Abstract
In Drosophila, the checkpoint protein-2 kinase (DmChk2) and its downstream effector protein, Dmp53, are required for DNA damage-mediated cell cycle arrest, DNA repair and apoptosis. In this study we focus on understanding the function of these two apoptosis inducing factors during ovarian development. We found that expression of Dmp53, but not DmChk2, led to loss of ovarian stem cells. We demonstrate that expression of DmChk2, but not Dmp53, induced mid-oogenesis cell death. DmChk2 induced cell death was not suppressed by Dmp53 mutant, revealing for the first time that in Drosophila, overexpression of DmChk2 can induce cell death which is independent of Dmp53. We found that over-expression of caspase inhibitors such as DIAP1, p35 and p49 did not suppress DmChk2- and Dmp53-induced cell death. Thus, our study reveals stage -specific effects of Dmp53 and DmChk2 in oogenesis. Moreover, our results demonstrate that although DmChk2 and Dmp53 affect different stages of ovarian development, loss of ovarian stem cells by p53 expression and mid-oogenesis cell death induced by DmChk2 do not require caspase activity.
Keywords: Drosophila melanogaster, ovary, Chk2, p53, caspase, cell death
Introduction
The DNA damage response is a signal transduction network which is activated upon recognition of DNA lesions to elicit appropriate cellular responses to these assaults (such as DNA repair, cell cycle arrest or apoptosis). Chk2, a checkpoint effector kinase, is activated mainly by Ataxia-telangiectasia mutated (ATM) protein in response to double strand DNA breaks (DSB's). Upon activation by ATM, Chk2 phosphorylates several proteins which in turn affect cell cycle checkpoint regulation, DNA repair, replication fork maintenance and apoptosis (1). During DNA damage-induced apoptosis, Chk2 promotes p53-dependent transcriptional responses mainly by phosphorylation of p53 (2–3) or by phosphorylation of other substrates that affect p53 stability (4–6). In mammals, it has also been shown that Chk2 may regulate DNA damage-induced apoptosis independently of p53, in part through the modification of the promyelocytic leukemia protein (7).
The Drosophila p53 homologue (Dmp53) is required for the induction of apoptosis following DNA damage events (8–10). Recently, it was shown that Dmp53 is also required for cellular differentiation in the Drosophila eye (11) and for compensatory proliferation due to cellular damage (12). During development, loss of Dmp53 activity affects fly longevity (13) and also programmed cell death of primordial germ cells (PGC's) (14). Recently, it was shown that Dmp53 in required for meiotic recombination in Drosophila (15) which is highly conserved. The Drosophila Chk2 homologue, DmChk2, is required for DNA damage-mediated cell cycle arrest and apoptosis (16–21). As shown for mammalian cells, the role of DmChk2 in mediating irradiation-induced apoptosis is dependent on Drosophila Dmp53 protein (17–18). During meiosis, DmChk2, but not Dmp53, was found to be essential for activation of a meiotic checkpoint, which occurs in double strand DNA repair enzyme mutants (22–23). DmChk2 was also essential for activation of a second meiotic checkpoint due to mutations in genes in the repeat associated small interfering RNA (RasiRNA) pathway (24–26).
As described above, both DmChk2 and Dmp53 had an important role during oogenesis, thus in this study we focused on analyzing the role of DmChk2 and Dmp53 in apoptosis processes during Drosophila ovarian development. We found that DmChk2 and Dmp53 could independently induce cell death in a stage and tissue-specific manner. Whereas expression of Dmp53 led to loss of ovarian stem cells, expression of DmChk2 led to cell death during mid-oogenesis. Moreover, expression of Dmp53 but not of DmChk2 in the somatic follicle cells affected egg chamber survival. Interestingly, we show that inhibition of caspase activity is not sufficient to suppress loss of germ cell by Dmp53 or mid-oogenesis cell death induced by DmChk2.
Materials and Methods
Drosophila stocks
The following mutant and transgenic flies were used: p53 (27), DmChk2 (lokp6) (22), Atg7d14, Atg7d77 (28), pUASp-DIAP1 (29). The UASp-p35 line was obtained from Andreas Bergmann (Houston, TX, USA). The GMR-Gal4, P{matalpha4-GAL-VP16}V2H, UASp-GFP-LC3; nanos-GAL4 and nanos-Gal4VP16 lines were all obtained from the Bloomington Stock Center. For expression in follicle cells CY2-Gal4 (30) and GR1-Gal4 (kindly provided by T. Schüpbach) were used. For LysoTracker and TUNEL experiments the driver was NGT40; nosGAL4VP16 (31).
Transgenic flies
To create HA-tagged DmChk2, the entire coding sequence of DmChk2 was amplified by PCR using modified primers to create a XbaI restriction site at the 5' end and a NotI site at the 3' end. The resulting PCR product was cut using XbaI and NotI and was cloned into HA-pBlueScript. The resulting pBlueScript vectors were cut using KpnI and NotI and the inserts were cloned into pUASp vectors. To make the pUASp- p53 or p49 fusion construct the entire coding sequence of either Dmp53 or p49 was amplified by PCR using modified primers to create a KpnI restriction site at the 5' end and a NotI site at the 3' end. The resulting PCR products of each gene were cut using KpnI and NotI and were cloned into pUASp.
P-element-mediated germ-line transformation of this construct was carried out according to standard protocols (32). Ten independent lines from each construct were established.
Ovary staining
Ovaries were dissected in phosphate-buffered saline (PBS), fixed in 200 µl 4% formaldehyde in PBS combined with 600µl heptane for 20 minutes, and washed in PBST (PBS + 0.3% Triton X-100). The ovaries were incubated with primary antibodies overnight at 4°C, and then with secondary antibodies for one hour. The ovaries were separated onto slides in 50% glycerol. The following antibodies were used at the designated dilutions: rabbit anti-vasa was used at 1:1000, mouse anti Adducin-like (1B1) was used at 1:20 (Hybridoma bank), mouse anti-HA monoclonal (Sigma) were used at a 1:10 dilution, mouse anti-p53 (17) were used at a 1:1000 dilution and rabbit anti-PH3 antibody (Upstate Biotech) was used at 1:1000 dilution. Cy2-, Cy3- and Cy5- conjugated secondary antibodies (Jackson Laboratories) were each used in 1:100 dilutions. For DNA staining, Hoechst stain (Molecular Probes) was used at a 1 µg/ml. Larval ovaries from GFP-LC3 flies were stained with rabbit anti-GFP (Santa-Cruz) at 1:100 dilution. Egg chambers were photographed using a Zeiss LSM510 laser-scanning confocal microscope. The TUNEL assay was performed as described (33) using the ApopTag Fluorescein Direct In Situ Apoptosis Detection Kit (Chemicon International). LysoTracker (Invitrogen) labeling was carried out as described (34). TUNEL and LysoTracker stained egg chambers were mounted in Vectashield with DAPI (Vector Labs) and observed on an Olympus DSU spinning disc microscope or Olympus FluoView® FV10i.
