Abstract
We report the case of a young man with a history of attention deficit/hyperactivity disorder and mild cognitive impairment who presented with chronic fatigue, anorexia and progressive darkening of the skin. On laboratory testing, severely depressed concentrations of morning cortisol, along with highly elevated values of adrenocorticotropic hormone (ACTH) revealed primary adrenal insufficiency as the primary cause of the patient’s symptomatology. Imaging of the brain showed altered signal intensities in the parieto-occipital regions of the brain. The demonstration of increased very long chain fatty acids (VLCFA) established the diagnosis of adolescent X-linked adrenoleukodystrophy (X-ALD). Presenting at an advanced yet slowly progressive stage the patient was not a suitable candidate for haematopoietic stem cell transplantation (HSCT), and treatment focused on hormone replacement therapy, family counselling and supportive care. On follow-up visits within the following year, fatigue had diminished and there was no evidence of progressive neurological deficits. However, exacerbation of the psychiatric symptomatology resulted in admittance to a psychiatric ward.
Background
X-linked adrenoleukodystrophy (X-ALD) is a peroxisomal disorder caused by a defect in the ABCD1 (ATP-binding cassette, subfamiliy D (ALD), member 1) gene, located at Xq28. For clinical reasons, several distinct phenotypes have been characterised. There are four main categories which include adrenoleukodystrophy (X-ALD), adrenomyeloneuropathy (AMN), Addison only, and asymptomatic.1 The ABCD1 gene encodes the ALD protein (ALDP) which serves as a half-sized peroxisomal transporter that undergoes dimerisation in order to shift very long chain fatty acids (VLCFA) into the peroxisome. Altered gene products of the ABCD1 protein may interfere with the transport of VLCFA into the peroxisome which in turn prevents VLCFA from β-oxidation and breakdown. Subsequently, VLCFA accumulate in the central nervous system, adrenal glands and testes.1,2 To date, more than 400 different mutations including missense (most prevalent), frameshift, insertion/deletion and splicing defects have been characterised (www.x-adl.nl).3 The recognition that no genotype–phenotype correlation could be identified led to the assumption that modifier genes exist. For example, the expression of BG1, a protein that activates VLCFA to VLCF-CoA, appears to correlate with the severity of the disease.4
The demyelinative process that is found in the cerebral hemispheres is the result of a vigorous inflammatory response which is characterised by dense infiltrates of perivascular CD8 cytotoxic T lymphocytes.5 Characterisation of the underlying pathology fostered the hypothesis that the autoimmune response represents a second hit that may be altered by therapeutic interventions such as haematopoietic stem cell transplantation.6 In addition to the inflammatory process described, a non-inflammatory distal axonopathy of the corticospinal tract is responsible for the progressive paraparesis in AMN.
The incidence has been estimated to be approximately 1:17000.7 As outlined in this case report, X-ALD may mimic common psychiatric disorders such as attention deficit/hyperactivity disorder. Furthermore, manifestations of ALD are often mistakenly attributed to other conditions such as multiple sclerosis or autoimmune adrenalitis.8 The diagnosis of X-ALD is commonly achieved by the demonstration of increased concentrations of VLCFA in the plasma. Additionally, genetic investigations are important to identify asymptomatic individuals.
