Abstract
PURPOSE
To evaluate the relationship between the 6-minute walk distance (6MWD) and survival in a cohort of patients with severe end-stage chronic obstructive pulmonary disease (COPD) who received inpatient pulmonary rehabilitation (IPR) from 1995 to 2007.
METHODS
We retrospectively analyzed 815 patients with severe end-stage COPD who received IPR. 6MWDs before and after IPR (pre-6MWD, post-6MWD) were compared to assess whether 6MWD was significantly changed after IPR. The Kaplan-Meier survival curves were constructed to show the relationship between survival and 6MWD. The age- and or comorbidities-adjusted Cox proportional hazard model was applied to assess association between the survival and the pre-6MWD, post-6MWD, or difference in 6MWD from the pre-6MWD to post-6MWD (Δ6MWD).
RESULTS
Baseline demographics demonstrated a median age 74.0 years, mostly women (60.1%), and white (89.9%) patients with significant comorbid diseases who were most recently hospitalized in acute care facilities (95.1%). IPR significantly increased the 6MWD (mean distance change: 86.4 m; 95% confidence interval [CI], 81.5–91.3 m). Pre-6MWD was not significantly associated with survival. However, post-6MWD was significantly associated with age- and comorbidity-adjusted survival (post-6MWD hazard ratio = 1.336; 95% CI, 1.232–1.449 [post-6MWD x m relative to post-6MWD 2x m]), and Δ6MWD was also significantly associated with age-, omorbidities-, and pre–6MWD-adjusted survival (Δ6MWD hazard ratio = 1.337; 95% CI, 1.227–1.457 [Δ6MWD x m relative to Δ6MWD 2x m]).
CONCLUSIONS
In patients with severe end-stage COPD, IPR significantly improved 6MWD, and the post-6MWD and Δ6MWD were positively associated with the length of survival.
Keywords: COPD, inpatient pulmonary rehabilitation, mortality, 6-minute walk test
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of morbidity and mortality in the United States.1 COPD is characterized by irreversible airflow obstruction and dyspnea,2–4 and patients with severe end-stage COPD usually develop recurrent exacerbations,5 peripheral muscle dysfunction,6–8 and significant exercise limitation.3,7 Even though COPD is considered irreversible, several studies have identified values for improving functional parameters including dyspnea and exercise capacity measured by walk distance,9–11 and dyspnea and exercise capacity are now considered independent predictors of clinical outcomes in COPD.12,13
Previously the 6-minute walk test has been demonstrated as a reliable assessment of functional exercise capacity to predict outcomes in patients with COPD.14–17 However, its role in the assessment of patients with severe end-stage COPD has not been well-defined. We proposed to demonstrate that the 6-minute walk distance (6MWD) is significantly associated with survival over time among patients with severe end-stage COPD who complete inpatient pulmonary rehabilitation (IPR). The investigation was a retrospective review of a cohort with available outcomes data over a span of 12 years.
METHODS
A retrospective analysis was conducted using data from the pulmonary rehabilitation program at HealthSouth Rehabilitation Hospital of Virginia (HSRHV) located in Richmond. The ethics committees of the University of Virginia and the HSRHV approved the study protocol. HealthSouth personnel managed data entry, and de-identified data were transferred to the University of Virginia for analyses without links and codes.
