patients whose biopsies we could evaluate, three exhibited histologic patterns in the pre-neoadjuvant therapy biopsies that were similar to the histological patterns seen in the radical prostatectomy specimens – cribriform architecture, vacuolated cytoplasm, and inconspicuous collapsed tumor glands. The results are summarized in Table 2. None of the pre-prostatectomy biopsies contained intraductal carcinoma.
Correlation with Pathologic/Other Endpoints
Prognostic Significance
At a median follow-up time of 65.1 months the overall estimated RFS at 2 years was 65.3% (95% CI 52.0–78.6%) and at 5 years was 48.8% (95% CI 34.1–63.5%). Of the 50 patients whose tissue was evaluated, 48 received at least 1 cycle of chemotherapy. These 48 patients were evaluated for clinical end-points.
In univariate analyses, intraductal carcinoma (p=0.001) and cribriform architecture (p=0.014) were associated with shorter RFS. In univariate analyses stratified for lymph node status, intraductal carcinoma (p=0.023) was associated with shorter RFS in lymph node negative patients, whereas cribriform architecture (p=0.036) was associated with shorter RFS in lymph node positive patients. These two histological patterns (intraductal carcinoma and cribriform architecture) were included in a multivariate analysis that included biopsy Gleason score, clinical T stage, serum PSA, and lymph node status (N0 vs. N1). In this model, cribriform architecture (HR 2.1, 95% CI 1.2 – 3.7), baseline PSA (HR 1.05, 95%CI 1.01–1.08, p=0.004) and lymph node metastases (HR 8.1, 95%CI 2.9–22.9, p<0.001) were associated with shorter RFS. Because lymph node status is a dominant predictor and because it may not always be known, we also examined these factors without including lymph node status in the model. In this analysis, in addition to baseline PSA (HR 1.04, 95% CI 1.01–1.08, p=0.007), both intraductal carcinoma pattern (HR 2.6, 95%CI 1.5–4.3, p<0.001) and cribriform architecture (HR 2.3, 95%CI 1.3–4.0, p=0.003) were independent predictors of progression-free survival.
In a multivariable logistic regression with lymph node status as the dependent variable and PSA, biopsy Gleason score, clinical T stage, intraductal carcinoma, and cribriform architecture as independent variables, only the presence of intraductal carcinoma (OR 4.6, 95%CI 1.5–14.2, p=0.007) correlated with lymph node metastases.
DISCUSSION
Many histological features of prostate cancer can serve as tissue biomarkers of tumor biology. For example, the microscopic architecture of primary prostate cancer (the Gleason score) provides a powerful predictor of both prognosis and of likelihood that primary therapy will be successful. Histological features also reflect and are characteristic of different systemic therapies. Androgen-deprived prostate cancer is characterized by inconspicuous, shrunken glands and small inconspicuous tumor cells that have pyknotic often hyperchromatic nuclei with inconspicuous nucleoli.16–20 Tumor cells subjected to high-dose radiation from brachytherapy are also typically inconspicuous, and have abundant, vacuolated cytoplasm.31, 32 Although histological changes of prostate cancer in patients treated with neoadjuvant chemotherapy have been previously described in a limited fashion,23 this study has found that two of these features – intraductal carcinoma and cribriform architecture – have apparent power in predicting outcome after neoadjuvant chemotherapy. The cribriform architecture retains its prognostic value in the context of a comprehensive multivariate analysis.
None of the histological features that we observed is unique to prostate cancer treated with mitoxantrone and docetaxel. Single small, inconspicuous tumor cells are seen in both androgen-deprivation and radiation therapy, as well as being a feature of one manifestation of Gleason pattern 5 primary prostate cancer. Collapsed, also relatively inconspicuous glands are also seen in both androgen-deprived and irradiated prostate cancer. Although prominent, vacuolated cytoplasm is a feature of brachytherapy, the nature of vacuolization is subtly different than that seen in androgen deprived prostate tissue. The vacuoles seen in the cancers in this study are round and clear. In contrast, the vacuoles in irradiated prostate cancer are irregular and often have a finely vesicular appearance.
A cribriform architecture, more frequent in our treated cancers, is architecturally indistinguishable from one variant (the cribriform variant) of Gleason pattern 4 carcinoma, although cytologically the tumor cells are distinctive, having small pyknotic nuclei and scanty, clear cytoplasm. The association of a cribriform architecture with a more malignant course of disease is of interest since it suggests that the cribriform variant of Gleason pattern 4 biologically differs from the collapsed gland variant of pattern 4. However, this hypothesis cannot be addressed in the present study since the sample size was small, particularly the number of pre-neoadjuvant therapy samples that had the cribriform variant of Gleason pattern 4 carcinoma.
