Abstract
This report explores the hypothesis that arterial stiffness indices, which predict cardiovascular disease, might also correlate with microalbuminuria (MA) in type 1 diabetes (T1D), and thus have potential for risk assessment. Three pulse wave analysis indices, measured using the SphygmoCor device, were evaluated on 144 participants with childhood-onset T1D. These variables, augmentation index (AIx) and augmentation pressure (AP), and subendocardial viability ratio (SEVR, an estimate of myocardial perfusion) were each analyzed cross-sectionally in relation to both prevalent MA (defined as albumin excretion rate (AER)=20–199 μg/min) and renal function (assessed by both estimated glomerular filtration rate (eGFR) and serum cystatin C). AP and SEVR were each univariately associated with AER, eGFR and cystatin C. Lower SEVR was also independently related to the presence of microalbuminuria and degree of albuminuria within normo- and microalbuminuric participants. SEVR, not AP, was independently and negatively associated with both measures of renal function. SEVR is a better predictor of AER than brachial blood pressure measures in those without clinical proteinuria, indicating a potential use for pulse wave analysis in the early detection of individuals at risk for cardiovascular and renal complication of T1D.
Index Words: pulse wave analysis, type 1 diabetes, microalbuminuria, renal disease, kidney function, arterial stiffness
INTRODUCTION
Diabetic nephropathy (DN) is a major complication of type 1 diabetes (T1D),1 and often leads to end-stage renal disease (ESRD).2 Known risk factors for DN in T1D include age, diabetes duration, poor glycemic control, dyslipidemia, and elevated blood pressure (brachial systolic and mean arterial pressure).3–5 However, these factors do not entirely explain the risk of nephropathy development or its progression. DN is also linked to other major T1D complications, such as retinopathy and cardiovascular disease.6–8 In the general population, reduced renal function has been associated with greater cardiovascular mortality,9, 10 increased left-ventricular mass in men,11 and subclinical atherosclerosis.12, 13 Albuminuria (a measure of renal damage) is also associated with increased risk of clinical cardiovascular disease (CVD) and mortality in a variety of populations, including T1D.10, 14, 15 Despite extensive studies, the underlying mechanism relating renal damage (and/or decreased renal function) to cardiovascular complications are not completely understood.
Pulse wave analysis (PWA) utilizing applanation tonometry measures variables associated with the forward propagation and reflection of the pulse wave. The pressure wave created by left ventricular contraction propagates forward until meeting sites of resistance, which reflect the wave backward. Stiffer artery walls result in earlier wave reflection.16 When the reflected wave returns during systole rather than diastole, as occurs when there is increased stiffness, systolic pressure is increased or “augmented”. PWA measures thus reflect arterial stiffness. One such measure, Augmentation Index (AIx), has been linked to progression to ESRD in patients with chronic kidney disease17 and was recently shown to be associated with glomerular filtration rate (GFR) in hypertensive patients.18 Pulse wave velocity (PWV), itself a direct measure of arterial stiffness, has also been shown to be a significant and independent correlate of eGFR,19 and to increase in a stepwise manner with advancing stages of chronic kidney disease.20 Measures of arterial stiffness are also associated with left ventricular diastolic function, cardiovascular events and mortality.21–23
The association between PWA measures and measures of renal function and/or renal damage has yet to be explored in a T1D population. Therefore, the aim of this study was to examine the relationship between PWA measures and measures of both renal damage (albumin excretion rate (AER)) and renal function (eGFR and cystatin C) in a population with childhood-onset T1D, to assess the potential of these measures in the early identification of those at increased renal (and CVD) risk.
METHODS
Pulse wave analysis via applanation tonometry was performed at the 18-year follow-up examination of participants in the Pittsburgh Epidemiology of Complications (EDC) Study with childhood-onset (age <17 years) T1D. This population (n=658) consists of individuals either diagnosed with T1D or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh between 1950 and 1980 and placed on insulin therapy at initial discharge.24 Baseline examination occurred between 1986 and 1988, with follow-up occurring biennially thereafter. The study protocol was approved by the University of Pittsburgh Institutional Review Board.
Questionnaires concerning demographic, health care, self-care, and medical history were sent to participants prior to their clinic visit. Self-reported smoking history (at least 100 cigarettes in lifetime), current smoking status and medication use were obtained. All medications were coded according to the World Health Organization’s Anatomical, Therapeutical, Chemical Classification/Defined Daily Doses (ATC/DDD) codes. Medications with potential effects on pulse wave analysis measures (angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), calcium channel blockers (CCB), beta blockers (BB), and nitrates)25 were of particular interest, and use of one or more of these medications was categorized as pulse wave drug (PWD) use.
