Abstract
A 44-year-old woman who had recently been on immunosuppressive therapy presented with malaise, cough, fever, weight loss, lymphadenopathy, severe hypercalcaemia and a paratracheal mass on imaging. The initial impression was of disseminated malignancy, and lymphoma was suspected. A mediastinal biopsy showed a mycobacterial spindle cell pseudotumour containing acid and alcohol fast bacilli (AAFB). Sputum microscopy demonstrated AAFBs, confirmed as Mycobacterium tuberculosis complex by PCR. Prolonged culture grew Mycobacterium microti, an organism often associated with disease in small rodents and llamas. M microti isolates from postmortem samples of an alpaca at a nearby farm were genetically indistinguishable. Although the patient had not visited the farm, concurrent illness in her adopted stray cat suggested a possible zoonotic connection. The patient responded to antituberculous therapy, and rehydration and pamidronate for hypercalcaemia. We believe the hypercalcaemia was caused by a similar mechanism to raised calcium levels sometimes seen in tuberculosis.
Background
This case report is important for a several reasons:
Human disease caused by Mycobacterium microti is unusual.
Cases of mycobacterial spindle cell pseudotumour are also very rare, and to our knowledge this is the first reported case associated with M microti.
To our knowledge, hypercalcaemia associated with M microti infection has not previously been described.
Intriguingly, this patient lived close to a farm where an alpaca died of a genetically indistinguishable organism suggesting a possible zoonotic connection.
Case presentation
In early 2007, a 44-year-old woman presented with fever, cough and weight loss. Clinical examination revealed cervical lymphadenopathy and hepatosplenomegaly. She had recently taken azathioprine (2005–2006) for oral lichen planus and had received frequent courses of oral corticosteroids since 1984 for chronic active hepatitis.
Over the next 2 months her symptoms worsened and she developed fatigue, anorexia, night sweats, weight loss, sputum production and dyspnoea. Her renal function deteriorated with urea rising to 29.1 mmol/litre and creatinine to 560 µmol/litre. She also developed severe hypercalcaemia (4.34 mmol/litre adjusted). Serum phosphate was slightly raised (1.47 mmol/litre) while parathyroid hormone concentration was suppressed (<8 ng/litre). 25-OH vitamin D, serum angiotensin-converting enzyme and creatine kinase concentrations were normal. A myeloma screen was negative.
Investigations
Chest x-ray and CT of the thorax displayed a right paratracheal mass and suggested right brachiocephalic vein infiltration/compression. An abdominal CT scan confirmed hepatosplenomegaly and retroperitoneal lymphadenopathy. There was no evidence of malignancy demonstrated in bronchoscopic samples (brushings/washings from right upper lobe), fine-needle aspiration from a cervical lymph node, or a mediastinoscopic nodal biopsy. The latter two specimens displayed occasional non-necrotising granulomata. Blood tests showed a C-reactive protein of 106 mg/litre and a white cell count of 13.4×109 cells/litre. Lactate dehydrogenase concentration was normal. A HIV test was negative, no immunoglobulin deficiency was identified, complement levels were normal, and although CD4 count was within normal range (0.46×109 cells/litre), CD8 levels were reduced at 0.15×109 cells/litre.
A radionuclide bone scan was non-diagnostic of a cause for hypercalcaemia. Renal ultrasound identified no obstruction, and blood, urine and stool cultures showed no significant growth.
Sputum microscopy demonstrated numerous AAFBs confirmed as belonging to Mycobacterium tuberculosis complex by PCR. Later, prolonged sputum solid media culture demonstrated growth of M microti, sensitive to pyrazinamide, rifampicin, ethambutol and isoniazid.