Tumor-associated, basophilic, mucin-like material, seen in two cases, is an infrequent feature that characterizes a minority of primary, untreated prostate cancers. The nested growth pattern that we found in one case, though non-specific, is one manifestation of neuroendocrine differentiation by tumors in many organ sites. This pattern is characterized by small, round aggregates of tumor cells. Finally, in one case many tumor cells were large with abundant eosinophilic cytoplasm and pleomorphic nuclei. This histologic set of features has been previously reported as a rare histological variant of prostate carcinoma that is associated with a poor outcome.33 Of note, although none of the six cases in the previous report were associated with neoadjuvant chemotherapy, two patients had received other modalities of neoadjuvant therapy – radiation and androgen deprivation, respectively.
Intraductal carcinoma has historically been a poorly defined and controversial entity. The controversy regards whether it is a variant of PIN or whether it represents a growth pattern of prostate adenocarcinoma that is invasive of prostate ducts and glands. Attempting to clarify this issue, several authors have articulated histological criteria that provide a reasonable basis for defining “intraductal carcinoma” as a distinct histopathological entity.25–27 Previous studies have found a correlation of IDC with both poor prognostic parameters (high Gleason score, elevated serum PSA, high tumor volume, extraprostatic extension of tumor) and increased frequency of biochemical evidence of recurrence. These correlations have led investigators to conclude that IDC generally represents a late event in the progression of prostate adenocarcinoma.
Intraductal carcinoma is an infrequent feature of prostate cancer, being represented in < 5% of primary prostate cancers. In general prostate carcinomas that have an intraductal component are of higher grade and greater volume. This pattern is predictive of more aggressive disease.25, 34, 35 We assessed the association of each pattern with likelihood of progression of cancer. Three patterns of histological features were too rare to have statistical predictive power in our series of patients: basophilic, mucin-like material; nested growth pattern; and, pleomorphic tumor cells. Hence, these patterns were omitted from further analysis. We found that the intraductal carcinoma pattern was an independent predictor of nodal metastases in our group of prostate cancer patients.
To assess whether the patterns seen in the radical prostatectomy specimens were induced by chemotherapy or whether they were innate features that were expressed in the treatment-naïve state of the tumor, we reviewed the corresponding pre-treatment biopsy slides in a blinded fashion. We found very little evidence of the intraductal, cribriform, inconspicuous cell and gland, and vacuolated cell patterns in the slides from the pre-chemotherapy core biopsy specimens. In fact, whereas 36/50 (72%) of the patients whose radical prostatectomy (post-chemotherapy) slides contained these histologic changes, only 3 of 22 (18%) of the biopsy slides contained similar patterns. There are several possible explanations for the difference in the frequency with which these histologic patterns are seen in prostatectomies compared with the biopsy specimens.
Selection bias
Since the slides we reviewed from the radical prostatectomy specimens were selected for having large areas of either normal or malignant prostate tissue, it is possible that we introduced some selection bias. Intraductal carcinoma has been previously shown to be found primarily within areas of invasive cancer 36, 37 and rarely in areas where carcinoma represents a minor fraction of prostate tissue and, as mentioned above, there is an association between cancer volume and presence of intraductal carcinoma.25, 37, 38 Further, biopsy specimens were only examined from a sub-set of patients for whom prostatectomy specimens were available for analysis.
Differences in the amount of tissue examined
In general, more tissue was histologically evaluated in the radical prostatectomy slides than in the core biopsy slides. There is an inherent limitation of core biopsies due to the small proportion of each cancer that is sampled with core biopsies. Therefore, it is possible that the histological patterns seen in the radical prostatectomy specimens were present, but were not sampled, in the pre-treatment biopsies.
Treatment with chemotherapy selected for survival of specific patterns
The intraductal carcinoma pattern has been previously associated with more aggressive prostate cancer. Therefore, it is conceivable that this pattern is more resistant to chemotherapy than the classically described Gleason patterns. Resistance to chemotherapy might have resulted in selection of this histological pattern following treatment. The same consideration pertains to the cribriform architecture, which is one variant of Gleason pattern 4, since Gleason pattern 4 is a histological marker of a cancer at higher risk of progression than are cancers that have a Gleason score equal to or less than 6.
The changes were induced by chemotherapy
It is also possible that some or all of these patterns represent a change in the histology of the prostate cancer caused by one or both of the chemotherapeutic agents. This possibility is unlikely since the two patterns that have been previously described (IDC-P and cribriform) occur in untreated prostate cancers. The one pattern that is quite distinctive is the large, pleomorphic tumor cell pattern, which was seen in one case (figure 1E).