During clinic visits, brachial systolic and diastolic blood pressures (SBP and DBP) were measured with the participant in a seated position after a 5-minute rest using a random zero sphygmomanometer, according to the Hypertension Detection and Follow-Up Program protocol.26 Hypertension (HTN) was defined as systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 80 mmHg, or the use of antihypertensive medications for the purpose of lowering blood pressure. Height and weight were measured and used to calculate body mass index (BMI, in kg/m2). Waist and hip circumferences were measured twice and if the two measures were not ≤0.5 cm apart, then a third measure was taken. Means of waist and means of hip measurements were used to calculate waist-to-hip ratio (WHR).
Total cholesterol was measured enzymatically.27 High-density lipoprotein cholesterol (HDL-c) levels were determined by a precipitation technique (heparin and manganese chloride) with modification of the Lipid Research Clinics method.28 Non-HDL-c levels were calculated by subtracting HDL-c from total cholesterol. Blood samples were analyzed for hemoglobin A1c (HbA1c) using the DCA 2000 analyzer (Bayer Diagnostics, Tarrytown, NY). Estimated glucose disposal rate (eGDR, a measure of insulin sensitivity) was calculated using a regression equation derived from hyperinsulinemic-euglycemic clamp studies of 24 subjects chosen to represent the full spectrum of insulin resistance: eGDR = 24.4 – 12.97(WHR) – 3.39(HTN) – 0.60(HbA1c).29
Both urinary and serum albumin were measured by immunonephelometry.30 Serum creatinine was assayed using an Ectachem 400 Analyzer (Eastman Kodak Co, Rochester, NY). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation31: 175 × serum creatinine (mg/dl)−1.154 × age−0.203 × [0.742 if female] × [1.21 if black]. Renal function categories were created based on MDRD eGFR calculations as follows: no renal insufficiency (eGFR≥90 ml/min/1.73m2), mild renal insufficiency (eGFR=60–89), moderate-severe insufficiency (eGFR=15–59), and ESRD (eGFR<15). Cystatin C was measured turbidimetrically on an Olympus AU 640 using reagents purchased from DakoCytomation N. America, Inc. (Carpiteria, CA). Cystatin C was categorized as high if ≥1.0 mg/l and normal if <1.0 mg/l. Albumin excretion rates (AER) were calculated using urinary albumin levels from at least 2 validated timed sample collections (24-hr, overnight, and/or timed samples during exam). Degree of albuminuria was categorized as normo- (AER<20 μg/min), micro- (20–200 μg/min) or macro-albuminuria (>200 μg/min). Those reporting a history of renal transplant or dialysis were considered to have a history of renal failure and were excluded from this analysis.
The 18-year follow-up exam (November 2004 – November 2006) consisted of 309 participants corresponding to 70.4% of those alive and locally residing. Pulse waveform analysis (PWA) testing began part way through the 18-year examination period (January 2006), and thus, 189 remaining participants were potentially available for PWA. Of these, 144 (76%) agreed to PWA testing. Aortic augmentation index (AIx), aortic augmentation pressure (AP) and subendocardial viability ratio (SEVR) were derived from radial artery waveform measures using the SphygmoCor Px version 7.01 (AtCor Medical, Sydney, Australia). Briefly, a high-fidelity micromanometer with afrequency response of >2 kHz (Millar Instruments, Houston, TX) was gently pressed over the right radial artery until a consistent waveform was produced. After obtaining ≥ 20 sequential waveforms, measurement was stopped. Central pressure values were estimated from radial measurements using the software’s mathematical transfer function;32, 33 the accuracy and reliability of which have been validated.34 As the left ventricle contracts, the pressure wave moves forward until meeting sites of resistance, which reflect the wave backward. Stiffer artery walls result in earlier wave reflection.16 Systolic pressure is augmented when the reflected wave returns during systole rather than diastole, and augmentation pressure (AP) is the portion of the central systolic pressure contributed by the early reflected wave. Augmentation index (AIx) is expressed as a percentage of the pulse pressure (AIx = AP/PP × 100) and reflects the level of augmentation measured. Heart rate is inversely associated with AIx and AP.35 Subendocardial viability ratio (SEVR) is the ratio of the diastolic area under the curve (AUC) of an arterial pulse wave to the systolic AUC.36 SEVR is a ratio of myocardial perfusion (as coronary artery perfusion takes place primarily during diastole) to myocardial contraction, and it serves as a non-invasive, tonometric measure of myocardial perfusion relative to cardiac workload. The SphygmoCor device provides a quality index (QI), which represents reproducibility of the waveform. PWA measures with a QI<80 were repeated. Only measures with a QI≥80 were included in this study.