This case report demonstrates that an increased level of awareness is essential when evaluating adolescents for symptoms of Addison’s disease or cognitive impairment. Apart from preventing overt adrenal crisis, early recognition is crucial since only boys presenting with mild symptoms represent potential candidates for bone marrow transplantation.9
Case presentation
A young man at the end of his teens was referred to our hospital because of suspected Addison’s disease. The patient had generalised darkening of the skin with hyperpigmentation in the creases of his palms and buccal mucosa. He had complained about progressive fatigue and decreased appetite for several months. He did not describe changes in his libido or hair growth and reported no gastrointestinal complaints. Furthermore, the patient did not have salt-craving or lightheadedness. During his adolescence, the patient was repeatedly evaluated for symptoms of distractibility, learning disabilities and disturbed personality. In addition, emotional liability and a history of visual hallucinations were described. Approximately 10 years before this evaluation, the patient was treated for suspected influenza A pneumonia and encephalitis at another hospital. Henceforth, the severe systemic infection with brain involvement served as a possible explanation for the psychiatric syndrome. When the patient was 15 years old, the diagnosis of attention deficit/hyperactivity disorder was made. One year before presenting at our hospital, mild cognitive impairment (verbal IQ 83, performance IQ 73) was diagnosed. An electroencephalogram (EEG) at the same time showed slowing over the right temporal lobe without epileptiform activity. However, there was no documented decline of cognitive functions. He smoked marihuana and drank alcohol socially. The patient was living in an institution for adolescents with psychosocial problems. The family history was hampered by the fact that the patient’s mother was raised in a foster family. From the history that could be obtained, no relatives with psychiatric, neurologic or endocrine disorder could be identified. On clinical examination, hyperpigmentation of the palmar crease and the buccal mucosa was noted. There was no orthostatic hypotension and no signs of altered mental status were seen. There was absent pubic hair growth (Tanner stage P0, G5). The testicular volume was estimated 15 ml bilaterally. Neurologic testing revealed a pathologic Romberg’s test and evidence of pallhypaesthesia in the lower extremities. The remainder of the examination was normal.
Investigations
On laboratory testing, serum concentrations for potassium (4.5 mmol/l, normal range 3.5–4.6 mmol/l), sodium (135 mmol/l, normal range 135–145 mmol/l) and calcium (albumin corrected, 2.2 mmol/l, reference range 2.25–2.7 mmol/l) were within normal limits, as were serum creatinine and a complete blood count. The measurement of the morning cortisol (morning cortisol <27.6 nmol/l, normal range 66.0–800.0 nmol/l) and adrenocorticotropin values (immeasurably high >275.3 pmol/l, normal <10.1 pmol/l)) revealed severe primary adrenal insufficiency. Adrenal antibodies (using Biognost indirect immunofluorescence test kit for the detection of human IgA, IgG and IgM antibodies against several adrenal antigens derived from monkeys) were negative. Analysis of steroid metabolites in the urine showed decreased concentrations of all measured androgen (including 17-OH pregnenolone, 11-0H androsterone, 16-OH-DHEA) and glucocorticoid metabolites, which rendered a specific defect in steroid synthesis unlikely. Furthermore, depressed testosterone concentrations were found (0.3 nmol/l, normal range 9.3–37.1 nmol/l) whereas the concentration for sex hormone binding globulin (SHBG) was slightly elevated (61.8 nmol/l, normal range 5.9–46.6 nmol/l). The testicular volume (15 ml bilaterally) was normal. Serum follicle stimulating hormone and luteinising hormone were within normal limits (1.8 U/l and 5.3 U/l, respectively), which pointed to a disturbed pituitary gonadotropin secretion, considering the low testosterone. Interestingly, cases of both hypogonadotropic and primary hypogandism are described in the literature.10,11 Completing the laboratory investigations, a mineralocorticoid status was performed that revealed normal values for aldosterone (125 pmol/l, normal 27–444 pmol/l) and renin (6.7 ng/l, normal 1.5–18 ng/l).
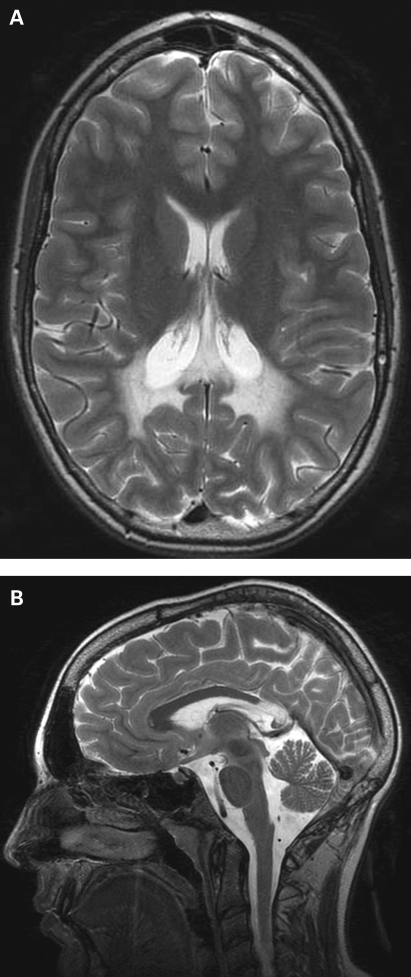
Magnetic resonance imaging (MRI) studies of the abdomen showed hypoplastic adrenal glands without structural abnormalities. On neuroimaging, symmetrical T2 weighted signal intensities without accumulation of contrast material in the parieto-occipital regions of the brain could be demonstrated (fig 1). There was no lesion of the hypothalamus or pituitary gland. Finally, increased concentrations of very long chain fatty acids in the plasma (C26:0 3.5 µmol/l (normal 0.3–1.9 µmol/l), C24:0/C22:0 ratio 1.52 (normal ratio 0.30–1.10), C26:0/C22:0 0.056 (normal ratio 0.002–0.025)) established the diagnosis of adolescent X-ALD. Clinical and laboratory evaluation of the patient’s mother was unremarkable. Because there were no additional relatives at risk we came to the conclusion that genetic investigations would not influence further clinical decisions.