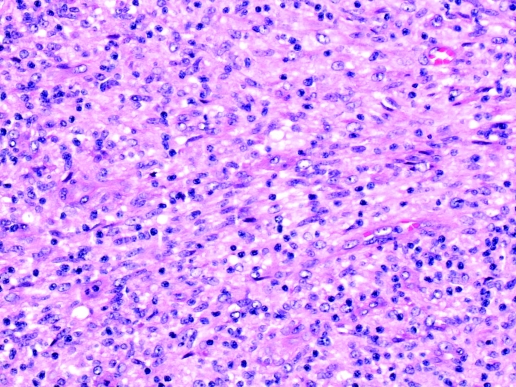
Biopsy obtained at mediastinotomy was macroscopically compatible with an enlarged lymph node. Microscopic appearances (figure 1) were of a multinodular proliferation of spindle cells with moderate numbers of interspersed small blood vessels, admixed with numerous lymphocytes and plasma cells. The lesion almost obliterated the nodal architecture leaving only a thin rim of normal residual lymphoid tissue. A few peripheral multinucleate giant cells and sparse aggregates of macrophages constituting poorly formed non-necrotic granulomata were identified. There was no evidence of malignancy.
Figure 1.
Histology of biopsy displaying mycobacterial spindle cell pseudotumour.
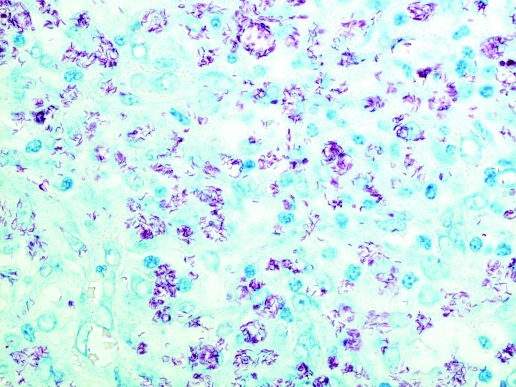
Spindle cells were CD68 positive on immunohistochemistry, confirming a macrophage-monocyte derived phenotype. Ziehl–Neelsen staining demonstrated numerous clusters of curved AAFB within spindle cells throughout the lesion (figure 2). Many cells showed cytoplasmic distension due to the large number of organisms. The histopathological appearances were those of a mycobacterial spindle cell pseudotumour (MSCP) and immunohistochemistry excluded other possibilities such as metastatic malignancies with a spindled morphology.
Figure 2.
Ziehl–Neelsen stained biopsy sample demonstrating numerous curved acid and alcohol fast bacilli.
In January 2007 a pregnant alpaca on a farm close to the patient’s residence developed diarrhoea and weight loss. Despite veterinary attention the alpaca’s condition deteriorated and she aborted on 19 March and died on 22 March 2007. A postmortem carried out by the Scottish Agricultural College showed both lungs were collapsed and oedematous containing a single abscess without evidence of pneumonia. In the abdomen there was a massive quantity of pale yellow fluid with extensive fibrin deposits arising from multifocal abscesses or tumour formation in the liver. These liver lesions contained central necrotic or inspissated areas and extended onto the serosal surface of the duodenum. Ziehl–Neelsen-stained smears of the liver lesions demonstrated numerous AAFBs resembling Mycobacterium spp. Genotyping confirmed the presence of M microti.
DNA was extracted from the human isolate at the Scottish Mycobacteria Reference Laboratory (SMRL), Edinburgh, UK and from the alpaca isolate at the Veterinary Laboratory Agency (VLA), Weybridge, UK. At SMRL spoligotyping was performed using a commercial assay available from Isogen Life Science (3600 BT; Maarssen, The Netherlands) and 15-locus Mycobacterial Interspersed Repetitive Unit-Variable Number Tandem Repeat (MIRU-VNTR) typing, performed using the current strategy for M tuberculosis.1 Spoligotyping and VNTR typing using six exact tandem repeat (ETR) loci (ETR-A to ETR-F) were performed on both isolates at VLA. The strains were indistinguishable by both methods. The spoligotype pattern was designated SB0112 by http://www.mbovis.org (VLA type 19). The 15-locus MIRU-VNTR profile was 123571 210414 23232 and the 6-locus ETR result was 12-3-5-7*-4-2.2. Both strains were deleted for RDmic, which identifies them as members of the M microti clade.2
Additionally, around the time of the patient’s illness, she adopted a stray cat that had been unwell with vomiting. The cat survived, but unfortunately no samples were available from this animal.