Each variable’s distributional characteristics including normality were assessed. AP, AER, cystatin C were not normally distributed. Student’s t-test (for parametric) and the Mann-Whitney U test (for non-parametric) were used to compare continuous variables between two groups. One-way ANOVA (parametric) or Kruskal-Wallis tests (non-parametric) were used for comparisons among >2 groups, and the χ2 test was used for categorical variables. Pearson’s and Spearman’s correlations were used as appropriate. Stepwise linear and logistic regression analyses were performed for continuous and binary outcomes, respectively. A significance level of <0.10 was used for entry into models. All continuous variables were standardized by subtracting the mean and dividing by the standard deviation of each variable. Models were adjusted for the potential confounders of PWA measures (height and heart rate for AIx and AP and heart rate only for SEVR). Age and sex were not available to multivariate models for MDRD eGFR as both of these factors are included in its calculation. Analyses were completed using SPSS v15 for Windows (SPSS, Chicago, IL).
RESULTS
Of the 144 EDC participants with PWA measures, 11 (7.6%) were excluded due to a history of renal failure (transplant or dialysis). Mean age ± SD and T1D duration for the remaining 133 participants were 44.3 ±7.4 and 36.1 ± 6.7, respectively. Of the 133, AER measurements were available for 130 (97.7%), MDRD eGFR could be calculated for 129 (97.0%), and cystatin C was available for 118 (88.7%) participants.
PWA Measures and Microalbuminuria
Ninety-one (70.0%) of the 130 with AER measures were normoalbuminuric, 25 (19.2%) had microalbuminuria (MA) and 14 (10.8%) had macroalbuminuria. Systolic and diastolic blood pressures increased significantly with increasing albuminuria category, as did HbA1c, WHR, serum creatinine, cystatin C and eGFR (Table 1). eGDR decreased with increasing albuminuria category. BMI, although having a borderline significant, positive linear trend with albuminuria category, did not significantly differ among the groups.
Table 1.
Characteristics of the Pittsburgh EDC Pulse Wave Analysis population by albuminuria categories and by renal function categories
| Albuminuria (μg/min) | eGFR (ml/min/1.73 m2) | Cystatin C (mg/l) | ||||||
|---|---|---|---|---|---|---|---|---|
| Normo (AER<20) | Micro (AER=20–200) | Macro (AER>200) | >90 | 60–89 | 15–59 | <1.0 | ≥1.0 | |
| N (%) | 91 (70.0) | 25 (19.2) | 14 (10.8) | 29 (22.5) | 75 (58.1) | 25 (19.4) | 98 (83.1) | 20 (16.9) |
| Augmentation Index (%) | 22.1±11.0 | 21.1±10.1 | 26.1±12.1 | 19.9±10.8b* | 22.3±10.8 | 27.2±9.22 | 21.3±10.6** | 28.1±12.1 |
| Augmentation Pressure (mmHg) | 7.54±4.62b** | 8.84±7.13 | 13.7±10.4 | 7.00±5.11b** | 8.24±5.97 | 11.9±7.41 | 7.71±4.88** | 13.6±9.98 |
| Subendocardial Viability Ratio (%) | 149.3±26.5b** | 126.7±30.3 | 131.6±35.6 | 147.2±24.1b | 143.1±32.2 | 134.4±36.0 | 143.9±29.4 | 133.6±42.1 |
| Age (years) | 43.3±6.57 | 46.8±8.69 | 44.2±7.41 | 40.6±4.37b*** | 44.2±7.83 | 49.3±5.90 | 43.7±7.12** | 49.2±6.58 |