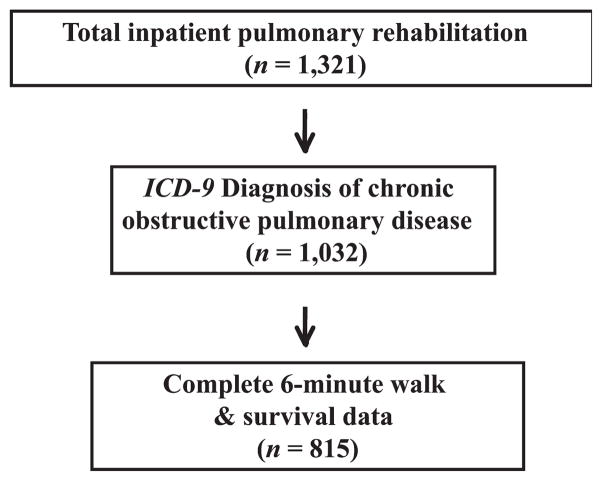
All patients who participated in the pulmonary rehabilitation program from January 1995 to March 2007 were captured, and demographic and clinical outcomes data of patients who completed IPR were entered into Microsoft Access Database (Microsoft Corp, Redmond, Washington). COPD was defined by the history of International Classification of Diseases, Ninth Revision (ICD-9) diagnosis (491 or 492) at the time of consultation for pulmonary rehabilitation. When patients were initially referred for pulmonary rehabilitation, complete pulmonary function test results and referring physician notes were reviewed to confirm a diagnosis of COPD in all patients. Admission criteria to IPR included (1) exercise intolerance defined as inability to walk 150 ft in 2 minutes without oxygen desaturation or to perform activities of daily living without severe dyspnea or oxygen desaturation; (2) existence of identifiable rehabilitation goals; (3) ability to participate in IPR; (4) readiness to undergo the rehabilitation program medically, cognitively, and emotionally; and (4) abstinence from smoking at the time of admission and during IPR. Approximately 80% of patients referred to the program were admitted. The remaining 20% were not for a variety of reasons including patient refusal or failure to meet the admission criteria. Among all entries, patients were excluded from our analyses if they did not carry a diagnosis of COPD on admission, or if 6MWD or mortality data were incomplete (Figure 1).
Figure 1.
Flow diagram of patient selection. ICD-9 indicates International Classification of Diseases, Ninth Revision.
Pulmonary Rehabilitation Program
The IPR program was designed for patients requiring inpatient 24 hours/day nursing care as well as daily medical evaluation and intervention by a board-certified rehabilitation physician. Each patient was required to be able to participate in rehabilitation for 3 hours each day, 5 days a week. Typically patients received equal time with physical therapy and occupational therapy. Speech language pathology was added for voice therapy, cognitive therapy, or dysphagia. Exercise regimens with physical therapy focused on ambulation, transfers, stair climbing, and increasing exercise tolerance. Occupational therapy focused on breathing techniques, energy conservation, activities-of-daily-living performance, adaptive equipment for home, and basic homemaking tasks. Patients also received nutritional intervention from certified dietitians focusing on dietary supplements and lifestyle modification. The decision to discharge a patient was determined by standard inpatient rehabilitation discharge criteria: (1) no longer need 24-hour nursing care, (2) physician decision, (3) patient deemed able to physically access home safely, (4) family/patient capable of managing care needs at home, and/or (5) patient determined to have reached maximum benefit from therapy.
Collection of Data and Outcomes Definitions
Patients were followed through the entire length of participation in pulmonary rehabilitation. On the day of admission, patients were oriented to the program. During the orientation period, patients were asked to complete a baseline 6-Minute Walk Test, which was repeated within 24 hours before discharge. Primary outcomes for effectiveness of pulmonary rehabilitation were the 6MWD and overall mortality after IPR. 6MWD was measured according to the American Thoracic Society guidelines.18 Mortality data were retrieved from US Social Security Death Index. All data entries were censored as of March 27, 2007, and statistical analyses were performed.