Host predisposition to intraductal carcinoma
That some, but not all, patients who otherwise shared all identifiable demographic and pathological features had distinctive post-chemotherapy histological patterns raises the question of host genome predisposition to these distinctive tumor architectures.
The association between cribriform architecture and risk of relapse as well as of IDC-P with nodal status should be evaluated in an independent set of prostate cancers, ideally in a group of untreated prostate cancer patients. Were the predictive power of these patterns to be validated, studies of the molecular phenotype of this pattern would be warranted to try to identify therapeutic molecular targets.
Acknowledgments
Supported in part by grant GIA US #16080 from Aventis Pharmaceuticals, grant #031.G0008 from OSI, Inc., grants 3M01RR00334-33S2 and 1 R01 CA119125-01 from the National Institutes of Health and Career Development award from Pacific Northwest Prostate Cancer SPORE
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Aus G, Abrahamsson PA, Ahlgren G, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002;90:561–566. doi: 10.1046/j.1464-410x.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 4.Meyer F, Moore L, Bairati I, et al. Neoadjuvant hormonal therapy before radical prostatectomy and risk of prostate specific antigen failure. J Urol. 1999;162:2024–2028. doi: 10.1016/S0022-5347(05)68092-5. [DOI] [PubMed] [Google Scholar]
- 5.Witjes WP, Schulman CC, Debruyne FM. Preliminary results of a prospective randomized study comparing radical prostatectomy versus radical prostatectomy associated with neoadjuvant hormonal combination therapy in T2-3 N0 M0 prostatic carcinoma. The European Study Group on Neoadjuvant Treatment of Prostate Cancer. Urology. 1997;49:65–69. doi: 10.1016/s0090-4295(97)00171-4. [DOI] [PubMed] [Google Scholar]
- 6.Civantos F, Sadek S, Obek C, et al. Neoadjuvant Hormonal Therapy Prior to Radical Prostatectomy. Mol Urol. 1999;3:201–204. [PubMed] [Google Scholar]
- 7.Klotz LH, Goldenberg SL, Jewett M, et al. CUOG randomized trial of neoadjuvant androgen ablation before radical prostatectomy: 36-month post-treatment PSA results. Canadian Urologic Oncology Group. Urology. 1999;53:757–763. doi: 10.1016/s0090-4295(98)00616-5. [DOI] [PubMed] [Google Scholar]
- 8.Schulman CC, Debruyne FM, Forster G, et al. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol. 2000;38:706–713. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 9.Clark PE, Peereboom DM, Dreicer R, et al. Phase II trial of neoadjuvant estramustine and etoposide plus radical prostatectomy for locally advanced prostate cancer. Urology. 2001;57:281–285. doi: 10.1016/s0090-4295(00)00914-6. [DOI] [PubMed] [Google Scholar]
- 10.Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 11.Garzotto M, Myrthue A, Higano CS, et al. Neoadjuvant mitoxantrone and docetaxel for high-risk localized prostate cancer. Urol Oncol. 2006;24:254–259. doi: 10.1016/j.urolonc.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M, Smith DC, El-Rayes BF, et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locallyadvanced prostate cancer. Urology. 2003;61:774–780. doi: 10.1016/s0090-4295(02)02519-0. [DOI] [PubMed] [Google Scholar]
- 13.Pettaway CA, Pisters LL, Troncoso P, et al. Neoadjuvant chemotherapy and hormonal therapy followed by radical prostatectomy: feasibility and preliminary results. J Clin Oncol. 2000;18:1050–1057. doi: 10.1200/JCO.2000.18.5.1050. [DOI] [PubMed] [Google Scholar]
- 14.Dreicer R, Magi-Galluzzi C, Zhou M, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–1142. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Oh WK, George DJ, Kaufman DS, et al. Neoadjuvant docetaxel followed by radical prostatectomy in patients with high-risk localized prostate cancer: a preliminary report. Semin Oncol. 2001;28:40–44. doi: 10.1016/s0093-7754(01)90153-8. [DOI] [PubMed] [Google Scholar]
- 16.Montironi R, Schulman CC. Pathological changes in prostate lesions after androgen manipulation. J Clin Pathol. 1998;51:5–12. doi: 10.1136/jcp.51.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy WM, Soloway MS, Barrows GH. Pathologic changes associated with androgen deprivation therapy for prostate cancer. Cancer. 1991;68:821–828. doi: 10.1002/1097-0142(19910815)68:4<821::aid-cncr2820680426>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Reuter VE. Pathological changes in benign and malignant prostatic tissue following androgen deprivation therapy. Urology. 1997;49:16–22. doi: 10.1016/s0090-4295(97)00164-7. [DOI] [PubMed] [Google Scholar]