Differential diagnosis
The patient’s presenting symptoms raised the possibility of malignancy. Lymphoma was the preferred diagnosis but inflammatory or infectious processes were also included in the original differential diagnosis, particularly in view of the history of immunosuppressive therapy. The investigations confirmed the latter, and a diagnosis of mycobacterial spindle cell pseudotumour secondary to M microti was made.
Treatment
The patient received 3 months of rifampicin, isoniazid, pyrazinamide and ethambutol, followed by 10 months of rifampicin and isoniazid. The renal failure and hypercalcaemia responded well to intravenous fluids and pamidronate.
Outcome and follow-up
At 4 months after stopping treatment, the mediastinal mass had largely resolved on chest x-ray, only an occasional cough remained and her weight had increased. Urea and creatinine levels had come down to 7.1 mmol/litre and 116 µmol/litre, respectively. Serum calcium level was 2.24 mmol/litre adjusted, C reactive protein (CRP) was <10 mg/litre and white cell count was 7.4×109 cells/litre. By the time that antimycobacterial therapy was discontinued, her liver was no longer palpable.
Discussion
MSCP is a rare condition usually associated with immunosuppression and characterised by a benign proliferation of spindle cells.3 Most previous cases have occurred in HIV-infected individuals4 but in this case, the likely cause of immunocompromise was previous use of immunosuppressant drugs. To date, approximately 22 human cases of MSCP have been described. Lymph nodes are frequently involved,5 although extranodal involvement has been described in a variety of tissues including brain and appendix.3,4 Most previous cases have been attributed to environmental mycobacteria, mainly Mycobacterium avium intracellulare.4 However, one case of pulmonary MSCP due to M tuberculosis has been reported.6
M microti, a rare member of the M tuberculosis complex, has a specific curved morphology.7,8 It can cause disease in various mammals including small rodents and llamas.8–10 Human disease is very rare, but may occur in immunocompromised and immunocompetent hosts, and is frequently pulmonary.9–12 The route of transmission to humans is unknown.8
The organism found in our patient was genetically indistinguishable to that found at postmortem in an alpaca from a nearby farm. Although the patient had no known direct contact with the infected alpaca, there appears to be some association in terms of time and place. Independent transmission to the patient and the alpaca from an external source, such as a rodent, cannot be ruled out. In addition, around this time the patient adopted a stray cat, which was reported as being unwell. Unfortunately no samples were available from the cat. Cats are considered important in the epidemiology of M microti,10 and it is possible that the stray cat may have been infected with M microti and thus played a role in the transmission between the alpaca and the patient.
In our patient’s case, we believe the severe hypercalcaemia was secondary to extrarenal 1α-hydroxylation of vitamin D to calcitriol. This is the suggested mechanism of hypercalcaemia in granulomatous disorders including tuberculosis.13–15 CD8 T lymphocytes in granulomata and activated alveolar macrophages are thought to be the source.13–16 Calcitriol has immunomodulatory functions, enhancing macrophages’ ability to kill mycobacteria, and retarding T cell γ-interferon synthesis.13–15 Calcitriol levels do not always correlate well with serum calcium concentration and may even be normal in tuberculosis.13,14 Calcitriol level was not measured in our patient. Dehydration and hypercalcaemia were the likely causes of renal deterioration.
Learning points
Mycobacterium microti, a member of the Mycobacterium tuberculosis complex, can occasionally cause human disease, usually on a background of immunocompromise.
M microti may cause zoonotic infection in humans.
Granulomatous disorders such as M microti infection, as well as tuberculosis can cause hypercalcaemia.
It is important to remember mycobacterial spindle cell pseudotumour (MSCP) as a cause of a mediastinal mass.