Figure 1.
T2 weighted images of the brain showing characteristic periventricular hyperintensities in the parieto-occipital region of the brain (A) and atrophic changes of the splenium corpori callosi (B), yet no active inflammatory changes are appreciated following the application of contrast material.
Differential diagnosis
As demonstrated by the severely decreased morning cortisol, the primary cause of the patient’s symptoms and the clinical presentation was attributed to primary adrenal insufficiency. Since the patient was not taking any medication, autoimmune adrenalitis was considered. Negative adrenal autoantibodies argued against this diagnosis since they are positive in 70% of the cases.12 Because autoimmune adrenalitis in young patients commonly occurs in the setting of the autoimmune polyglandular syndrome type 1 (APS1) and cannot be entirely ruled out by antibody testing, evidence for hypoparathyroidism and mucocutaneous candidiasis was sought. However, normal values of serum calcium rendered hypoparathyroidism unlikely. In addition to that, there were no clinical signs of chronic mucocutaneous candidiasis identifiable.
Owing to the patient’s hypogonadism, we turned next to the question of whether congenital adrenal hypoplasia was responsible for the patient’s presentation. The X-linked forms of this disorder are caused by mutations in the DAX-1 gene (dosage sensitive sex reversal, adrenal hypoplasia gene on the X chromosome, gene 1). The DAX-1 gene is crucial for normal gonadal and adrenal development and plays an important role in the expression of several CYP450 steroidogenic enzymes. Patients with DAX-1 mutations typically present with signs and symptoms of salt wasting, along with glucocorticoid deficiency and hypogonadotropic hypogonadism.13 However, there was no clinical or laboratory evidence of mineralocorticoid deficiency.
Furthermore, a neoplastic process was considered, yet MRI studies of the abdomen ruled out structural abnormalities of the adrenal glands. Finally, results of the laboratory testing (normal white blood count 4.3×109/l, C reactive protein <5 mg/l) made a severe systemic infection such as tuberculosis unlikely. The neuroradiological studies, along with the psychiatric symptomatology, and the subtle neurologic deficits pointed to X-linked ALD as the unifying diagnosis. The demonstration of increased very long chain fatty acids confirmed the suspected neurodegenerative disorder.
Treatment
Glucocorticoid replacement therapy was initiated. Next, the implementation of dietary therapy with Lorenzo’s oil (4:1 mixture of glyceryl trioleate and glyceryl trierucate) that lowers VLCFA in the plasma was evaluated since it has been shown to exert a preventive effect in asymptomatic boys.14 However, there is evidence that dietary therapy with Lorenzo’s oil does not have a favourable effect on the rate of progression in patients who present at a symptomatic stage.15 Moreover, dietary therapy which restricts the content of VLCFA as a sole therapy is not successful.16 A trial observing biochemical and clinical effects on patients with X-ADL who were treated with lovastatin showed reduction of VLCFA concentrations.17 In our opinion, further clinical investigations are needed to confirm the evidence for treatment with lovastatin.