Statistical Methods
Demographics and past medical history were described as a mean ± standard deviation or percent. Pre- and postrehabilitation 6MWD (m) was analyzed by way of a linear mixed-effects model. Nonparametric survival curves were estimated via the Kaplan-Meier estimator. Age-alone-adjusted and age- and comorbidity-adjusted log-hazard ratios were estimated via the Cox proportional hazard model. Corresponding hazard ratios were obtained by the inverse exponential transformation of the log-hazard ratio. The Grambsch and colleagues weighted residual proportional hazard test was utilized to evaluate the Cox-proportional hazard assumption.19 To avoid violating the Cox proportional hazard assumption, 6MWD data utilized as covariate information were transformed to the natural logarithmic scale. The Cox model was designed to evaluate hazard ratios between survival and 3 distinctive assessments of the 6MWD: pre-6MWD (at the beginning of pulmonary rehabilitation); post-6MWD (at completion of pulmonary rehabilitation); and change in 6MWD (Δ6MWD) (post-6MWD minus pre-6MWD). For the pre-and post-6MWD, we compared how doubling 6MWD impacted age-adjusted versus age- and comorbidity-adjusted hazard ratios. In assessing the hazard ratio between the survival and Δ6MWD, the hazard ratio was adjusted with age alone; age and comorbidities; or age, comorbidities, and pre-6MWD. The statistical software packages SAS, Version 9.3 EA (SAS Institute Inc, Cary, North Carolina) and S-Plus, Version 7.3 (Insightful Inc, Seattle, Washington) were utilized to conduct the statistical analyses.
RESULTS
A total of 1,321 patient entries to IPR were captured for analyses. From these entries, we selected entries corresponding to ICD-9 diagnosis of COPD with complete 6-minute walk test and survival data (alive or deceased) (Figure 1). This subset of data consisted of 815 inpatient entries, which had complete comorbidity data, vital statistics, baseline and final 6MWD data, and mortality data. This group was primarily women (60.1%), white (89.9%), married (44.4%), widowed (39.6%), and mostly from other acute care facilities (95.1%), with a mean age of 73.4 years (Table 1). More than half of the patients had a high school or less education. The 9 most prevalent comorbid diseases were pneumonia (23.8%), coronary artery disease (22.4%), diabetes (20.5%), arrhythmia (19.9%), depression/anxiety (15.8%), degenerative joint disease/osteoarthritis (14.7%), cancer (12.5%), respiratory failure related to other than coronary artery bypass graft (CABG) surgery (10.6%), and postoperative CABG respiratory failure (8.8%). In combination, the description of patients demonstrated the presence of severe, end-stage COPD and other multiple comorbid diseases, which necessitated pulmonary rehabilitation in an inpatient facility.
Table 1.
BASELINE CHARACTERISTICS AND COMORBIDITIES
| Characteristic | |
|---|---|
| Age, y, mean (SD) | 73.4 (8.6) |
| Male/female, n (%) | 325 (39.9)/490 (60.1) |
| Race (white), n (%) | 730 (89.9) |
| Marital status, n (%) | |
| Single | 41 (5.1) |
| Married | 360 (44.4) |
| Divorced/separated | 88 (10.9) |
| Widowed | 321 (39.6) |
| Admission source, n (%) | |
| Home | 29 (4.1) |
| Acute care facility | 680 (95.1) |
| Other rehabilitation | 6 (0.8) |
| Educational status, n (%) | |
| Up through eighth grade | 93 (12.1) |
| Some high school | 106 (13.8) |
| High school graduate | 280 (36.6) |
| Some college | 137 (17.9) |
| College graduate | 127 (16.6) |
| Postgraduate | 23 (3.0) |
| Past medical history,a n (%) | |
| Pneumonia | 194 (23.8) |
| Coronary heart disease | 182 (22.3) |
| Diabetes | 167 (20.5) |
| Arrhythmias | 162 (19.9) |
| Depression/Anxiety | 129 (15.8) |
| Degenerative joint disease | 120 (14.7) |
| Cancer | 102 (12.5) |
| Respiratory failure | 86 (10.6) |
| Postoperative respiratory failure | 72 (8.8) |
| Discharge disposition | |
| Home | 728 (89.3) |
| Transferred to another rehabilitation facility | 68 (8.3) |
| Skilled nursing facility | 7 (.9) |
| Transferred back to acute care facility | 12 (1.5) |
Denotes the set of 9 most prevalent comorbidities that were included as adjustment factors in the survival analysis.