| Diabetes Duration (years) | 35.2±6.09 | 39.1±7.38 | 35.1±6.77 | 33.8±5.34b** | 36.2±6.70 | 39.3±7.17 | 36.0±6.33 | 37.9±7.20 |
| Sex (% male) | 46.2 | 60.0 | 57.1 | 58.6** | 53.3 | 28.0 | 55.1 | 40.0 |
| HbA1c (%) | 7.33±1.15b** | 8.00±1.70 | 8.28±1.30 | 7.71±1.62 | 7.52±1.27 | 7.43±0.98 | 7.53±1.28 | 7.56±1.34 |
| eGDR (mg/kg/min) | 8.33±1.97c*** | 6.82±2.27 | 5.56±2.21 | 7.88±2.36 | 7.92±2.09 | 7.28±2.28 | 7.94±2.09** | 6.64±2.51 |
| Systolic Blood Pressure (mmHg) | 109.3±11.5c*** | 115.7±13.1 | 139.5±15.5 | 108.5±13.4b** | 114.4±14.3 | 120.4±20.5 | 112.4±14.3** | 124.3±19.3 |
| Diastolic Blood Pressure (mmHg) | 65.4±8.23b*** | 63.3±9.12 | 75.6±11.7 | 67.2±7.07 | 65.7±9.30 | 66.6±11.8 | 65.6±8.42 | 66.7±13.9 |
| Heart Rate (bpm) | 76.7±13.0 | 82.6±12.4 | 79.9±14.0 | 78.0±13.1 | 79.1±13.7 | 76.2±11.0 | 79.0±12.9 | 74.2±13.1 |
| Non-HDL-c (mg/dL) | 111.1±27.3 | 107.0±34.0 | 144.9±52.8 | 111.3±28.1 | 116.3±33.1 | 108.4±38.1 | 111.5±31.9 | 119.2±40.8 |
| HDL-c (mg/dL) | 60.3±16.7 | 55.5±15.8 | 57.115.9 | 59.3±17.2 | 60.0±16.7 | 56.3±15.2 | 58.6±16.3 | 57.2±16.1 |
| Body Mass Index (kg/m2) | 26.7±4.34a | 27.3±4.89 | 28.9±3.99 | 26.5±4.46a | 26.9±4.39 | 28.6±4.65 | 26.7±4.26 | 27.1±4.20 |
| Waist-to-Hip Ratio | 0.85±0.09b** | 0.91±0.09 | 0.90±0.7 | 0.86±0.07b** | 0.86±0.09 | 0.89±0.11 | 0.87±0.09 | 0.90±0.10 |
| Albumin Excretion Rate (μg/min) | 5.29 (3.8–6.9)c*** | 5.88 (4.0–24.1) | 22.0 (8.5–241.1) | 6.09 (4.0–17.8)*** | 93.7 (22.5–608.9) | |||
| Serum Creatinine (mg/dL) | 0.93±0.17c*** | 1.12±0.42 | 1.32±0.39 | 0.80±0.10c*** | 0.97±0.13 | 1.37±0.46 | 0.94±0.16*** | 1.42±0.50 |
| Cystatin C (mg/l) | 0.80±0.12c*** | 0.98±0.34 | 1.24±0.50 | 0.74±0.09c*** | 0.82±0.12 | 1.19±0.36 | ||
| MDRD eGFR (ml/min/1.73 m2) | 78.4±15.1c*** | 69.7±19.9 | 56.7±18.0 | 78.9±14.1*** | 49.0±14.6 | |||
Data are presented as either n (%), mean±SD, or median (IQR).
Comparisons within albuminuria, MDRD eGFR, or Cystatin C categories:
p<0.10,
p<0.05,
p<0.001.
significant linear trend, p<0.10,
significant linear trend, p<0.05,
significant linear trend, p<0.001
Abbreviations: EDC, Epidemiology of Diabetes Complications; AER, albumin excretion rate; eGDR, estimated glucose disposal rate; HDL-c, high density lipoprotein cholesterol; MDRD, Modification of Diet in Renal Disease; eGFR, estimated glomerular filtration rate.
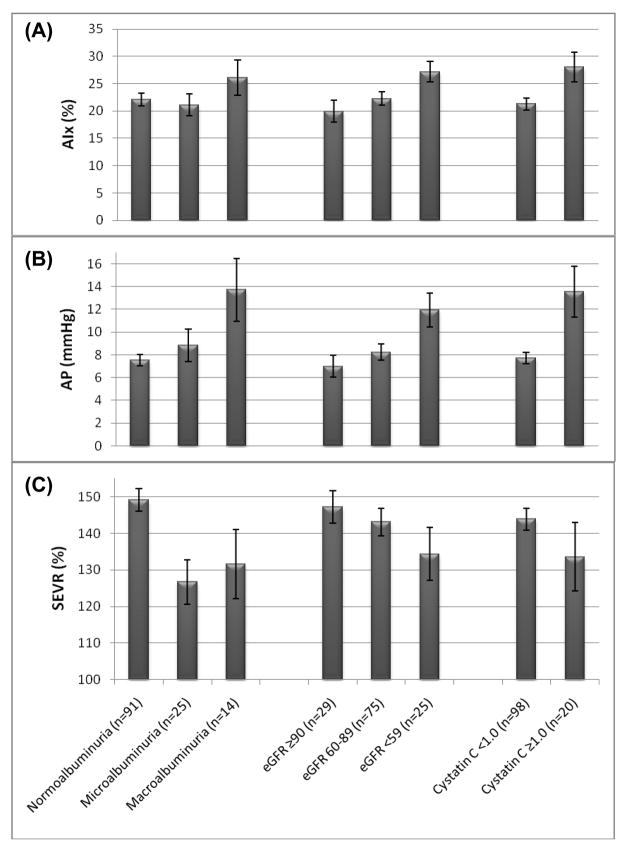
AIx did not significantly differ by albuminuria category and did not show a significant linear trend (Table 1 and Figure 1A). AP increased with increasing albuminuria category (p=.07) and showed a significant, positive linear trend (p=.01) (Table 1 and Figure 1B). SEVR differed more significantly by albuminuria category (p=.002) and showed a significant, inverse linear trend (p=.002) (Table 1 and Figure 1C). Similar data are presented in Tables 1 and 2 with renal function as the outcome. In unadjusted, univariate correlations, only SEVR was correlated with AER (r= −.35; p<.001). After adjustment for heart rate (and height for LnAP and AIx), SEVR remained significantly correlated with AER (r= −.22; p=.01), and LnAP became positively correlated with AER (r=.28; p=.001) (Table 2).