Scanning Electron Microscopy
Adult Drosophila were fixed and dehydrated by immersion in increasing concentrations of alcohol (25%, 50%, 75%, 2x100% each for 10 minutes). The samples were then completely dehydrated using increasing concentrations of hexamethyldisilazane (HMDS) in alcohol (50%, 75%, 2x100%, each for 2 hours), air dried overnight, placed upon stubs, coated with gold, and examined with a scanning electron microscope (JEOL model JSM-5610LV).
Results
Over-expression of Dmp53, but not DmChk2, leads to ovarian stem cell loss and over expression of DmChk2, but not Dmp53, leads to mid-oogenesis cell death
To study the effect of overexpression of DmChk2 and Dmp53 in vivo we used the UAS-Gal4 binary system (35) in which ubiquitous or tissue-specific expression can be induced. We cloned the full length DmChk2 and Dmp53 into the pUASp vector allowing expression in both somatic and germline tissues (36). To test the functionality of these constructs we determined whether expression of DmChk2 could enhance Dmp53-mediated apoptosis in the eye (17–18). We found that overexpression of Dmp53 in the eye using GMR-Gal4 resulted in a reduced eye size with partial fusion of the ommatidia and some remaining bristles due to apoptosis (Fig. S1 C). Expression of DmChk2 in the Drosophila eye had no effect on eye morphology (Fig. S1 B). Co-expression of DmChk2 and Dmp53 resulted in a considerably more severe phenotype with almost complete loss of the eye compared to flies expressing Dmp53 alone (Fig S1 D). Thus, similar to previous findings (17–18), we found that Dmp53-induced apoptosis was enhanced by DmChk2 expression, showing that DmChk2 regulates Dmp53 mediated apoptosis. Thus, our DmChk2 and Dmp53 constructs and transgenic flies are functional and suitable for further investigation of the role of DmChk2 and Dmp53 during oogenesis.
To test the role of DmChk2 and Dmp53 in apoptosis during oogenesis we expressed these proteins in the Drosophila germline. We found that females over-expressing Dmp53 by nanos-Gal4VP16, which drives expression during ovarian stem cell development and also during oogenesis (37), are agametic with rudimentary ovaries probably due to loss of ovarian stem cells (Fig. 1B). We showed that over-expression of DmChk2 with the same driver had no effect on stem cell development but led to massive cell death of stage 8/9 egg chambers in mid-oogenesis (56%, n=456) (Fig. 1C), visualized by nurse cell DNA condensation. These results show ovarian germline stem cells are sensitive to the level of Dmp53 but not of DmChk2.
Figure 1. Effects of Dmp53 and DmChk2 over-expression using nanos-Gal4VP16 in germline.
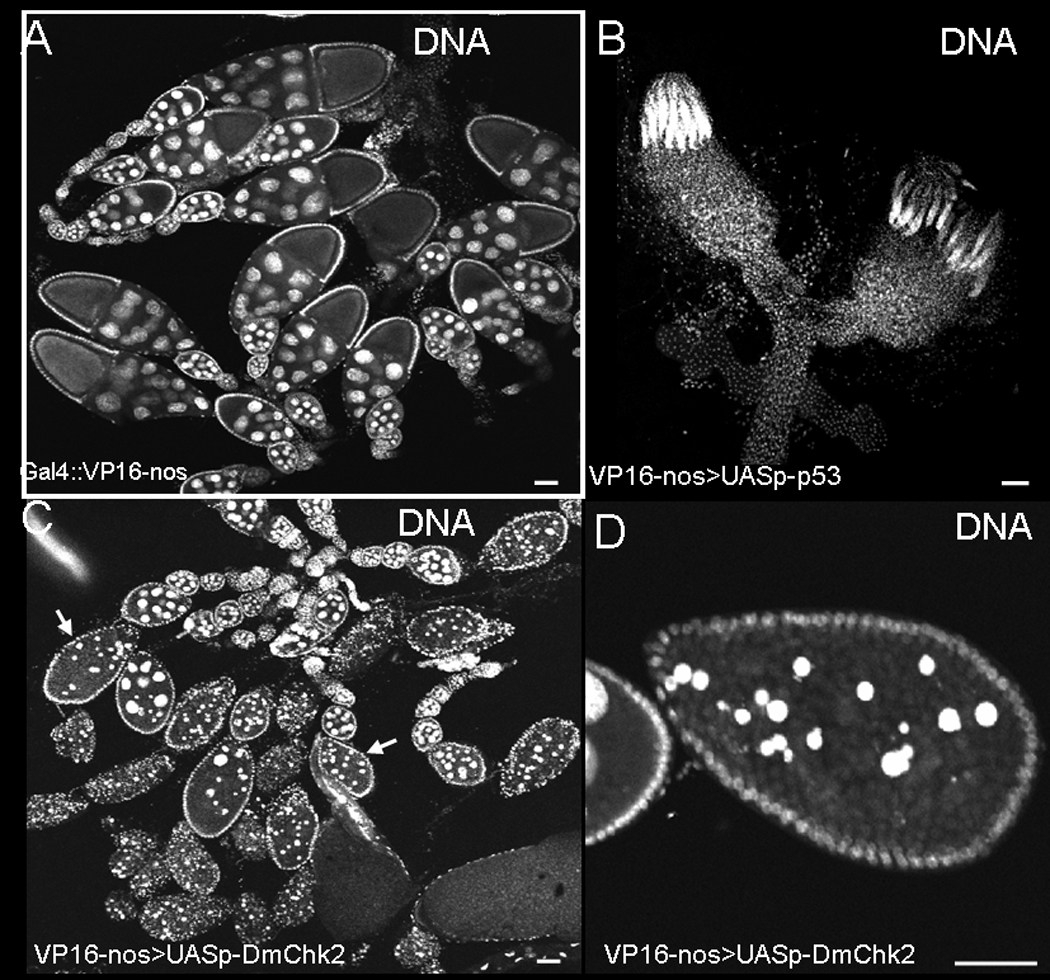
Egg chambers were stained with Hoechst. A) nanos-Gal4VP16 Drosophila ovaries. B) Over-expression of Dmp53 in the germline using the above Gal4 driver resulted in small ovaries probably due to loss of germline stem cells. C) Over-expression of DmChk2 in the germline leads to massive egg chamber death; degenerating egg chambers are indicated by arrows. D) Closer examination of a degenerating egg chamber over-expressing DmChk2. Scale bar is 50µm.