6MWD
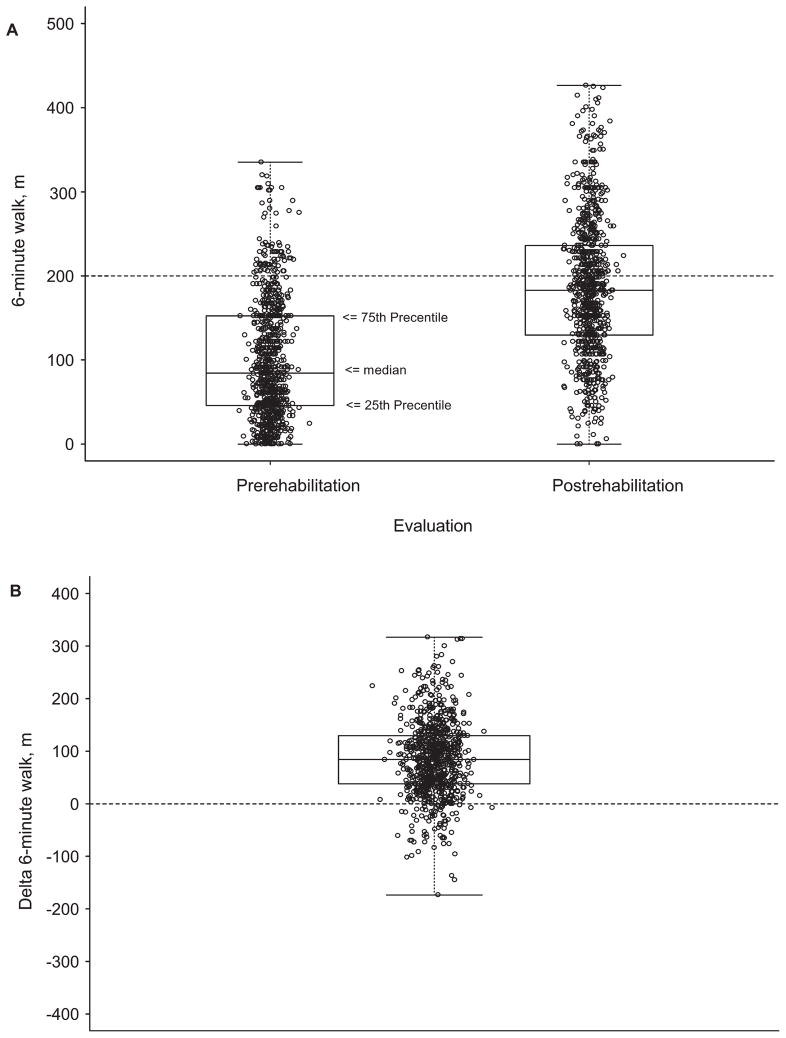
The IPR increased mean 6MWD from the baseline, 99.6 m (SD = 69.7; 95% confidence interval [CI], 94.8–104.4 m) to 186.0 m (SD = 82.26; 95% CI, 180.3–191.6 m) (Figure 2A). The mean improvement in Δ6MWD was 86.39 m (95% CI 81.5–91.3 m, P < .001) (Figure 2B).
Figure 2.
6MWD distance (m). (A) Prerehabilitation 6MWD and Postrehabilitation 6MWD. The horizontal dotted line represents reference line at 200 m. (B) Change in 6MWD from prerehabilitation to postrehabilitation. 6MWD indicates 6-minute walk test.