Figure 1.
Augmentation Index (mean and standard error (SE)) (Panel A), Augmentation Pressure (mean and SE) (Panel B), and Subendocardial Viability Ratio (mean and SE) (Panel C) by albuminuria and renal function categories in the Pittsburgh EDC Pulse Wave Analysis study population.
Table 2.
Bivariate correlations and heart rate-adjusted partial correlations between AIx, AP, and SEVR with AER, eGFR and cystatin C in the Pittsburgh EDC Pulse Wave Analysis study population
| Variables | Albumin Excretion Rate (μg/min) | MDRD eGFR (ml/min/1.73 m2) | Cystatin C (mg/l) |
|---|---|---|---|
| AIx | −.074 | −.210* | .108 |
| AP | .050 | −.252** | .209* |
| SEVR | −.354*** | .212* | −.186* |
| Heart Rate-adjusted Correlations | |||
| Variables | LnAERb | MDRD eGFR | Cystatin C |
| AIx | .138 | −.175* | .170 |
| LnAPa,b | .281** | −.249** | .273** |
| SEVRa | −.223* | .257** | .283** |
p<0.05,
p<0.01,
p<0.001.
also height adjusted;
Due to use of Pearson’s partial correlations, non-parametric variables (AER and AP) were natural logarithmically transformed.
Abbreviations: AIx, augmentation index; AP, augmentation pressure; SEVR, subendocardial viability ratio; AER, albumin excretion rate; MDRD, Modification of Diet in Renal Disease; eGFR, estimated glomerular filtration rate.
PWA Measures and Glomerular Filtration Rate
Increased AIx (p=.09), AP (p=.03), age, SBP, WHR, AER, serum creatinine and cystatin C accompanied decreased eGFR category (Table 1). SEVR, although showing a linear trend of borderline significance (p=.07), did not differ significantly between the eGFR groups (p=.17). AIx and AP showed an inverse linear trend (p=.005), both increasing with decreasing eGFR category (Table 1, Figures 1A and 1B, respectively). AIx, AP and SEVR were all significantly correlated with MDRD eGFR both univariately and in heart rate- and height-adjusted correlations (Table 2).
PWA Measures and Cystatin C
Cystatin C was significantly correlated with AP and SEVR, but not with AIx, in both univariate and heart rate- and height-adjusted correlations (Table 2). Those with high cystatin C (≥1.0) did however have significantly higher AIx (28.1±12.1 vs. 21.3±10.6; p<.05, Figure 1A) and AP (13.6±10.0 vs. 7.71±4.9; p<.01, Figure 1B), compared to those with normal cystatin C levels (<1.0). SEVR, although about 10 units lower in the higher cystatin C group, was not significantly different between the groups (Figure 1C). Other factors univariately associated with high cystatin C were older age (but not longer diabetes duration), lower eGDR, higher SBP (but not DBP), and expectedly, higher AER, serum creatinine and lower eGFR (all p<.001) (Table 1).
Subendocardial Viability Ratio and Renal Measures
To determine if PWA measures were associated with early renal damage, multivariate logistic regression was performed. Those with macroalbuminuria (n=14) were excluded, and normoalbuminuria and microalbuminuria were modeled. AP did not remain significantly associated with presence of MA in multivariate models, nor was it associated with AER level in linear regression analysis. However, in multivariate analysis, each standardized unit decrease in SEVR was associated with a 65% increased risk for MA (p=.005) (Table 3, Model 1). In fact, SEVR preferentially entered multivariate models for MA over brachial SBP and DBP measures. Lower eGDR was also associated with presence of MA multivariately (OR=0.52; 95%CI: 0.30–0.89; p=.017). In linear regression for AER, among those with AER <200 μg/min, SEVR was again significant and entered the model preferentially over SBP and DBP. Lower HDL-c was also associated with increased AER in this model (Table 4).
Table 3.
Multivariate logistic regression model for renal measures in the Pittsburgh EDC Pulse Wave Analysis study population
| Model 1a | Model 2b | Model 3b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Microalbuminuriac | GFR < 60 ml/min/1.73 m2 | Cystatin C ≥ 1.0 mg/l | |||||||
| Variable | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| SEVR | 0.35 | 0.17–0.73 | .005 | 0.44 | 0.22–0.89 | .02 | 0.31 | 0.13–0.73 | .007 |
| eGDR | 0.52 | 0.30–0.89 | .017 | --- | --- | --- | --- | --- | --- |
| Heart Rate | 0.72 | 0.38–1.38 | .32 | 0.42 | 0.20–0.88 | .02 | 0.20 | 0.07–0.57 | .003 |
| ACEI/ARB Use | --- | --- | --- | 3.57 | 1.18–10.8 | .02 | 5.65 | 1.21–26.4 | .03 |
| Hypertension | --- | --- | --- | --- | --- | --- | 6.35 | 1.88–21.5 | .003 |
Odds Ratios are per standardized unit.
