Abstract
Stomach carcinogenesis involves mucosal and luminal changes that favor spontaneous disappearance of Helicobacter pylori. Therefore, the association between the infection and cancer risk might typically be underestimated. As acquisition of the infection almost invariably occurs before adulthood, the serostatus at age 16–40 should best reflect the lifetime occurrence of the infection. We therefore conducted a case-control study nested within a historic cohort of about 400,000 individuals who donated sera before age 40 to either of two large Swedish Biobanks between 1968 and 2006, and whose records were linked to complete nationwide registers. For each stomach adenocarcinoma case occurring at least 5 years after serum donation 2 controls were selected matched on age, sex and year of donation and biobank. Serum immunoglobulin G antibodies against H. pylori cell-surface antigens (Hp-CSAs) were measured with an enzyme–linked immunosorbent assay and antibodies against CagA with an immunoblot assay. Conditional logistic regression models were used to estimate odds ratios (ORs) for stomach adenocarcinoma among H. pylori infected relative to uninfected. We confirmed 59 incident cases of stomach adenocarcinoma (41 non-cardia tumors) during follow-up. ORs for non-cardia stomach adenocarcinoma among subjects with Hp-CSA antibodies (regardless of CagA serostatus), antibodies against CagA (regardless of Hp-CSA serostatus), and antibodies to both, relative to those who were seronegative to both, were 17.1 (95% confidence interval [CI] 4.0–72.9), 10.9 (95% CI 3.2–36.9), and 48.5 (95% CI 5.8–407.4), respectively. H. pylori infection is a much stronger risk factor for non-cardia stomach adenocarcinoma than initially realized. However, further studies are needed to answer whether it is a necessary cause, as the possibility of misclassification of H. pylori status could not be ruled out in our study.
Introduction
Although the incidence of stomach adenocarcinoma has declined in the industrialized world, most likely due to the declining rates of Helicobacter pylori (H. pylori) infections, it is still the fourth most common type of cancer, and due to the poor prognosis the second most common cause of cancer-related deaths worldwide [1], [2]. Notwithstanding its epithet as the by far strongest established risk factor for distal stomach cancer [3], H. pylori infection has remained a dark horse in regard to its true attributable fraction, in turn determined by the true strength of the association. Serological tests of antibodies against H. pylori cell surface antigens (Hp-CSAs) and CagA encoded by cytotoxin-associated gene-A, known to cause more extensive inflammation in the stomach mucosa, have long been employed to explore this association. However, chronic atrophic gastritis, occurring at a rate of 0.9–3.6 per 100 person-years among untreated infected individuals [4] and probably at a much higher rate among those predestined to develop stomach cancer, destroys the microorganism's natural niche and may lead to disappearance of H. pylori and decreasing antibody titres. This might have resulted in underestimation of the strength of the association in previous studies. To measure H. pylori infection status in early adulthood till 40 years of age, when essentially all infected people have already acquired their infection [5] but chronic atrophic gastritis is still rare [6], would be critical for accurately measuring the true association. We therefore conducted a case-control study nested within a historic cohort of about 400 000 individuals who donated serum samples to either of two large Swedish Biobanks at young ages between 1968 and 2006 to estimate the true strength of the H. pylori-stomach cancer relationship.
Methods
The cohort included all individuals who donated serum before 40 years of age to either of the two Swedish Biobanks – the Swedish Institute for Infectious Disease Control Biobank (since 1957) and Malmö Microbiology Biobank (since 1969).
Study biobanks
Swedish Institute for Infectious Disease Control Biobank
The Swedish Institute for Infectious Disease Control has performed a series of population-based, nationwide investigations of the immunity against infections in the Swedish population [7]. For decades, Swedish Institute for Infectious Disease Control has also served as reference laboratory for many microbiological analyses. As a part of the quality control and documentation system, part of the samples that have been analyzed have been stored at −20°C. Samples have been stored since 1957 and complete series of samples are stored since 1977. The biobank included serum samples from 93 462 unique individuals (with valid National Registration Numbers [NRNs] – individually unique personal identifiers assigned to all Swedish residents shortly after birth or immigration) collected between age 16 and 40. The samples selected for the study cohort arrived in the biobank between 1968-03-19 and 2001-12-31.