Kaplan-Meier Curves of 6MWD and Length of Survival
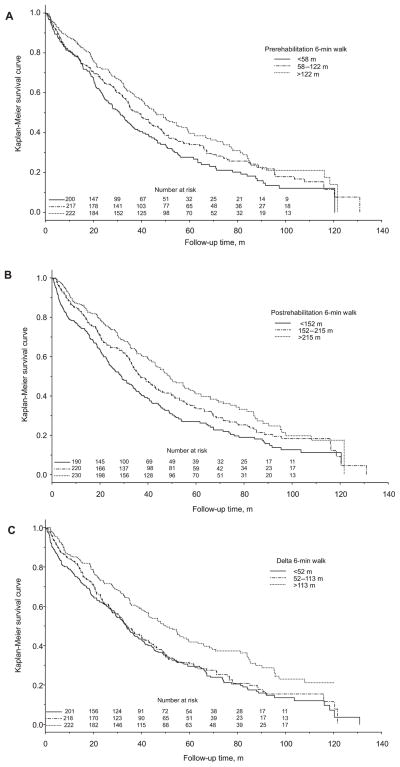
The pre-6MWD, post-6MWD, and Δ6MWD were divided into tertiles according to magnitude. After stratified according to these tertiles, but not adjusted for ages and comorbidities, Kaplan-Meier survival curves were constructed (Figures 3A–3C). For pre-6MWD, the upper tertile was greater than 122 m, middle tertile 58 to 122 m, and lower tertile less than 58 m with respective median survival times of 45.9 months (95% CI, 40.0–54.6), 38.3 months (95% CI, 33.5–44.5), and 31.0 months (95% CI, 24.9–35.9). For post-6MWD, the upper tertile was greater than 215 m, middle tertile 152 to 215 m, and lower tertile less than 152 m with respective median survival times of 49.1 months (95% CI, 43.0–56.8), 36.8 months (95% CI, 33.1–43.5), and 28.9 months (95% CI, 23.8–33.6). For Δ6MWD, the upper tertile was greater than 113 m, middle tertile 52 to 113 m, and lower tertile less than 52 m with respective median survival times of 50.4 months (95% CI, 42.9–58.8), 34.4 months (95% CI, 29.7–40.7), and 33.7 months (95% CI, 29.1–39.0). Survival curves of the pre-6MWD and post-6MWD demonstrated that patients in the upper tertiles had longer length of survival than the middle tertiles, and the middle tertiles longer than the lower tertiles (P = .003 and <.001, respectively) (Figures 3A and 3B). However, survival curves of the Δ6MWD demonstrated that those in the upper tertile of the Δ6MWD had longer length of survival than the middle and lower tertiles (P < .001), but the survival curves of the middle and lower tertiles were similar (Figure 3C).
Figure 3.
Unadjusted Kaplan-Meier curves of 6MWD and survival (all subjects subgrouped into tertiles according to the 6MWD). (A) Pre-6MWD and survival. (B) Post-6MWD and survival. (C) Change in 6MWD and survival. 6MWD indicates 6-minute walk distance.
Cox Proportional Hazard Ratio of 6MWD and Survival
To further characterize the relationships between the length of survival and the 6-minute walk test, pre-6MWD, post-6MWD, and Δ6MWD data were treated as continuous predictors, and age-only-adjusted and age- and comorbidity-adjusted Cox proportional hazard ratios were estimated (Table 2). This Cox model tested the effects of 6MWD on length of survival of 2 patients if their 6MWDs had a 2-fold difference. According to this Cox model, those who had a shorter 6MWD were expected to have shorter survival, thus higher hazard ratios, than those who walked longer. In the age-adjusted Cox model, 2-fold differences in pre-6MWD were found to be minimally associated with the length of survival (hazard ratio = 1.056; 95% CI, 1.000–1.116). After adjusting for age and the 9 most prevalent comorbidities, the relationship was minimally changed and still marginal at best (hazard ratio = 1.058; 95% CI, 1.001–1.118). Conversely, in the age-only adjusted Cox model, 2-fold differences in post-6MWD were found to be significantly associated with the length of survival (hazard ratio = 1.337; 95% CI, 1.234–1.448). This relationship still remained significant and strong even after the hazard ratio was adjusted for age and the 9 most prevalent comorbidities (hazard ratio = 1.336; 95% CI, 1.232–1.449).