Variables available to Model 1: age, SEVR, systolic BP, diastolic BP, eGDR, WHR, BMI, heart rate, ACEI/ARB use, HDL-cholesterol, non-HDL-cholesterol, smoking history, anti-lipidemic agent use
Variables available to Models 2 & 3: Model 1 variables (except age) + hypertension status (BP>130/80 or medication)
Microalbuminuria vs. Normoalbuminuria
Abbreviations: SEVR, subendocardial viability ratio; eGDR, estimated glucose disposal rate; ACEI/ARB, ACE inhibitor/Angiotensin II receptor blocker; BP, blood pressure; BMI, body mass index; WHR, waist-to-hip ratio
Table 4.
Multivariate linear regression model for albumin excretion rate in those within the normal and microalbuminuric range
| Variables | β | standard error | p-value |
|---|---|---|---|
| SEVR | −11.3 | 3.71 | .003 |
| HDL-c | −6.56 | 2.64 | .015 |
| Heart Rate | −5.76 | 3.68 | .13 |
All variables are standardized to the study population
Variables available to the model: age, subendocardial viability ratio (SEVR), systolic BP, diastolic BP, waist-to-hip ratio, estimated glucose disposal rate, HDL-cholesterol, non-HDL-cholesterol, smoking history, ACE inhibitor/Angiotensin II receptor blocker use, heart rate, body mass index, anti-lipidemic agent use
A comparison between those with moderately to severely impaired renal function (eGFR<60) and those with normal to mildly impaired renal function (eGFR≥60) showed a significant difference in AIx (p=.02) and AP (p=.006), but not SEVR (p=.13). However, multivariately, each standard deviation decrease in SEVR was associated with a 56% increased risk for low eGFR (<60) (p=.02), was selected over brachial SBP and DBP measures, and remained significantly associated with low eGFR after adjusting for heart rate and ACEI/ARB medication use. No other factors were significantly associated with eGFR in this model (Table 3, Model 2). Neither AIx nor AP was significant in multivariate models for low eGFR. Linear regression for eGFR in the total population showed results similar to logistic regression for AIx, AP and SEVR. However, SBP diminished the statistical significance of the SEVR–eGFR relationship in the linear model.
Results of logistic regression for cystatin C were similar to those seen for low eGFR. AP, although significant in a model only adjusting for heart rate and height, did not remain significant multivariately. In multivariate models for high cystatin C, one standardized unit decrease in SEVR translated into a 69% increased risk for high cystatin C (Table 3, Model 3). In a model allowing for brachial SBP and DBP, both SEVR and SBP were significantly associated with high cystatin C. However, the model with hypertension status instead of SBP was a better fitting model (Table 3, Model 3). Adjustment for PWD use instead of ACEI/ARB use did not substantially change any of the models in Table 3. Neither age nor sex entered models for cystatin C (data not shown).
DISCUSSION
The prominent findings of the present study are that pulse wave analysis measures –augmentation pressure (a measure of arterial stiffness) and subendocardial viability ratio (an estimate of myocardial perfusion), in particular – are associated with both renal damage (even at the microalbuminuric level) and poor renal function in type 1 diabetes. SEVR’s relationship with both renal function and renal damage remained significant in multivariate models, and SEVR was preferred over brachial SBP and DBP in models predicting albumin excretion rate, microalbuminuria, high cystatin C and low eGFR.
