Abstract
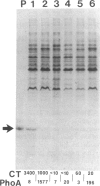
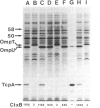
The transposon TnphoA was used to generate fusions between phoA, the gene for alkaline phosphatase (PhoA), and genes encoding proteins that are secreted by Vibrio cholerae. One of the PhoA+ mutants isolated showed a dramatic reduction in its ability to colonize the intestines of suckling mice. This mutant no longer produced a 20.5-kDa protein (TcpA) that we show is the major subunit of a V. cholerae pilus. Amino-terminal sequence analysis of the TcpA pilus subunit showed that it shares amino acid homology with the pilins produced by several other pathogenic bacteria. The TcpA pilus was coordinately expressed with cholera toxin under various culture conditions, and this effect appeared to be dependent on the transcriptional activator encoded by the toxR gene. We conclude that the toxR gene plays a central role in the transcriptional regulation of multiple virulence genes of V. cholerae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Miller V. L., Mekalanos J. J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1986;40:577–605. doi: 10.1146/annurev.mi.40.100186.003045. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., West P. A., Small E. B., Huq M. I., Colwell R. R. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984 Aug;48(2):420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs C. F., Schoolnik G., Koomey J. M., Hardy J., Rothbard J., Falkow S. Cloning and sequencing of a Moraxella bovis pilin gene. J Bacteriol. 1985 Jul;163(1):132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Simple method for purifying choleragenoid, the natural toxoid of Vibrio cholerae. Infect Immun. 1977 Jun;16(3):789–795. doi: 10.1128/iai.16.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry P. A., Pearlstone J. R., Smillie L. B., Paranchych W. Amino acid sequence of pilin isolated from pseudomonas aeruginosa PAK. FEBS Lett. 1983 Jan 24;151(2):253–256. doi: 10.1016/0014-5793(83)80080-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevenson G., Leavesley D. I., Lagnado C. A., Heuzenroeder M. W., Manning P. A. Purification of the 25-kDa Vibrio cholerae major outer-membrane protein and the molecular cloning of its gene: ompV. Eur J Biochem. 1985 Apr 15;148(2):385–390. doi: 10.1111/j.1432-1033.1985.tb08850.x. [DOI] [PubMed] [Google Scholar]
- Todd W. J., Wray G. P., Hitchcock P. J. Arrangement of pili in colonies of Neisseria gonorrhoeae. J Bacteriol. 1984 Jul;159(1):312–320. doi: 10.1128/jb.159.1.312-320.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]