Malmö Microbiology Biobank
The biobank contains the samples submitted to the Department of Clinical Microbiology at MAS University Hospital in Malmö, where saving of a part of the sample has been necessary for clinical diagnostic and documentation purpose. The majority of the samples are serum samples that have been submitted for diagnosis of blood-borne viral infections, e.g. hepatitis virus, (over 500,000 samples). About 100,000 samples in the biobank emanate from population-based serological screening for viral infections and rubella immunity during pregnancy. The population served is currently the entire region of Skåne, where the Department of Clinical Microbiology is currently the only clinical virological laboratory. The operation of the biobank is certified by the Swedish National Accreditation Agency and follows ISO17025 procedures. The Malmö Microbiology Biobank included serum samples from 374 598 unique individuals (with valid NRNs) collected between age 16 and 40. The samples selected for the study cohort arrived in the biobank between 1969-02-10 and 2001-12-31.
Follow-up
Linkage to complete nationwide registers, including the Swedish Cancer Register, Causes of Death Register and Migration Register, provided follow-up data from serum collection until first diagnosis of stomach cancer, emigration, death, or December 31, 2006, whichever occurred first. The NRNs, used both in the biobanks and the registers, ensured precise matches. To verify the validity of the NRNs, further linkage to the Total Population Register was performed. If a NRN could not be found in the Total Population Register, Causes of Death Register or Migration Register, it was deemed to be invalid and the serum sample, along with the record, was not included in our analysis. Stomach adenocarcinoma cases were identified through the nationwide Cancer Register, founded in 1958 and with an estimated completeness of 96–98% [8], [9]. The Cancer Register recorded neoplasms using ICD-7 classification through the entire study period. A separate code for gastric cancer in the cardia was introduced first in 1969 and was widely used since 1970. The histological type of each recorded tumour was also recorded by a histology code, albeit these codes did not distinguish between Laurén's intestinal and diffuse types of adenocarcinoma [10]. In order to reduce the possibility of misclassification of H. pylori infection status associated with stomach cancer development, stomach cancer cases occurring within 5 years of index serum collection were disregarded. For each stomach cancer case we selected 2 controls matched on age, sex, year of serum sample collection and biobank using incidence density sampling.
Review of medical records
To further verify the diagnosis and specify the gastric subsite, all medical records collected from corresponding treatment units were reviewed by a specialist in gastrointestinal surgery (ON) blinded to H. pylori status and earlier reported ICD code in the Swedish Cancer Register.
Laboratory analysis
Immunoglobulin G antibodies against Hp-CSAs were measured with an enzyme–linked immunosorbent assay (ELISA) (Biohit, Finland). The sensitivity and specificity for the assay was reported as 96% and 94%, respectively [11]. Antibodies against CagA were measured with an immunoblot assay (Helicoblot 2.1, Genelabs Diagnostics, Singapore) according to manufacturer's instructions [12].
Pepsinogen (PG) levels in sera were measured using an ELISA method (Biohit, Finland) according to the manufacturer's instructions. Presence of severe or moderate corpus atrophic gastritis was defined as PGI below 25 ug/l or PGI:PGII ratio less than 3. The sensitivity and specificity was reported as 71% and 98% in a validation study performed in Sweden [13]. All laboratory analyses were carried out in a blinded fashion by trained personnel. The quality control samples provided with the kits were included on each assay plates with additional quality control samples using pooled serum samples from the laboratory.
Statistical analysis
The odds ratios (ORs) and their corresponding 95% confidence intervals (95% CIs) of developing stomach adenocarcinoma associated with H. pylori infection was estimated using conditional logistic regression models. Since controls were individually matched to the cases with regard to age, sex, calendar year of serum collection, and biobank, these factors were inherently adjusted for. All statistical analyses were performed using Stata/SE 9.2 (StataCorp, college Station, TX, USA). The study was approved by the Regional Research Ethics Vetting Board in Stockholm, Sweden.