Subsequently, the indication for haematopoietic stem cell transplantation was considered. This therapy is regarded as the treatment of choice for patients presenting with mild cerebral involvement (mild cerebral changes on MRI, performance IQ >80).18 The underlying mechanism that alters disease progression is poorly understood. Probable determinants encompass immunosuppression along with replacement of microglia by donor derived cells.19
However, based on the neuroradiologic assessment (severity score according to Loes 11), the neuropsychological function and the functional level as a composite, it was concluded that the patient would not be a suitable candidate for haematopoietic stem cell transplantation (HSCT). Therefore, further therapy focused on hormone replacement therapy, supportive care, and family counselling.
Outcome and follow-up
On follow-up visits within the following year, fatigue had diminished and there was no evidence of progressive neurological deficits. Another MRI of the brain 6 months after the initial study appeared unchanged. However, exacerbation of the psychiatric symptomatology resulted in admittance to a psychiatric ward.
Discussion
This case report illustrates some of the challenges that may be encountered when diagnosing X-ALD. The underlying pathology of the chronic-progressive psychiatric symptomatology remained elusive until overt adrenal insufficiency prompted clinical reevaluation. With hindsight, there are several unusual features of this case that contributed to the delayed diagnosis. First, there was an atypically slow progression of the psychiatric symptomatology without overt neurologic deficits. Secondly, consistent with the remittent clinical course, no evidence of an ‘active’ intracerebral inflammation with breakdown of the blood–brain barrier was found on the MRI studies of the brain. Finally, the diagnosis of encephalitis served as an explanation for the cognitive disorder that was not questioned upon reevaluation.
Slow onset Addison’s disease is most commonly attributed to autoimmune adrenalitis. In a retrospective review of 86 cases of Addison’s disease in Nottingham, 81 (93%) were caused by autoimmune pathology and only three cases of adrenoleukodystrophy were identified.20 However, the prevalence in paediatric populations presenting with Addison’s disease may be higher. In a study conducted by Jorge et al, X-ALD was identified as the underlying disorder in five of 24 boys presenting with Addison’s disease.21
Little is known about the incidence of psychiatric symptoms in patients with newly diagnosed X-ALD. In a survey of 109 reported cases, Kitchin and colleagues showed that in patients with onset of cerebral X-ALD before 21 years of age, 16 of 69 (23%) presented exclusively with psychiatric problems, and 38 of 69 (55%) patients presented with some psychiatric problems. Among this cohort, dementia, learning difficulties and behavioral changes were the most prevalent presenting psychiatric problems.22 Furthermore, it is important to note that the risk of developing cerebral X-ALD peaks in childhood (the risk is estimated to be 40% at the age of 12 years and decreases thereafter steadily to become 10% after the age of 15).23
Learning points
X-ALD must be considered in young male patients with Addison’s disease or therapy refractory psychiatric symptoms; continuing psychiatric and somatic re-evaluation is crucial.
Yielding a prevalence comparable to other neurodegenerative diseases such as phenylketonuria, X-ALD may occur at a much higher prevalence (1:17000) than previously estimated.24
The clinical presentation of X-ALD is highly variable. Psychiatric symptoms and primary adrenal insufficiency are among the most common presenting features.
Diagnosis is achieved by the demonstration of elevated VLCFA in the plasma. MRI studies, along with neurologic and psychiatric evaluation, are important to determine the stage at the time of presentation. In addition to that, genetic studies should be offered for potential carriers.