Since expression of Dmp53 using the nanos-Gal4VP16 driver affected stem cell development, we could not use this driver for studying the role of Dmp53 during mid-oogenesis. To test the role of DmChk2 and Dmp53 specifically during mid-oogenesis we decided to use the P{matalpha4-GAL-VP16}V2H flies which drives expression during mid to late oogenesis and is loaded into eggs. We found that over-expression of DmChk2 in germ cells using the above driver caused cell death of stage 8/9 egg chambers (52%, n=378) (Fig. 2B), as again was evident by nurse cell DNA condensation. Most importantly, we found that over-expression of Dmp53 in the germ line using this driver had no effect on survival of the germline (Fig. 2A). To check the expression of Dmp53 we stained the ovaries with p53 monoclonal specific antibody (18) and found that p53 expressed in the ovaries and is localized to the cell nucleus (data not shown). To verify these results we repeated these experiments with all of our transgenic lines (DmChk2 and Dmp53) and found similar results. These results suggest that over-expression of DmChk2, but not Dmp53, is sufficient for induction of cell death during mid-oogenesis.
Figure 2. Effects of Dmp53 and DmChk2 over-expression using P{matalpha4-GAL-VP16} V2H in the germline.
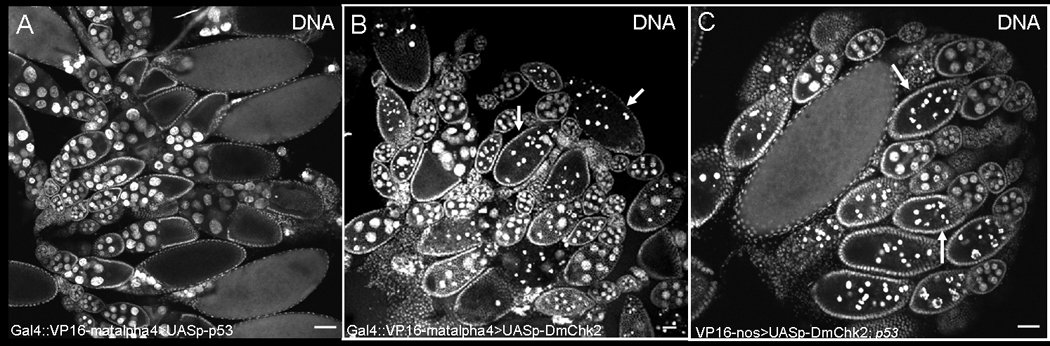
A) DNA staining of egg chambers from P{matalpha4-GAL-VP16}V2H:UASp-p53 fly. B) DNA staining of egg chambers from P{matalpha4-GAL-VP16}V2H : UASp-HA-Chk2 fly. Degenerating egg chambers of stages 8–9 are indicated by arrows. C) DNA staining of egg chambers from P{matalpha4-GAL-VP16}V2H/ UASp-HA-Chk2; p53 flies. Degenerating egg chambers of stages 8–9 are indicated by arrows. Scale bar is 50µm.
Mid-oogenesis DmChk2-mediated cell death is not suppressed by mutation in Dmp53
The up-regulation of Dmp53 under apoptotic conditions and the finding that Dmchk2 regulates irradiation-induced, Dmp53-mediated apoptosis in Drosophila (17) suggested that DmChk2 may also regulate p53 during mid-oogenesis cell death. Thus, we studied the ability of Dmp53 mutants to suppress Chk2-induced cell death and found that mutations in Dmp53 did not block DmChk2-dependent cell death (Fig. 2C). Ovaries from flies over-expressing DmChk2 in the germline showed 52% degenerating stage 8/9 egg chambers (n=129), and the level of cell death of egg chambers was similar (54%, n=86) in Dmp53 mutant background. These results indicate that cell death caused by DmChk2 during mid-oogenesis is Dmp53 independent.
Over expression of Dmp53 but not of DmChk2 in the somatic follicle cells leads to egg chamber cell death
It was shown that induction of egg chamber apoptosis in Drosophila could be achieved not only in the germline but also in the follicle cell layer (38). Thus, we decided to check whether expression of either Dmp53 or DmChk2 in the follicle cell layer could also induce death of the egg chamber. We found that whereas over-expression of DmChk2 using several follicle cell specific Gal4 drivers had no effect on either follicle or germline cell survival (Fig. 3B); expression of Dmp53 led to the degeneration of mid-oogenesis egg chambers (Fig. 3C). These results suggest that DmChk2 induced cell death is germline-specific but Dmp53 could induce cell death in the somatic follicle cells.
Figure 3. Over-expression of Dmp53 but not of DmChk2 in the follicle somatic cells using GR1-Gal4 driver induced germline cell death.
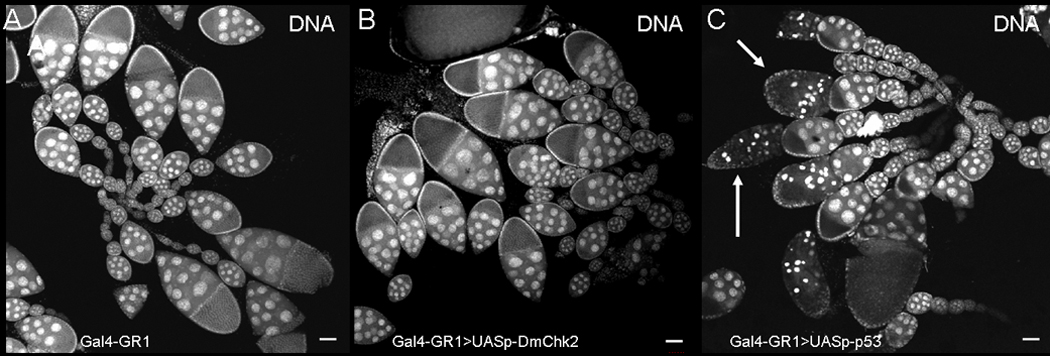
Egg chambers were stained with Hoechst. A) Egg chambers from GR1-GAL4 flies. B) Expressing DmChk2 in the follicle cell does not affect egg chamber survival. C) Expressing Dmp53 in the follicle cells lead to egg chamber death (marked with arrows).