Results
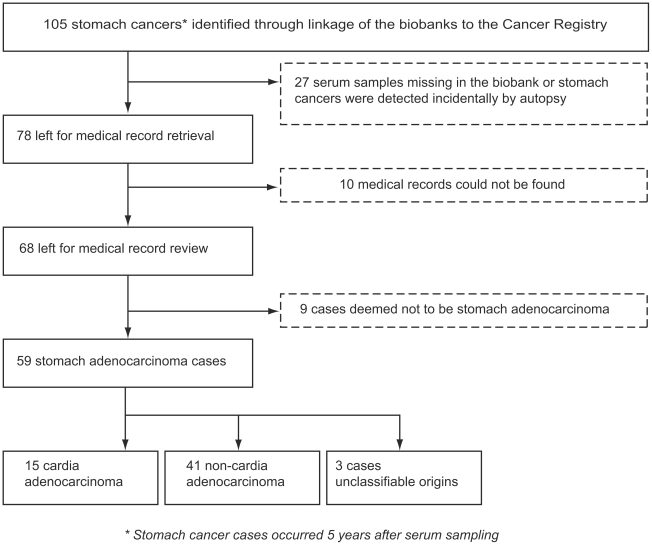
Figure 1 shows the flowchart for identification of stomach adenocarcinoma cases. In total 105 stomach cancers (ICD-7 code 151) which occurred at least 5 years after blood sample collection were identified during follow-up. Among these, 27 were excluded due to either missing serum samples or the fact that the cancers were found incidentally at autopsy. For the remaining 78 cases, we successfully retrieved medical records related to cancer diagnosis for 68 cases. After review, 9 cases were deemed to be tumors other than stomach adenocarcinoma. Among the remaining 59 stomach adenocarcinoma cases, 15 stomach adenocarcinoma cases were determined to be cardia adenocarcinoma and 41 as non-cardia stomach adenocarcinoma. In 3 stomach adenocarcinoma cases it was not possible to determine the exact origin in the stomach.
Figure 1. Flowchart for identifying stomach adenocarcinoma cases.
In total 105 stomach cancers (ICD-7 code 151) which occurred at least 5 years after blood sample collection were identified during follow-up. Among these, 27 were excluded due to either missing serum samples or the fact that the cancers were found incidentally at autopsy. For the remaining 78 cases, medical records related to cancer diagnosis for 68 cases were successfully retrieved. After review, 9 cases were deemed to be tumors other than stomach adenocarcinoma. Among the remaining 59 stomach adenocarcinoma cases, 15 stomach adenocarcinoma cases were determined to be cardia adenocarcinoma and 41 as non-cardia stomach adenocarcinoma. In 3 stomach adenocarcinoma cases it was not possible to determine the exact origin in the stomach.
Some basic characteristics of cases and their matched controls are summarized in Table 1 . As expected, there were no differences in the sex distribution and mean age at blood sample collection between cases and controls. The mean age at diagnosis of stomach adenocarcinoma was 47.3 years (range 25–68). The mean time between serum collection and stomach adenocarcinoma diagnosis was 16.5 years (range 5–33). Presence of severe or moderate corpus atrophic gastritis at time of initial serum collection was more common among stomach adenocarcinoma cases (8.5%) as compared with matched controls (0.9%).
Table 1. Characteristics of the stomach adenocarcinoma cases and their matched controls.
| Characteristics | Stomach adenocarcinoma cases (n = 59) | Controls (n = 117) |
| Men (%) | 30 (50.9) | 60 (51.2) |
| Age at sample collection, mean (SD), range | 30.8 (6.1), 16–40 | 30.9 (6.0), 16–40 |
| Age at cancer diagnosis, mean (SD), range | 47.3 (9.4), 25–68 | |
| Years between serum collection and cancer diagnosis, mean (SD), range | 16.5 (7.2), 5–33 | |
| Severe or moderate corpus atrophy*, N (%) | 5 (8.5) | 1 (0.9) |
* Defined as serum pepsinogen I<25 ug/l or pepsinogen I:II ratio <3 at time of initial serum collection.