Acknowledgments
We are very grateful to Susan Duthie (TB control sister, NHS Grampian), Christine Doig (Scottish Mycobacteria Reference Laboratory), Salmah Bakar (Pathologist, NHS Grampian), Jim Dale (Veterinary Laboratories Agency) and Douglas Gray (Scottish Agriculture College). Part of this work was funded by the Department of Environment, Food and Rural Affairs, UK.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Supply P, Lesjean S, Savine E, et al. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol 2001; 39: 3563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith NH, Gordon SV, de la Rua-Domenech R, et al. Bottlenecks and broomsticks: the molecular evolution of Mycobacterium bovis. Nat Rev Microbiol 2006; 4: 670–81 [DOI] [PubMed] [Google Scholar]
- 3.Basílio-de-Oliveira CA, Eyer-Silva WA, Valle HA, et al. Mycobacterial spindle cell pseudotumor of the appendix vermiformis in a patient with AIDS. Braz J Infect Dis 2001; 5: 98–100 [DOI] [PubMed] [Google Scholar]
- 4.Morrison A, Gyure KA, Stone J, et al. Mycobacterial spindle cell pseudotumor of the brain: a case report and review of the literature. Am J Surg Pathol 1999; 23: 1294. [DOI] [PubMed] [Google Scholar]
- 5.Chen KT. Mycobacterial spindle cell pseudotumor of lymph nodes. Am J Surg Pathol 1992; 16: 276–281 [DOI] [PubMed] [Google Scholar]
- 6.Sekosan M, Cleto M, Senseng C, et al. Spindle cell pseudotumors in the lungs due to Mycobacterium tuberculosis in a transplant patient. Am J Surg Pathol 1994; 18: 1065–8 [DOI] [PubMed] [Google Scholar]
- 7.Smith NH, Crawshaw T, Parry J, et al. Mycobacterium microti: more diverse than previously thought. J Clin Microbiol 2009; 47: 2551–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiss HK, Feldhues R, Niemann S, et al. Landouzy septicemia (sepsis tuberculosa acutissima) due to Mycobacterium microti in an immunocompetent man. Infection 2005; 33: 393–6 [DOI] [PubMed] [Google Scholar]
- 9.Deforges L, Boulouis HJ, Thibaud JL, et al. First isolation of Mycobacterium microti (llama-type) from a dog. Vet Microbiol 2004; 103: 249–53 [DOI] [PubMed] [Google Scholar]
- 10.Xavier Emmanuel F, Seagar AL, Doig C, et al. Human and animal infections with Mycobacterium microti, Scotland. Emerg Infect Dis 2007; 13: 1924–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horstkoffe MA, Sobottka I, Schewe CK, et al. Mycobacterium microti llama-type infection presenting as pulmonary tuberculosis in a human immunodeficiency virus-positive patient. J Clin Microbiol 2001; 39: 406–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer K, van Soolingen D, van Embden J, et al. Mycobacterium microti: more widespread than previously thought. J Clin Microbiol 1998; 36: 2793–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med 2000; 6: 442–7 [DOI] [PubMed] [Google Scholar]
- 14.Yen TH, Huang JY, Wu CH, et al. Severe hypercalcaemia with normal serum calcitriol in a diabetic patient with chronic renal failure, autoimmune hepatitis and disseminated tuberculosis. Nephrol Dial Transplant 2000; 15: 2046–49 [DOI] [PubMed] [Google Scholar]
- 15.Simon A, Chalumeau M, Mougenot B, et al. Severe hypercalcaemia and acute renal failure: atypical complications of generalized tuberculosis. Acta Paediatr 2006; 95: 1517–18 [DOI] [PubMed] [Google Scholar]
- 16.Roussos A, Lagogianni I, Gonis A, et al. Hypercalcaemia in Greek patients with tuberculosis before the initiation of anti-tuberculosis treatment. Respir Med 2001; 95: 187–90 [DOI] [PubMed] [Google Scholar]