Univariately, AIx was not associated with AER (continuously or categorically), was only borderline significantly associated with eGFR (continuously and categorically), and was associated with high cystatin C compared to normal but not with cystatin C continuously. In the Hoorn Study, there were significant increases in aortic AIx with increasing albuminuric quartile (measured using urinary albumin-to-creatinine ratio (ACR) in a general population).37 However, after adjusting for age, sex, glucose tolerance status and mean arterial pressure, AIx was no longer significantly associated with ACR. This study also found no significant relationship between AIx and eGFR (also MDRD calculated), consistent with the present study. It is important to note, however, that the Hoorn Study did find an association between other measures of vascular stiffness and both ACR and eGFR. Likewise, in the present study, higher augmentation pressure (AP) was associated with higher AER and higher albuminuric category, as well as with eGFR and cystatin C. The difference in the associations of AIx and AP with renal damage and renal function may be due to the notion that AIx has limitations in its use as an arterial stiffness index in some populations due to its calculation: AP ÷ PP. Simultaneous rises in both AP and PP can result in a stable AIx, thereby reducing its usefulness as a surrogate for change in central pressure waveforms.38 This limitation is especially apparent in older populations, for which AP may be a more suitable measure of arterial stiffness,39 and may occur earlier in T1D populations, especially in those who have higher pulse pressures and accelerated vascular aging.38, 40
To date, we are unaware of any studies that have examined the relationship between SEVR and renal function or renal damage. Our findings suggest that estimated myocardial perfusion is reduced in those with greater damage and reduced function. This is even true when comparing microalbuminuric T1D participants to those within the normal range. This finding is consistent with the fact that albuminuria, even at the micro level, is associated with CAD in T1D and other populations.41–44
In our renal function analyses, we looked at both eGFR and cystatin C, because eGFR is used in current clinical practice, and recent findings suggest that cystatin C may be superior to serum creatinine in assessing GFR, especially in T1D populations.45, 46 AP was associated with both eGFR and cystatin C, but the addition of SBP to the multivariate models eliminated the statistical significance of the association. This is not surprising since AP represents the increase of central systolic blood pressure due to early return of the reflected pulse wave which would be represented as an increase in brachial SBP. Yoshida et al. found an association, albeit weak, between increased brachial-ankle PWV, another measure of arterial stiffness, and MDRD eGFR in those with normal to mild (eGFR = 60–90 ml/min/1.73 m2) renal function impairment.47 Wang et al. found a stepwise increase in PWV with decreasing chronic kidney disease category.20 Although we also found an association between arterial stiffness (using AP) and renal function, the only significant association was found when comparing those with normal and mild impairment to those with moderate and severe impairment. In other regression models, both linear in overall or in subgroups of the population (no/mild impairment vs. moderate/severe) and logistic models for mild impairment compared to normal function, neither AP nor SEVR was associated with eGFR outcomes.
Chade et al. showed that low MDRD eGFR (<60) was univariately associated with coronary microvascular dysfunction (defined as coronary flow reserve (CFR) < 2.5, evaluated using intracoronary adenosine).48 This is consistent with our finding that SEVR is associated with eGFR. However, in multivariate analysis, the association did not remain significant. The attenuation of the association between renal function and reduced CFR occurred after adjusting for age and gender. In our multivariate analysis detailed in Table 3 (Model 2), we did not make age or sex available to the eGFR models, since age and sex are used in the calculation of MDRD eGFR. When we allowed for these two variables, they did enter the models with over-inflated odds ratios. By not allowing age and sex to distort the analysis, we find that SEVR is associated with renal function as measured by eGFR, which is confirmed by our cystatin C analysis, which allowed for both age and sex, and SEVR was significantly related to high cystatin C.
This study is not without limitations. First, the sample size was relatively limited. However, this is the largest study to date to assess PWA measures (AIx, AP, and SEVR) in type 1 diabetes. Another limitation is that compared to those at the 18-year follow-up who did not have PWA measures, the PWA study group had significantly lower follow-up AER measures and waist-to-hip ratio, and therefore, may represent a healthier segment of our type 1 diabetes population (data not shown). Finally, this study examines cross-sectional associations between PWA measures and renal measures, and therefore, prospective studies are necessary to confirm these results. These limitations, however, are more likely to hinder finding significant relationships than demonstrate false relationships.
In summary, higher augmentation pressure and lower subendocardial viability ratio are associated with both renal damage and renal function in T1D. Of great importance is the positive relationship between SEVR and AER in those with no or mild renal damage, which appears to be a better predictor of AER than SBP. These findings underscore the potential value of PWA in the early detection of those at renal and cardiovascular disease risk. Hopefully, this will also lead to a better understanding of the entire pulse wave, rather than just using brachial SBP and DBP, and its role in renal dysfunction and damage. Pulse wave analysis can be quickly and easily measured in a clinical setting and thus might be a feasible addition to assess renal complication risk and facilitate earlier intervention (e.g., ACEI or ARB use). Prospective research is needed to confirm these cross-sectional findings.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01-DK034818). AMS was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (F30-DK082137).
Footnotes
The authors have no relevant conflict of interest to disclose.