ORs for all stomach adenocarcinoma among subjects with Hp-CSA antibodies (regardless of CagA serostatus) and antibodies against CagA (regardless of Hp-CSA serostatus) were 4.1 (95% CI 1.9–8.5) and 3.5 (95% CI 1.7–7.1), respectively ( Table 2 ). We further analyzed data by combining Hp-CSA and CagA antibodies, and used subjects seronegative for both as the unexposed reference group. Compared with this ‘clean’ reference group, those seropositive with either Hp-CSA or CagA antibodies had a more than 3-fold increased risk of developing stomach adenocaricnoma. The increased risk was more pronounced among the group seropositive with both Hp-CSA and CagA antibodies (OR = 5.5, 95% CI 2.3–12.9) ( Table 2 ).
Table 2. H. pylori -CSAs and CagA seropositivity and the risk of stomach adenocarcinoma overall, cardia site and non-cardia site.
| Stomach adenocarcinoma | Cardia adenocarcinoma | Non-cardia adenocarcinoma | ||||||||
| Serological test results | Case | Control | OR*(95% CI) | Case | Control | OR*(95% CI) | Case | Control | OR*(95% CI) | |
| Hp-CSAs antibodies | Negative | 20 | 76 | Ref. | 12 | 21 | Ref. | 6 | 51 | Ref. |
| Positive | 39 | 41 | 4.1 (1.9–8.5) | 3 | 9 | 0.5 (0.1–2.8) | 35 | 30 | 17.1 (4.0–72.9) | |
| CagA antibodies | Negative | 19 | 70 | Ref. | 11 | 19 | Ref. | 7 | 49 | Ref. |
| Positive | 40 | 47 | 3.5 (1.7–7.1) | 4 | 11 | 0.6 (0.2–2.5) | 34 | 32 | 10.9 (3.2–36.9) | |
| Hp-CSAs – CagA antibodies | Hp-CSAs and CagA antibodies negative | 15 | 61 | Ref. | 10 | 18 | Ref. | 4 | 41 | Ref. |
| Hp-CSAs or CagA antibodies positive | 44 | 56 | 3.3 (1.6–6.7) | 5 | 12 | 0.8 (0.2–2.7) | 37 | 40 | 9.7 (2.9–32.9) | |
| Hp-CSAs and CagA antibodies positive | 35 | 32 | 5.5 (2.3–12.9) | 2 | 8 | 0.3 (0.03–2.6) | 32 | 22 | 48.5 (5.8–407.4) | |
* Odds ratios (ORs) were derived from conditional logistic regression models.
When analysis was limited to 41 cases of non-cardia stomach adenocarcinoma, ORs were 17.1 (95% CI 4.0–72.9) and 10.9 (95% CI 3.2–36.9) among carriers of antibodies to Hp-CSAs and CagA, respectively. The corresponding results for cardia adenocarcinoma were 0.5 (95% CI 0.1–2.8) and 0.6 (95% CI 0.2–2.5), respectively ( Table 2 ). Compared with the ‘clean’ reference group, those seronegative for both Hp-CSA and CagA antibodies, subjects seropositive with either Hp-CSA or CagA antibodies had a close to 10-fold increased risk (95% CI 2.9–32.9), and the increased risk further rose to close to 50-fold among those seropositive with both antibodies (95% CI 5.8–407.4). The corresponding results for cardia adenocarcinoma were 0.8 (95% CI 0.2–2.7) and 0.3 (95% CI 0.03–2.6), respectively ( Table 2 ).
Discussion
Although H. pylori was classified as a class I carcinogen for stomach cancer by the International Agency for Research on Cancer (IARC) already in 1994 [14], the strength of the association remains uncertain. In an early meta-analysis of 42 studies that were typically of a cross-sectional nature (with H. pylori diagnosis at time of cancer diagnosis), the summary odds ratio was only 2.04 [15]. This is probably due to the fact that stomach adenocarcinoma develops late in life (55+), and the histological changes in the stomach during cancer development might lead to spontaneous disappearance of the bacteria. H. pylori have been shown to promote the production of inflammatory mediators such as IL-1β and TNF-α, both potent suppressors of stomach juice secretion. The increase in stomach pH may lead to spread of H. pylori from the antrum to the corpus, resulting in enhanced inflammation in the mucosa of the corpus, followed by parietal cell destruction and irreversible hypochlorhydria [2], [16].