Stem cell loss by Dmp53 and mid-oogenesis cell death mediated by DmChk2 are not suppressed by over-expression of caspase inhibitors
To determine whether cell death occurring during mid-oogenesis in flies over-expressing DmChk2 in the germline was dependent only on caspase activity, we co-expressed the caspase inhibitor, DIAP1, in the germline along with DmChk2. Expression of pUASp –DIAP1 (Fig. 4A) did not suppress mid-oogenesis DmChk2 mediated cell death (63%, n=150). Since DIAP1 and another caspase inhibitor, p35, may inhibit different caspases (39), we decided to check whether expression of other caspase inhibitors, p35 or p49 (40–41), could suppress DmChk2 mid-oogenesis cell death. We found that overexpression of p35 (Fig. 4B, 51%, n=87) or p49 (Fig. 4C, 55%, n=98) did not affect cell death caused by DmChk2. These results suggest that DmChk2 expression leads to mid-oogenesis cell death that is not dependent on caspase activity.
Figure 4. DmChk2-mid oogenesis cell death is caspase-independent.
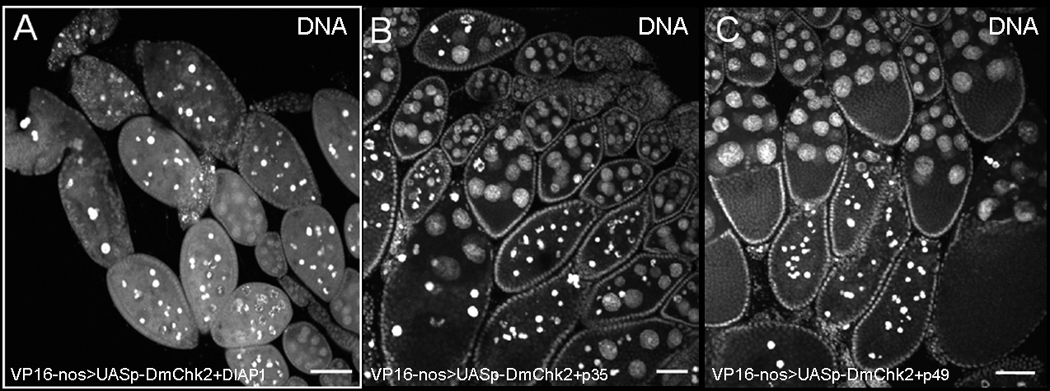
Egg chambers were stained with Hoechst. A) Egg chambers from flies over-expressing DmChk2 and DIAP1 undergo cell death similar to egg chambers expressing DmChk2 alone. Over-expressing p35 (B) or p49 (C) does not suppress DmChk2-mid oogenesis cell death. Scale bar is 50µm.
Next we analyzed whether ovarian stem cell loss is dependent on caspase activity by expressing either DIAP1, p35 or p49 (Fig. 5) together with Dmp53. We found that similar to DmChk2, these caspase inhibitors did not suppress Dmp53 ovarian stem cell loss (Fig. 5). Thus, our results show that the cell death effects of over-expression of Dmp53 and DmChk2 in the germline do not require caspase activity.
Figure 5. Expression of caspase inhibitors do not suppress p53-mediated stem cell loss.
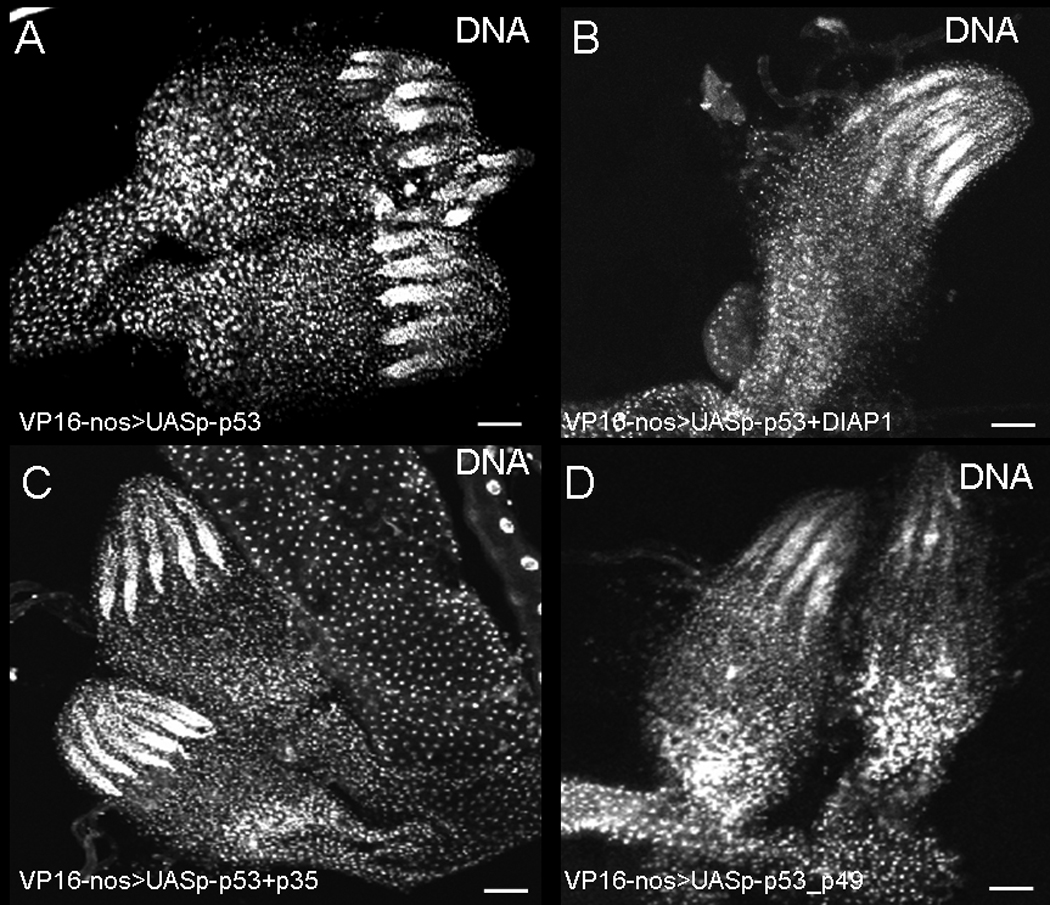
Ovaries were stained with Hoechst. A) ovaries from nanos-Gal4VP16; pUASp53. Expression of p53 led to loss of stem cells. Over-expressing DIAP1 (B) or p35 (C) or p49 (D) does not suppress Dmp53-stem cell loss.