Table 2.
COX PROPORTIONAL HAZARD MODEL SUMMARY FOR AGE AND/OR COMORBIDITY-ADJUSTED INFLUENCE OF PRE-6MWD AND POST-6MWD ON THE RISK OF DEATH AND FOR AGE-, COMORBIDITY-, AND PRE–6MWD-ADJUSTED INFLUENCE OF Δ6MWD ON THE RISK OF DEATH
| 6MWD ratio | Estimate β | Lower 95%, confidence limit | Upper 95%, confidence limit | |
|---|---|---|---|---|
| Age-adjusted 6MWD and risk of death | ||||
| Pre-6MWD | ||||
| Log-hazard | x m:2x m | 0.056 | 0.001 | 0.111 |
| Hazard | x m:2x m | 1.058 | 1.001 | 1.118 |
| Post-6MWD | ||||
| Log-hazard | x m:2x m | 0.291 | 0.211 | 0.370 |
| Hazard | x m:2x m | 1.337 | 1.234 | 1.448 |
| Δ6MWD | ||||
| Log-hazard | x m:2x m | 0.060 | −0.007 | 0.127 |
| Hazard | x m:2x m | 1.062 | 0.993 | 1.136 |
| Age- and comorbidity-adjusted 6MWD and risk of death | ||||
| Pre-6MWD | ||||
| Log-hazard | x m:2x m | 0.056 | 0.001 | 0.111 |
| Hazard | x m:2x m | 1.058 | 1.001 | 1.118 |
| Post-6MWD | ||||
| Log-hazard | x m:2x m | 0.290 | 0.209 | 0.371 |
| Hazard | x m:2x m | 1.336 | 1.232 | 1.449 |
| Δ6MWD | ||||
| Log-hazard | x m:2x m | 0.055 | −0.013 | 0.122 |
| Hazard | x m:2x m | 1.056 | 0.987 | 1.130 |
| Age-, comorbidity-, and pre–6MWD-adjusted 6MWD and risk of death | ||||
| Δ6MWD | ||||
| Log-hazard | x m:2x m | 0.292 | 0.207 | 0.376 |
| Hazard | x m:2 x m | 1.339 | 1.231 | 1.457 |
Abbreviation: 6MWD, indicates 6-minute walk distance.
In addition, the magnitude of 6MWD improvement (Δ6MWD) was considered as a direct beneficial effect of IPR. To elucidate the effects of the IPR on the survival of patients with severe end-stage COPD, the Cox model was applied to the Δ6MWD and survival as described previously. In the age-adjusted Cox model, 2-fold differences in the Δ6MWD were weakly associated with survival (hazard ratio = 1.062; 95% CI, 0.993–1.136). This relationship still remained marginal when adjusted for the age and the 9 most prevalent comorbidities (hazard ratio = 1.056; 95% CI, 0.987–1.130). Because greater than 95% of patients with COPD in the database were directly admitted from acute care facilities, the pre-6MWD was considered as a composite measure of effects from preexisting COPD and acute illness–related hospitalization. Consequently, the Cox model of the Δ6MWD and survival was further adjusted with the pre-6MWD in addition to the age and comorbidities. As expected, adjustment with the pre-6MWD demonstrated highly significant association between the Δ6MWD and survival (hazard ratio = 1.337; 95% CI, 1.227–1.457). The Cox model of the Δ6MWD and survival demonstrated that the magnitude of the Δ6MWD after IPR was highly associated with the length of survival after adjusted for age, comorbidities, and pre-6MWD in patients with severe end-stage COPD.