Already in 1994 a combined analysis of 3 prospective studies found that excess risks increased with increasing time window between sample collection and cancer diagnosis [17]. This finding was corroborated later by a pooled analysis of 12 prospective studies [18], in which a six-fold increased risk for non-cardia adenocarcinoma was found in patients whose samples were collected 10 years or more before cancer diagnosis, while the relative risk within 10 years after serotesting was 2.4. Similarly, in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) prospective cohort study, a close to 8-fold relative risk for non-cardia stomach cancer associated with H. pylori infection was reported [19]. H. pylori strains containing the CagA gene are known to cause more extensive inflammation in the stomach mucosa and antibodies against CagA persist long after eradication [20], [21]. In a Swedish population based case-control study [22], when Hp-CSA-/CagA+ subjects (presumably with “burnt-out” H. pylori infection) were removed from the seronegative reference group, subjects seropositive with Hp-CSAs had an OR of 21.0. In particular, the group with Hp-CSA-/CagA+, compared with the reference group seronegative with both tests, has an odds ratio as high as 68.0. This is consistent with a recent Japanese cohort study among middle-aged subjects [23]. There, a more than 100-fold excess risk of stomach cancer was noted in the group seronegative with H. pylori antibodies but with a low pepsinogen I:II ratio. Some studies even suggested that H. pylori infection might be a necessary cause for non-cardia adenocarcinoma. In a small case-control study performed in Germany, after excluding cases with blood samples collected more than 90 days after gastrectomy, T4 stage, Hp-/CagA+, or borderline IgG results, all the remaining 32 non-cardia stomach adenocarcinoma cases were seropositive with H. pylori infection [24]. Similarly, in a small cohort study, in which H. pylori status was ascertained by histological examination, rapid urease test, and serologic test, 3% of infected subjects but none of the uninfected developed a stomach cancer, after a mean follow-up time of 7.8 years [25]. Another study conducted in Taiwan with a similar study design also reported none of stomach malignancies detected among the uninfected subjects after a mean follow-up of 6.3 years [26].
In summary, several of the more recent studies suggested that the strength of the H. pylori-noncardia stomach adenocarcinoma association might range between a 6-fold to infinite (necessary cause). However, previous studies have various limitations, including small sample size, blood samples collected at old age or at cancer diagnosis, and misclassification of anatomic site of stomach cancer. Our study, based on two large biobanks in Sweden, was able to identify a relative large cohort of subjects, who donated blood samples between ages 16–40, which is the best time window to capture the lifetime occurrence of the infection, as acquisition of the infection almost invariably occurs before adulthood. Further, we retrieved and reviewed medical records for all stomach cancer cases, and managed to clarify the cardia/noncardia origin in all but 3 cases. Our results suggested that the strength of the association between H. pylori infection and risk of non-cardia stomach adenocarcinoma was greater than what was reported before in some previous prospective studies among older subjects. However, our data do not provide clear support for the hypothesis that H. pylori is a necessary cause of non-cardia stomach adenocarcinoma. Despite small number of cardia adenocarcinoma, our results were consistent with previous report of a null association with H. pylori infection in low-risk areas [27].