Expression of p53 lead to loss of PGC's during larval development
We showed that expression of Dmp53 led to loss of ovarian stem cells which was not suppressed by expression of caspase inhibitors. To determine how these stem cells were lost, we studied the effects of Dmp53 on PGC's development. First we analyzed whether expression of Dmp53 affects PGC's survival. We found that larval gonads from flies expressing Dmp53 had significantly lower numbers of PGC's (number of PGC's= 36±20; Fig. 6B) compared to wild type (number of PGC's=97±10, Fig. 6A), as revealed by using Vasa antibody as a marker for germ cells. Thus, our results showed that expression of Dmp53 led to loss of PGC's.
Figure 6. Expression of Dmp53 in the germline lead to loss of primordial germ cells (PGC's).
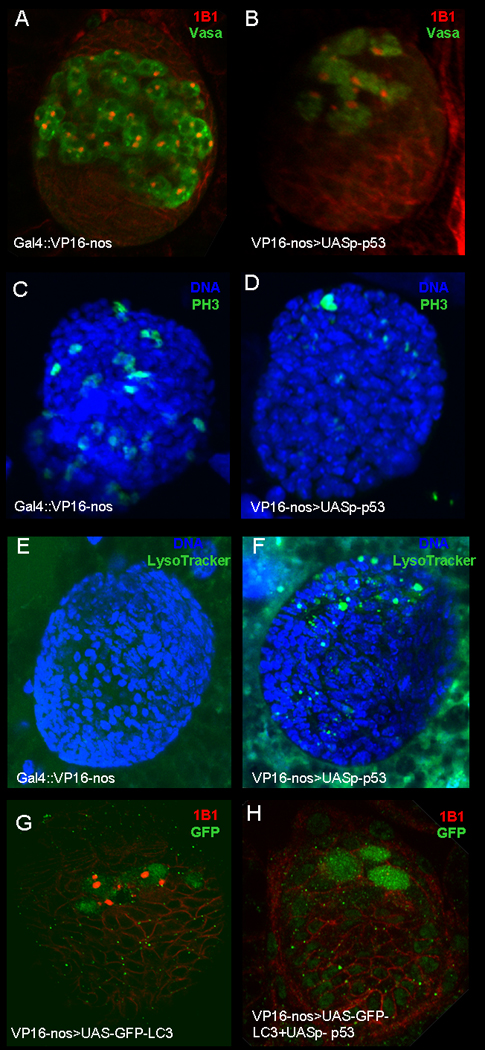
Gonads from third instar larvae were stained with germ cell marker, vasa (A–B; Green), anti-Adducin-like antibodies (A–B and G–H; Red), anti-phospho-histone 3 (C–D, Green), Lysotracker (E–F, Green), anti-GFP (G–H) and DAPI (C–F, Blue). A) nanos-Gal4VP16 ovary. B) Ovary from nanos-Gal4VP16; UASp- p53 fly, over-expressing of Dmp53 lead to loss pf PGC's. C) nanos-Gal4VP16. D) Ovary from nanos-Gal4VP16; UASp- p53 fly, over-expressing of Dmp53 lead to decrease in numbers of proliferating PGC's. E) Ovary from nanos-Gal4VP16. F) Ovary from nanos-Gal4VP16; UASp- p53 fly, LysoTracker positive puncta (arrows) have begun to accumulate in the ovary. G) Ovary from nanos-Gal4VP16; GFP-LC3 stained with anti-GFP. H) Ovary from nanos-Gal4VP16/UASp-p53; GFP-LC3 stained with anti-GFP.
Next, we studied whether over-expression of Dmp53 in the germline affected PGC proliferation by staining the ovaries for a mitotic marker, phosphorylated histone-3 (PH3). In ovaries from Dmp53 over-expression flies (Fig 6D) we detected a significantly lower number of PGC stained for anti- PH3 as compared to wild type (Fig. 6C).
We also analyzed whether loss of PGC's is accompanied by increased apoptosis. To accomplish this, we stained ovaries from flies over-expressing Dmp53 with terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick end labeling (TUNEL). We were unable to detect positive TUNEL staining in these ovaries (data not shown). Since we were unable to detect TUNEL staining in ovaries expressing Dmp53, we checked whether loss of PGC's by Dmp53 led to other forms of cell death, such as autophagic cell death. We used LysoTracker (LT) Red, which labels lysosomes and autolysosomes, and GFP-LC3 as a reporter for autophagy (42). We found that larval ovaries from flies expressing Dmp53 in the germline showed a significant increase in LT positive dots in the PGC area (Fig. 6F, number of ovaries= 28) as compared to wild type larval ovaries (Fig. 6E, number of ovaries=30). However, when we tested for activation of autophagy by p53 in the ovaries using the GFP-LC3 marker, we found that larval ovaries from flies expressing p53 with GFP-LC3 (Fig. 6H) had diffuse GFP-LC3 staining as in ovaries expressing GFP-LC3 alone (Fig. 6G), suggesting that expression of p53 did not led to activation of autophagy.
Mid-oogenesis DmChk2-mediated cell death is characterized by DNA fragmentation
Next we examined DNA fragmentation in DmChk2 over-expressing egg chambers using TUNEL. As previously shown, we found that 84% of degenerating mid-stage egg chambers from nutrient deprived females (Fig. 7A) stained positively for TUNEL (n=108 egg chambers with highly condensed nurse cell chromatin). Similarly, 82% of degenerating egg chambers were TUNEL-positive in DmChk2 (Fig. 7E) over-expression females (n=94). Our results suggest that over-expression of DmChk2 leads to both chromatin condensation and DNA fragmentation.