DISCUSSION
This observational cohort study has significant findings regarding the 6-minute walk test and survival in patients with severe end-stage COPD. First, 6MWD in patients with severe end-stage COPD was significantly improved following IPR. Second, 6MWD at completion of IPR was more strongly associated with survival than 6MWD at the beginning of the IPR. Third, the magnitude of 6MWD improvement after IPR was strongly associated with survival only when adjusted for baseline functional status (pre-6MWD). This indirectly suggests that IPR improved survival in patients with severe end-stage COPD by increasing functional capacity measured by the 6-minute walking test. Even though this study was not a randomized controlled trial and was possibly confounded by uncontrolled variables such as the natural history of recovery from an acute illness, results are provocative. Findings are specific to IPR and are uniquely different from out-patient pulmonary rehabilitation.
Limitation of exercise capacity is a hallmark of disability in COPD and is associated with poor health–related quality of life, increased morbidity, and higher mortality.7,14,20–22 This association is more notable among patients with severe end-stage COPD because activity tolerance is significantly limited. This study demonstrated that even in severely disabled COPD patients, pulmonary rehabilitation effectively improved exercise capacity measured by 6MWD and that 6MWD was strongly associated with survival. These findings are in agreement with several previously published studies of patients with COPD who received pulmonary rehabilitation.4,14,23–26 However, a larger number of subjects in the present study offer stronger evidence for the 6-minute walk test to predict mortality among patients with severe end-stage COPD. In addition, this study is one of the largest studies to investigate the beneficial role of the IPR in patients with severe end-stage COPD to this date.27–29
Of the 3 6MWD measurements tested in this study, pre-6MWD was deemed not only the most important but also the most confounded assessment. Because most of the subjects were admitted directly from acute care hospitals or skilled nursing facilities, pre-6MWD was thought to be significantly confounded by detrimental influences of acute illness and hospitalization on activity tolerance. As expected, it was only weakly associated with survival. This demonstrated that the functional capacity assessed by 6MWD in the presence of acute illness before administering pulmonary rehabilitation is inaccurate and unreliable in predicting mortality among patients with severe end-stage COPD. This is an important observation because many patients with severe end-stage COPD are often denied from IPR precisely because of severe limitation in exercise tolerance or poor functional capacity. If exercise capacity measured by the 6-minute walk test was used to screen for eligibility for pulmonary rehabilitation, a significant number of patients with severe end-stage COPD could be denied from a therapy, which could provide a significant chance of survival benefit. Unfortunately our study and database were not designed to directly ask and test such an important clinical question. Instead, future randomized controlled studies are necessary to answer how to evaluate and screen patients with COPD and severe functional limitations for IPR.
Post-6MWD was strongly associated with survival in the present study. This demonstrated that IPR may effectively ameliorate detrimental effects of acute illness and that post-6MWD may be an accurate marker of clinical improvement and survival after IPR in patients with severe end-stage COPD. Our study demonstrated that Δ6MWD is also strongly associated with survival only when the Cox model was adjusted with the pre-6MWD. Presuming that pre-6MWD is a marker of the baseline exercise capacity in our population, this was a logical finding that suggested that there may be a subgroup of patients with severe end-stage COPD who may benefit more from IPR than others. Again this study was not designed to test such hypothesis, but future prospective study is necessary to further investigate this issue.
CONCLUSIONS
The present study indicates that the 6-minute walk test following IPR is a marker of activity tolerance and is also a clinical marker, which may predict the risk of mortality in this patient population. A similar role for the 6-minute walk test has already been proposed as a part of BODE score.16,17,26 However, the present study is the first study to demonstrate a strong positive association between post-6MWD and survival among patients with severe end-stage COPD who received IPR. With limited healthcare resources, future studies can be directed to identify a subset of patients with COPD who may more likely experience significant improvement in the post-6MWD so that our clinical approach to treat these patients can be better guided. For example, when IPR is considered for sicker patients with severe end-stage COPD, those who will more likely gain in the post-6MWD should be offered pulmonary rehabilitation whereas those who will unlikely gain can be counseled on alternative treatment goals including transplant or palliative care. Finally, randomized, prospective studies are needed to confirm how improvement in the patients with severe end-stage COPD would translate into savings in healthcare expenditure.