Despite our efforts to capture H. pylori infection status in the best time window, we could not exclude the possibility of misclassification of H. pylori infection status. Several lines of evidence support this suspicion, as among the four non-cardia adenocarcinoma cases without any evidence of H. pylori infection, one had moderate/severe corpus atrophy at time of serum sampling, suggesting (albeit not proving) previous occurrence of H. pylori at some point. Linkage to the Inpatient Register also found that this patient had been hospitalized for viral hepatitis before the date of blood sampling. For the other 3 cases, one had been hospitalized for chronic nephritis before blood sampling, which might also lead to inaccurate measurement of H. pylori antibodies. Another limitation of our study is that, due to the age structure of our cohort subjects, most stomach cancer cases were of young age. The association between H. pylori infection and stomach cancer might differ as diffuse type is more common among young cases, although previous studies found similar associations for both diffuse and intestinal types [19], [22]. Further, although the cases and controls were matched on age, sex, calendar year of serum collection and biobank, we lacked the information on other potential confounders, like socioeconomic status, smoking and diet, etc. Thus, the possibility of residual confounding cannot not be ruled out.
Our results add confidence to the conclusion that H. pylori infection is a much stronger risk factor for non-cardia stomach adenocarcinoma than initially realized, but it is probably not a necessary cause. However, since misclassification of H. pylori status may have occurred also in this study, the association might be even stronger than indicated by our data.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by a grant from Martin Rind Stiftelse and partly by a grant from the Swedish Research Council (K2009-69X-15372-05-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 4.Adamu MA, Weck MN, Gao L, Brenner H. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439–448. doi: 10.1007/s10654-010-9482-0. [DOI] [PubMed] [Google Scholar]
- 5.Malaty HM, Nyren O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2003;8(Suppl 1):8–12. doi: 10.1046/j.1523-5378.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 6.de Vries AC, Kuipers EJ. Epidemiology of premalignant gastric lesions: implications for the development of screening and surveillance strategies. Helicobacter. 2007;12(Suppl 2):22–31. doi: 10.1111/j.1523-5378.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiland O, Berg JV, Bottiger M, Lundbergh P. Prevalence of antibody against hepatitis A in Sweden in relation to age and type of community. Scand J Infect Dis. 1980;12:171–174. doi: 10.3109/inf.1980.12.issue-3.03. [DOI] [PubMed] [Google Scholar]
- 8.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23:305–313. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 9.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48:27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 10.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Newell DG, Ormand JE, Carpenter HA, Wilson WR, et al. Serodiagnosis of Helicobacter pylori: comparison of enzyme-linked immunosorbent assays. J Clin Microbiol. 1991;29:1635–1639. doi: 10.1128/jcm.29.8.1635-1639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veijola L, Oksanen A, Sipponen P, Rautelin H. Evaluation of a commercial immunoblot, Helicoblot 2.1, for diagnosis of Helicobacter pylori infection. Clin Vaccine Immunol. 2008;15:1705–1710. doi: 10.1128/CVI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43:1448–1455. doi: 10.1080/00365520802273025. [DOI] [PubMed] [Google Scholar]
- 14.IARC Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 15.Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373–2379. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 16.Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765–773. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman D, Webb P, Parsonnet J. H pylori and gastric cancer. Lancet. 1994;343:243–244. [PubMed] [Google Scholar]
- 18.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–1452. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, et al. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaamas K, Held M, Wadstrom T, Lipping A, Kurtenkov O. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by ELISA and immunoblotting. Int J Cancer. 1996;67:1–5. doi: 10.1002/(SICI)1097-0215(19960703)67:1<1::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Ekstrom AM, Held M, Hansson LE, Engstrand L, Nyren O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784–791. doi: 10.1053/gast.2001.27999. [DOI] [PubMed] [Google Scholar]
- 23.Yanaoka K, Oka M, Yoshimura N, Mukoubayashi C, Enomoto S, et al. Risk of gastric cancer in asymptomatic, middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. Int J Cancer. 2008;123:917–926. doi: 10.1002/ijc.23571. [DOI] [PubMed] [Google Scholar]
- 24.Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159:252–258. doi: 10.1093/aje/kwh039. [DOI] [PubMed] [Google Scholar]
- 25.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 26.Hsu PI, Lai KH, Hsu PN, Lo GH, Yu HC, et al. Helicobacter pylori infection and the risk of gastric malignancy. Am J Gastroenterol. 2007;102:725–730. doi: 10.1111/j.1572-0241.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- 27.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2010 doi: 10.1007/s10552-010-9707-2. [DOI] [PubMed] [Google Scholar]