Figure 7. DmChk2-mediated cell death is characterized by DNA fragmentation.

All egg chambers were stained with DAPI (Blue) and TUNEL (Green). A) A control degenerating egg chamber from a nutrient deprived fly has condensed and fragmented nurse cell nuclei. B) TUNEL-positive puncta are detected in the same egg chamber. C) Merge of A–B. D) A degenerating egg chamber from a female over-expressing DmChk2 has condensed and fragmented nurse cell nuclei. E) The degenerating egg chamber over-expressing DmChk2 also has TUNEL-positive puncta. F) Merge of D–E. Scale bar is 50µm. All images were taken at the same magnification.
Mid-oogenesis DmChk2-mediated cell death shows delayed autophagy induction
It has previously been shown that autophagy is induced during mid-oogenesis cell death following nutrient deprivation (43–44). Thus, we asked whether over-expression of DmChk2 in the germline also regulated autophagy in mid-stage egg chambers using LT. As previously shown (43–44), degenerating egg chambers of wild-type nutrient-deprived flies displayed increasing amounts of LT staining as the nurse cell nuclei became condensed and began to fragment (Fig. 8A–C). In flies over-expressing DmChk2, LT staining was seen much later during degeneration, when the nurse cell nuclei had fragmented into tiny pieces (Fig. 8D–F). To quantify this delay in lysosome increase, we staged degenerating egg chambers carefully with respect to the degree of nuclear condensation and fragmentation. While 100% (n=11) of control NGT;nosGAL4 egg chambers at stage 8–9 showing fragmented nurse cell nuclei were LT-positive, only 20% (n=62) of DmChk2-expressing egg chambers were LT-positive at that stage. At later stages of degeneration, when nurse cell nuclei were largely degraded, 100% (n=14) of control egg chambers and 96% (n=28) of DmChk2-expressing egg chambers were LT-positive. Because autophagy mutants have been shown to partially disrupt normal mid-oogenesis cell death (43–44), we tested whether DmChk2-mediated cell death required the autophagy machinery. We found that a mutation in Atg7 did not suppress DmChk2-mediated cell death (data not shown). Taken together, these findings indicate that DmChk2 does not induce autophagic cell death.
Figure 8. Lysosome activity is delayed in flies over-expressing DmChk2.
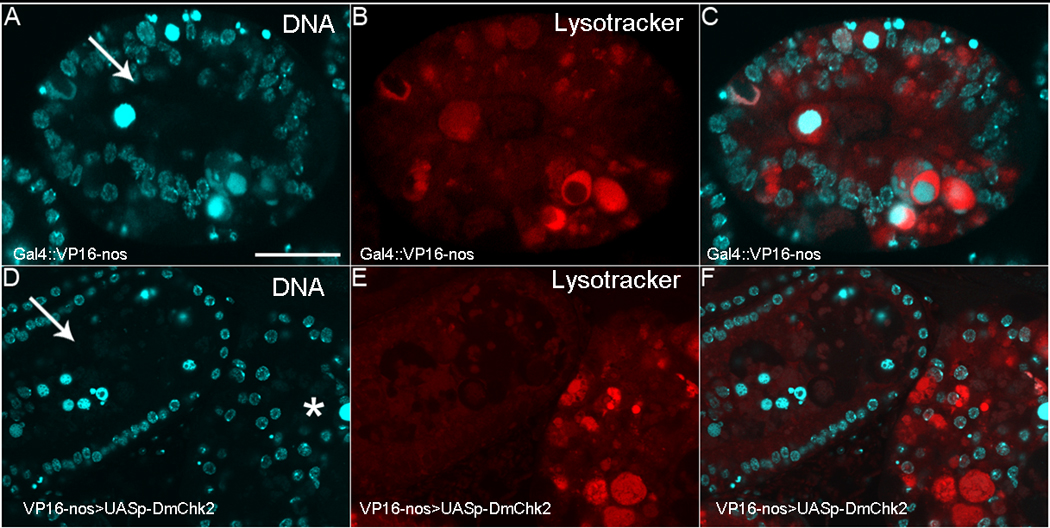
All egg chambers were stained with DAPI (Blue) and LysoTracker (Red). A) Control NGT;nosGAL4 egg chamber from a nutrient deprived fly with condensed nurse cell nuclei that have fragmented. B) LysoTracker positive puncta have begun to accumulate in this egg chamber. C) Merge of A–B. D) Egg chambers over-expressing DmChk2 at different stages of degeneration (arrow, middle and *, late) E) LysoTracker staining can only be seen in the later degenerating egg chamber (*). F) Merge of D–E. Scale bar is 20µm. All egg chambers were taken at the same magnification.
Discussion
In this study we focused on analyzing the role of DmChk2 and Dmp53, apoptosis-inducing factors during oogenesis in Drosophila. We demonstrated, for the first time in Drosophila, that expression of DmChk2 alone induced a tissue specific massive cell death; expression of DmChk2 in the germline had a specific cell death effect on mid-oogenesis egg chambers but not on ovarian stem cells. In support of these results, it was shown that the level of DmChk2 transcripts increase in response to nutrient deprivation that leads to mid-oogenesis cell death (45), suggesting an important role of DmChk2 in the induction of cell death in response to starvation. In contrast to DmChk2, expression of Dmp53, a Chk2-dependent effector protein, in the germline, affected stem cell survival but not mid-oogenesis egg chambers. Thus, to that end it seems that the only tissue that is refractory to DmChk2 but not to Dmp53 levels are mid-oogenesis egg chambers. Moreover, DmChk2 mid-oogenesis induced cell death is not dependent on Dmp53. Thus, our study reveals in Drosophila a novel role of DmChk2 in inducing cell death in a Dmp53-independent manner.
Surprisingly, egg chamber cell death induced by expressing DmChk2 was not prevented by co-expression of caspase inhibitors. Two other major forms of cell death are autophagic cell death and necrosis (46). We found that DmChk2-induced cell death was associated with a delay in autophagy induction and occurred normally in an Atg7 mutant background, indicating that Chk2-induced cell death is not occurring by autophagic cell death. Further, the cell death observed was not morphologically necrotic, and looked indistinguishable from apoptosis. Taken together, Chk2-induced cell death appears to occur by a caspase-independent apoptosis-like death.