- 19.Tetu B, Srigley JR, Boivin JC, et al. Effect of combination endocrine therapy (LHRH agonist and flutamide) on normal prostate and prostatic adenocarcinoma. A histopathologic and immunohistochemical study. Am J Surg Pathol. 1991;15:111–120. doi: 10.1097/00000478-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Trummel DE, McCabe KM, Cina SJ. Characteristics of hormone-treated prostate carcinoma: stressing the need for clinician-pathologist communication. Mil Med. 2000;165:294–297. [PubMed] [Google Scholar]
- 21.Vailancourt L, Ttu B, Fradet Y, et al. Effect of neoadjuvant endocrine therapy (combined androgen blockade) on normal prostate and prostatic carcinoma. A randomized study. Am J Surg Pathol. 1996;20:86–93. doi: 10.1097/00000478-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Bullock MJ, Srigley JR, Klotz LH, et al. Pathologic effects of neoadjuvant cyproterone acetate on nonneoplastic prostate, prostatic intraepithelial neoplasia, and adenocarcinoma: a detailed analysis of radical prostatectomy specimens from a randomized trial. Am J Surg Pathol. 2002;26:1400–1413. doi: 10.1097/00000478-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Magi-Galluzzi C, Zhou M, Reuther AM, et al. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer. 2007;110:1248–1254. doi: 10.1002/cncr.22897. [DOI] [PubMed] [Google Scholar]
- 24.Beer TM, Garzotto M, Lowe BA, et al. Phase I study of weekly mitoxantrone and docetaxel before prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2004;10:1306–1311. doi: 10.1158/1078-0432.ccr-1021-03. [DOI] [PubMed] [Google Scholar]
- 25.Rubin MA, de La Taille A, Bagiella E, et al. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol. 1998;22:840–848. doi: 10.1097/00000478-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Cohen RJ, Wheeler TM, Bonkhoff H, et al. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med. 2007;131:1103–1109. doi: 10.5858/2007-131-1103-APOTIH. [DOI] [PubMed] [Google Scholar]
- 27.Tavora F, Epstein JI. High-grade prostatic intraepithelial neoplasialike ductal adenocarcinoma of the prostate: a clinicopathologic study of 28 cases. Am J Surg Pathol. 2008;32:1060–1067. doi: 10.1097/PAS.0b013e318160edaf. [DOI] [PubMed] [Google Scholar]
- 28.Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol. 2006;19:1528–1535. doi: 10.1038/modpathol.3800702. [DOI] [PubMed] [Google Scholar]
- 29.Christensen WN, Steinberg G, Walsh PC, et al. Prostatic duct adenocarcinoma. Findings at radical prostatectomy. Cancer. 1991;67:2118–2124. doi: 10.1002/1097-0142(19910415)67:8<2118::aid-cncr2820670818>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 31.Siders DB, Lee F. Histologic changes of irradiated prostatic carcinoma diagnosed by transrectal ultrasound. Hum Pathol. 1992;23:344–351. doi: 10.1016/0046-8177(92)90080-m. [DOI] [PubMed] [Google Scholar]
- 32.Bostwick DG, Egbert BM, Fajardo LF. Radiation injury of the normal and neoplastic prostate. Am J Surg Pathol. 1982;6:541–551. doi: 10.1097/00000478-198209000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Parwani AV, Herawi M, Epstein JI. Pleomorphic giant cell adenocarcinoma of the prostate: report of 6 cases. Am J Surg Pathol. 2006;30:1254–1259. doi: 10.1097/01.pas.0000209841.77595.4b. [DOI] [PubMed] [Google Scholar]
- 34.Cohen RJ, Chan WC, Edgar SG, et al. Prediction of pathological stage and clinical outcome in prostate cancer: an improved pre-operative model incorporating biopsy-determined intraductal carcinoma. Br J Urol. 1998;81:413–418. doi: 10.1046/j.1464-410x.1998.00530.x. [DOI] [PubMed] [Google Scholar]
- 35.Cohen RJ, McNeal JE, Baillie T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate. 2000;43:11–19. doi: 10.1002/(sici)1097-0045(20000401)43:1<11::aid-pros3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Dawkins HJ, Sellner LN, Turbett GR, et al. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate. 2000;44:265–270. doi: 10.1002/1097-0045(20000901)44:4<265::aid-pros1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol. 1996;20:802–814. doi: 10.1097/00000478-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox G, Soh S, Chakraborty S, et al. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol. 1998;29:1119–1123. doi: 10.1016/s0046-8177(98)90423-3. [DOI] [PubMed] [Google Scholar]