References
- 1.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Hay JG, Stone P, Carter J, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J. 1992;5:659–664. [PubMed] [Google Scholar]
- 4.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 5.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154(4 pt 1):959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 6.Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:102–108. doi: 10.1164/rccm.200202-080OC. [DOI] [PubMed] [Google Scholar]
- 7.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–980. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 8.Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 9.Reardon J, Awad E, Normandin E, Vale F, Clark B, ZuWallack RL. The effect of comprehensive outpatient pulmonary rehabilitation on dyspnea. Chest. 1994;105:1046–1052. doi: 10.1378/chest.105.4.1046. [DOI] [PubMed] [Google Scholar]
- 10.Vale F, Reardon JZ, ZuWallack RL. The long-term benefits of outpatient pulmonary rehabilitation on exercise endurance and quality of life. Chest. 1993;103:42–45. doi: 10.1378/chest.103.1.42. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5 pt 1):1489–1497. doi: 10.1164/ajrccm.157.5.9708010. [DOI] [PubMed] [Google Scholar]
- 12.Guell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117:976–983. doi: 10.1378/chest.117.4.976. [DOI] [PubMed] [Google Scholar]
- 13.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 14.ZuWallack RL. Functional status and survival in COPD. Monaldi Arch Chest Dis. 2003;59:230–233. [PubMed] [Google Scholar]
- 15.Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD. 2005;2:125–129. doi: 10.1081/copd-200050527. [DOI] [PubMed] [Google Scholar]
- 16.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 17.Cote CG, Pinto-Plata VM, Marin JM, Hekach H, Dordelly LJ, Celli BR. The modified BODE index: validation with mortality in COPD. Eur Respir J. 2008;32:1269–1274. doi: 10.1183/09031936.00138507. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. ATS Statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51:1469–1482. [PubMed] [Google Scholar]
- 20.Mannino DM, Brown C, Giovino GA. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med. 1997;156(3 pt 1):814–818. doi: 10.1164/ajrccm.156.3.9702026. [DOI] [PubMed] [Google Scholar]
- 21.Kyroussis D, Polkey MI, Keilty SE, et al. Exhaustive exercise slows inspiratory muscle relaxation rate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:787–793. doi: 10.1164/ajrccm.153.2.8564133. [DOI] [PubMed] [Google Scholar]
- 22.Killian KJ. Limitation to muscular activity in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:6–7. doi: 10.1183/09031936.04.00038104. [DOI] [PubMed] [Google Scholar]
- 23.Lisboa BC, Barria PP, Yanez VJ, Aguirre ZM, Diaz PO. [Six minutes walk for the assessment of patients with chronic obstructive pulmonary disease] Rev Med Chil. 2008;136:1056–1064. [PubMed] [Google Scholar]
- 24.Budweiser S, Heidtkamp F, Jorres RA, et al. Predictive significance of the six-minute walk distance for long-term survival in chronic hypercapnic respiratory failure. Respiration. 2008;75:418–426. doi: 10.1159/000109662. [DOI] [PubMed] [Google Scholar]
- 25.Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–1785. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- 26.Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26:630–636. doi: 10.1183/09031936.05.00045505. [DOI] [PubMed] [Google Scholar]
- 27.Lacasse Y, Martin S, Lasserson TJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura Medicophys. 2007;43:475–485. [PubMed] [Google Scholar]
- 28.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Clini EM, Romagnoli M. Inpatient pulmonary rehabilitation: does it make sense? Chron Respir Dis. 2005;2:43–46. doi: 10.1191/1479972305cd069oa. [DOI] [PubMed] [Google Scholar]