We found that over-expression of Dmp53 led to loss of ovarian stem cells. It was previously shown that during gonad formation, Dmp53 is required for programmed cell death of PGC's (14). In Dmp53 mutant, an excess of PGC's were found ectopic of the gonads. The role of Dmp53 in this process was found to be due to germ cell death but not of PGC's migration. Interestingly enough, in contrast to our results, it was reported that p53 expression in wild-type embryos using the same Gal4 line used by us (nanos-Gal4VP16) did not affect survival of PGCs in the gonads (14). The difference between our results and the previous findings is likely due to the use of different pUAS vectors. In this study, we express Dmp53 using the pUASp vector, which allow expression in both somatic and germline tissues (36), whereas Yamada et al. expressed Dmp53 in the pUAST vector which drives expression only in somatic cells.
We demonstrated that loss of ovarian stem cell by Dmp53 was not suppressed by expression of caspase inhibitors. Moreover, we were unable to detect DNA fragmentation by TUNEL in PGC's from larval ovaries expressing Dmp53. A similar type of cell death was seen with overexpression of lipid phosphate phosphatases Wun or Wun2 in somatic tissues (47–51). Wun/Wun2- induced germ cell death is not affected by the expression of the inhibitor-of-apoptosis proteins. Moreover, cells dying in response to Wun/Wun2-mediated signals were negative for TUNEL staining and did not label for another marker of apoptosis, cleaved caspase 3. Thus, Dmp53 andWun/Wun2 induces cell death of the PGC's in a caspase independent manner. To check what other cell death mechanism led to the loss of PGC's by Dmp53, we stained larval ovaries with the lysosomal marker, LT and autophagy marker, GFP-LC3. Using LT and GFP-LC3 staining we detect that activation of a lysosomic process is accompanying the loss of PGC's by Dmp53. Thus, our results revealed that lysosomal cell death may contribute to loss of PGC's by Dmp53.
Supplementary Material
(A–D) Scanning electron micrographs of eyes from GMR-Gal4 flies crossed to a wildtype fly (A), UASp-DmChk2 fly (B), UASp-Dmp53 fly (C), or UASp-Dmp53 UASp-DmChk2 fly (D). Expression of DmChk2 in the Drosophila eye had no effect on eye morphology (Fig. 1B). We found that overexpression of Dmp53 in the eye using GMR-Gal4 result in a reduced eye size with partial fusion of the ommatidia and some remaining bristles due to apoptosis (Fig. 1C). Co-expression of DmChk2 and Dmp53 resulted in a considerably more severe phenotype with almost complete loss of the eye compared to flies expressing Dmp53 alone (Fig 1D). We repeated these experiments using all of our transgenic flies and found similar phenotypes.
Acknowledgments
We thank Trudi Schüpbach, Andreas Bergmann, Thomas Neufeld, Michael Brodsky and the Bloomington stock center for generously providing fly strains and reagents. This research was supported by Israel Cancer association grant (to U.A) and NIH R01 GM60574 (to K.M.).
References
- 1.Stracker TH, Usui T, Petrini JH. Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (Amst) 2009;8:1047–1054. doi: 10.1016/j.dnarep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Gilkes DM, Pan Y, Lane WS, Chen J. ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 2005;24:3411–3422. doi: 10.1038/sj.emboj.7600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBron C, Chen L, Gilkes DM, Chen J. Regulation of MDMX nuclear import and degradation by Chk2 and 14-3-3. EMBO J. 2006;25:1196–1206. doi: 10.1038/sj.emboj.7601032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereg SY, Lam A, Teunisse S, Biton E, Meulmeester L, Mittelman G, et al. Differential roles of ATM- and Chk2-mediated phosphorylations of Hdmx in response to DNA damage. Mol Cell Biol. 2006;26:6819–6831. doi: 10.1128/MCB.00562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Kuo C, Bisi JE, Kim MK. PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat Cell Biol. 2002;4:865–870. doi: 10.1038/ncb869. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 9.Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S, et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell. 2000;101:91–101. doi: 10.1016/S0092-8674(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 10.Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A, et al. Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:7301–7306. doi: 10.1073/pnas.97.13.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y, Lee TV, Xu D, Chen Z, Lamblin AF, Steller H, et al. Dual roles of Drosophila p53 in cell death and cell differentiation. Cell Death Differ. 2010;17:912–921. doi: 10.1038/cdd.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer JH, Chang C, Morris SN, Hozier S, Andersen S, Waitzman JS, et al. Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling. Proc Natl Acad Sci U S A. 2007;104:13355–13360. doi: 10.1073/pnas.0706121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada Y, Davis KD, Coffman CR. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the Outsiders monocarboxylate transporter. Development. 2008;135:207–216. doi: 10.1242/dev.010389. [DOI] [PubMed] [Google Scholar]
- 15.Lu WJ, Chapo J, Roig I, Abrams JM. Meiotic recombination provokes functional activation of the p53 regulatory network. Science. 2010;328:1278–1281. doi: 10.1126/science.1185640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Xin S, Du W. Drosophila Chk2 is required for DNA damage-mediated cell cycle arrest and apoptosis. FEBS Lett. 2001;508:394–398. doi: 10.1016/s0014-5793(01)03103-9. [DOI] [PubMed] [Google Scholar]
- 17.Peters M, DeLuca C, Hirao A, Stambolic V, Potter J, et al. Chk2 regulates irradiation-induced, p53-mediated apoptosis in Drosophila. Proc Natl Acad Sci U S A. 2002;99:11305–11310. doi: 10.1073/pnas.172382899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, et al. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Du W. Drosophila chk2 plays an important role in a mitotic checkpoint in syncytial embryos. FEBS Lett. 2003;545:209–212. doi: 10.1016/s0014-5793(03)00536-2. [DOI] [PubMed] [Google Scholar]