To date, the only effective treatment is HSCT,25,26 and potential candidates are boys with mild cerebral disease.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Moser HW, Mahmoud A, Raymond GV. X-linked adrenoleukodystrophy. Nature Clinical Practice Neurology 2007; 3: 140–51 [DOI] [PubMed] [Google Scholar]
- 2.Moser HW, Moser AB, Smith KD, et al. Adrenoleukodystrophy: phenotypic variability and implications for therapy. J Inherit Metab Dis 1992; 15: 645–64 [DOI] [PubMed] [Google Scholar]
- 3.X-linked Adrenoleukodystrophy Database (www.x-ald.nl) [Google Scholar]
- 4.Asheuer M, Bieche I, Laurendeau I, et al. Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X-linked adrenoleukodystrophy. Human Molecular Genetics 2005; 14: 1293–303 [DOI] [PubMed] [Google Scholar]
- 5.Moser HW. Therapy of X-linked adrenoleukodystrophy. NeuroRx 2006; 3: 246–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmood A, Dubey P, Moser HW, et al. X-linked adrenoleukodystrophy: therapeutic approaches to distinct phenotypes. Pediatr Transplant 2005; 9: 55–62 [DOI] [PubMed] [Google Scholar]
- 7.Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol 2001; 49: 512–7 [PubMed] [Google Scholar]
- 8.Moser HW, Raymond GV, Dubey P. New approaches to a neurodegenerative disease. JAMA 2005; 294: 3131–4 [DOI] [PubMed] [Google Scholar]
- 9.Moser HW, Mahmoud A, Raymond GV. X-linked adrenoleukodystrophy. Nature Clinical Practice Neurology 2007; 3: 140–51 [DOI] [PubMed] [Google Scholar]
- 10.Frank K, Schrecker O, Brosi K, et al. Adrenomyeloneuropathy, a rare cause of primary adrenal cortex insufficiency. Dtsch Med Wochenschr 1986; 111: 1519–22 [DOI] [PubMed] [Google Scholar]
- 11.Vorgerd M, Fuchs S, Tegenthoff M, et al. A missense point mutation (Ser515Phe) in the adrenoleukodystrophy gene in a family with adrenomyeloneuropathy: a clinical, biochemical, and genetic study. J Neurol Neurosurg Psychiatry 1995; 58: 229–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335: 1206–12 [DOI] [PubMed] [Google Scholar]
- 13.Reutens AT, Achermann JC, Ito M, et al. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab 1999; 84: 504–11 [DOI] [PubMed] [Google Scholar]
- 14.Moser HW, Raymond GV, Lu SE, et al. Follow-up of 89 Lorenzo’s oil treated asymptomatic adrenoleukodystrophy patients. Arch Neurl 2005; 62: 1073–80 [DOI] [PubMed] [Google Scholar]
- 15.Moser HW. Therapy of X-linked adrenoleukodystrophy. NeuroRx 2006; 3: 246–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins PA, Naidu S, Moser HW: Adrenoleukodystrophy: biochemical procedures in diagnosis, prevention and treatment. J Inherit Metab Dis 1987; 10: 46–53 [DOI] [PubMed] [Google Scholar]
- 17.Pai GS, Khan M, Barbosa E, et al. Lovastatin therapy for X-linked adrenoleukodystrophy: clinical and biochemical observations in 12 patients. Mol Genet Metab 2000; 69: 312–22 [DOI] [PubMed] [Google Scholar]
- 18.Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 2004; 104: 881–8 [DOI] [PubMed] [Google Scholar]
- 19.Moser HW, Mahmoud A, Raymond GV. X-linked adrenoleukodystrophy. Nature Clinical Practice Neurology 2007; 3: 140–51 [DOI] [PubMed] [Google Scholar]
- 20.Kong MF, Jeffcoate W. Eigthy-six cases of Addison’s disease. Clin Endocrinol 1994; 41: 757–61 [DOI] [PubMed] [Google Scholar]
- 21.Jorge P, Quelhas D, Oliveira P, et al. X-linked adrenoleukodystrophy in patients with idiopathic Addison disease. Eur J Pediatr 1994; 153: 594–7 [DOI] [PubMed] [Google Scholar]
- 22.Kitchin W, Cohen-Cole SA, Mickel SF: Adrenoleukodystrophy: frequency of presentation as psychiatric disorder. Biol Psychiatry 1987; 22: 1375–87 [DOI] [PubMed] [Google Scholar]
- 23.Cox CS, Dubey P, Raymond GV, et al. Cognitive evaluation of neurologically asymptomatic boys with x-linked adrenoleukodystrophy. Arch Neurol 2006; 63: 69–73 [DOI] [PubMed] [Google Scholar]
- 24.Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol 2001; 49: 512–7 [PubMed] [Google Scholar]
- 25.Shapiro E, Krivit W, Lockman L, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet 2000; 356: 713–8 [DOI] [PubMed] [Google Scholar]
- 26.Peters C, Charnas LR, Tan Y, et al. Cerebral x-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 2004; 104: 881–8 [DOI] [PubMed] [Google Scholar]