- 20.Masrouha N, Yang L, Hijal S, Larochelle S, Suter B. The Drosophila chk2 gene loki is essential for embryonic DNA double-strand-break checkpoints induced in S phase or G2. Genetics. 2003;163:973–982. doi: 10.1093/genetics/163.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takada S, Kelkar A, Theurkauf WE. Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell. 2003;113:87–99. doi: 10.1016/s0092-8674(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 22.Abdu U, Brodsky M, Schupbach T. Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol. 2002;12:1645–1651. doi: 10.1016/s0960-9822(02)01165-x. [DOI] [PubMed] [Google Scholar]
- 23.Staeva-Vieira E, Yoo S, Lehmann R. An essential role of DmRad51/SpnA in DNA repair and meiotic checkpoint control. EMBO J. 2003;22:5863–5874. doi: 10.1093/emboj/cdg564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klattenhoff C, Bratu DP, McGinnis-Schultz N, Koppetsch BS, Cook HA, Theurkauf WE. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell. 2007;12:45–55. doi: 10.1016/j.devcel.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Pane A, Schüpbach T. cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint Activation in Drosophila. Current Biology. 2007;17:1–6. doi: 10.1016/j.cub.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pane A, Wehr K, Schüpbach T. zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev Cell. 2007;12:851–862. doi: 10.1016/j.devcel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rong YS, Titen SW, Xie HB, Golic MM, Bastiani M, Bandyopadhyay P, et al. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juhász G, Erdi B, Sass M, Neufeld TP. atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007;21:3061–3066. doi: 10.1101/gad.1600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson JS, Barkett M, McCall K. Stage-specific regulation of caspase activity in Drosophila oogenesis. Dev Biol. 2003;260:113–123. doi: 10.1016/s0012-1606(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 30.Queenan AM, Ghabrial A, Schüpbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124(19):3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- 31.Cox RT, Spradling AC. A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development. 2003;130:1579–1590. doi: 10.1242/dev.00365. [DOI] [PubMed] [Google Scholar]
- 32.Spradling AC. P element-mediated transformation. In: Roberts DB, editor. Drosophila - A Practical Approach. Oxford: IRL Press; 1986. pp. 175–197. [Google Scholar]
- 33.McCall K, Pritchett TL, Peterson JS. Erhardt P, Toth A, editors. Detection of cell death in Drosophila. Apoptosis: Methods and Protocols, 2nd Ed. Methods in Molecular Biology. 2009;559:343–356. doi: 10.1007/978-1-60327-017-5_24. [DOI] [PubMed] [Google Scholar]
- 34.Bass BP, Tanner EA, Mateos San Martín D, Blute T, Kinser RD, et al. Cell-autonomous requirement for DNaseII in non-apoptotic cell death. Cell Death Differ. 2009;16:1362–1371. doi: 10.1038/cdd.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 36.Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 37.Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- 38.Chao S, Nagoshi RN. Induction of apoptosis in the germline and follicle layer of Drosophila egg chambers. Mech Dev. 1999;88:159–172. doi: 10.1016/s0925-4773(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 39.Baum JS, Arama E, Steller H, McCall K. The Drosophila caspases strica and dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ. 2007;14:1508–1517. doi: 10.1038/sj.cdd.4402155. [DOI] [PubMed] [Google Scholar]
- 40.Zoog SJ, Schiller JJ, Wetter JA, Chejanovsky N, Friesen PD. Baculovirus apoptotic suppressor P49 is a substrate inhibitor of initiator caspases resistant to P35 in vivo. EMBO J. 2002;21:5130–5140. doi: 10.1093/emboj/cdf520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jabbour AM, Ekert PG, Coulson EJ, Knight MJ, Ashley DM, Hawkins CJ. The p35 relative, p49, inhibits mammalian and Drosophila caspases including DRONC and protects against apoptosis. Cell Death Differ. 2002;9:1311–1320. doi: 10.1038/sj.cdd.4401135. [DOI] [PubMed] [Google Scholar]
- 42.Rusten TE, Lindmo K, Juhasz G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev. Cell. 2004;7:179–192. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Hou YC, Chittaranjan S, Barbosa SG, McCall K, Gorski SM. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J Cell Biol. 2008;182:1127–1139. doi: 10.1083/jcb.200712091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nezis IP, Lamark T, Velentzas AD, Rusten TE, Bjorkoy G, Johansen T, et al. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy. 2009;5:298–302. doi: 10.4161/auto.5.3.7454. [DOI] [PubMed] [Google Scholar]
- 45.Terashima J, Bownes M. A microarray analysis of genes involved in relating egg production to nutritional intake in Drosophila melanogaster. Cell Death Differ. 2005;12:429–440. doi: 10.1038/sj.cdd.4401587. [DOI] [PubMed] [Google Scholar]
- 46.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starz-Gaiano M, Lehmann R. Moving towards the next generation. Mech Dev. 2001;105:5–18. doi: 10.1016/s0925-4773(01)00392-6. [DOI] [PubMed] [Google Scholar]
- 48.Burnett C, Howard K. Fly and mammalian lipid phosphate phosphatase isoforms differ in activity both in vitro and in vivo. EMBO Rep. 2003;4:793–799. doi: 10.1038/sj.embor.embor900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanyu-Nakamura K, Kobayashi S, Nakamura A. Germ cell-autonomous Wunen2 is required for germline development in Drosophila embryos. Development. 2004;131:4545–4553. doi: 10.1242/dev.01321. [DOI] [PubMed] [Google Scholar]
- 50.Renault AD, Sigal YJ, Morris AJ, Lehmann R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 2004;305:1963–1966. doi: 10.1126/science.1102421. [DOI] [PubMed] [Google Scholar]
- 51.Sano H, Renault AD, Lehmann R. Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J. Cell Biol. 2005;171:675–683. doi: 10.1083/jcb.200506038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–D) Scanning electron micrographs of eyes from GMR-Gal4 flies crossed to a wildtype fly (A), UASp-DmChk2 fly (B), UASp-Dmp53 fly (C), or UASp-Dmp53 UASp-DmChk2 fly (D). Expression of DmChk2 in the Drosophila eye had no effect on eye morphology (Fig. 1B). We found that overexpression of Dmp53 in the eye using GMR-Gal4 result in a reduced eye size with partial fusion of the ommatidia and some remaining bristles due to apoptosis (Fig. 1C). Co-expression of DmChk2 and Dmp53 resulted in a considerably more severe phenotype with almost complete loss of the eye compared to flies expressing Dmp53 alone (Fig 1D). We repeated these experiments using all of our transgenic flies and found similar phenotypes.