References
- 1.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006 May;55(5):1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association: clinical practice recommendations 2002: Diabetic Nephropathy. Diabetes Care. 2002 Jan;25( Suppl 1):S85–89. doi: 10.2337/diacare.25.2007.s1. [DOI] [PubMed] [Google Scholar]
- 3.Coonrod BA, Ellis D, Becker DJ, et al. Predictors of microalbuminuria in individuals with IDDM. Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 1993 Oct;16(10):1376–1383. doi: 10.2337/diacare.16.10.1376. [DOI] [PubMed] [Google Scholar]
- 4.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004 May 8;328(7448):1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yishak AA, Costacou T, Virella G, et al. Novel predictors of overt nephropathy in subjects with type 1 diabetes. A nested case control study from the Pittsburgh Epidemiology of Diabetes Complications cohort. Nephrol Dial Transplant. 2006 Jan;21(1):93–100. doi: 10.1093/ndt/gfi103. [DOI] [PubMed] [Google Scholar]
- 6.Kostraba JN, Klein R, Dorman JS, et al. The epidemiology of diabetes complications study. IV. Correlates of diabetic background and proliferative retinopathy. Am J Epidemiol. 1991 Feb 15;133(4):381–391. doi: 10.1093/oxfordjournals.aje.a115892. [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Borch-Johnsen K, Molarius A, et al. Incidence of cardiovascular disease in Type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia. 1998 Jul;41(7):784–790. doi: 10.1007/s001250050988. [DOI] [PubMed] [Google Scholar]
- 8.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med. 2004 Sep 27;164(17):1917–1924. doi: 10.1001/archinte.164.17.1917. [DOI] [PubMed] [Google Scholar]
- 9.Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int. 2002 Oct;62(4):1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 10.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008 May 15;167(10):1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 11.Henry RM, Kamp O, Kostense PJ, et al. Mild renal insufficiency is associated with increased left ventricular mass in men, but not in women: an arterial stiffness-related phenomenon--the Hoorn Study. Kidney international. 2005 Aug;68(2):673–679. doi: 10.1111/j.1523-1755.2005.00445.x. [DOI] [PubMed] [Google Scholar]
- 12.Fox CS, Larson MG, Keyes MJ, et al. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: the Framingham Heart Study. Kidney Int. 2004 Nov;66(5):2017–2021. doi: 10.1111/j.1523-1755.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 13.Maahs DM, Ogden LG, Kretowski A, et al. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes. 2007 Nov;56(11):2774–2779. doi: 10.2337/db07-0539. [DOI] [PubMed] [Google Scholar]
- 14.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992 Aug 8;340(8815):319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 15.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005 Aug 16;112(7):969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 16.O’Rourke MF. Wave travel and reflection in the arterial system. J Hypertens Suppl. 1999 Dec;17(5):S45–47. [PubMed] [Google Scholar]
- 17.Taal MW, Sigrist MK, Fakis A, Fluck RJ, McIntyre CW. Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract. 2007;107(4):c177–181. doi: 10.1159/000110678. [DOI] [PubMed] [Google Scholar]
- 18.Mule G, Cottone S, Cusimano P, et al. Inverse relationship between ambulatory arterial stiffness index and glomerular filtration rate in arterial hypertension. Am J Hypertens. 2008 Jan;21(1):35–40. doi: 10.1038/ajh.2007.10. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa N, Takahashi F, Chinda J, et al. A newly estimated glomerular filtration rate is independently associated with arterial stiffness in Japanese patients. Hypertens Res. 2008 Feb;31(2):193–201. doi: 10.1291/hypres.31.193. [DOI] [PubMed] [Google Scholar]
- 20.Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005 Mar;45(3):494–501. doi: 10.1053/j.ajkd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002 Oct 15;106(16):2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 22.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003 May;63(5):1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 23.Abhayaratna WP, Barnes ME, O’Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006 Nov 15;98(10):1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Orchard TJ, Dorman JS, Maser RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care. 1990 Jul;13(7):741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci (Lond) 2007 Aug;113(4):157–170. doi: 10.1042/CS20070080. [DOI] [PubMed] [Google Scholar]
- 26.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Preventive medicine. 1976 Jun;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 27.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- 28.Warnick GR, Albers JJ. Heparin--Mn2+ quantitation of high-density-lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978 Jun;24(6):900–904. [PubMed] [Google Scholar]
- 29.Olson JC, Erbey JR, Williams KV, et al. Subclinical atherosclerosis and estimated glucose disposal rate as predictors of mortality in type 1 diabetes. Ann Epidemiol. 2002 Jul;12(5):331–337. doi: 10.1016/s1047-2797(01)00269-1. [DOI] [PubMed] [Google Scholar]
- 30.Ellis D, Coonrod BA, Dorman JS, et al. Choice of urine sample predictive of microalbuminuria in patients with insulin-dependent diabetes mellitus. Am J Kidney Dis. 1989 Apr;13(4):321–328. doi: 10.1016/s0272-6386(89)80039-3. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007 Apr;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 32.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997 Apr 1;95(7):1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 33.O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. British journal of clinical pharmacology. 2001 Jun;51(6):507–522. doi: 10.1046/j.0306-5251.2001.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998 Dec;16(12 Pt 2):2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. The Journal of physiology. 2000 May 15;525(Pt 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckberg GD, Archie JP, Fixler DE, Hoffman JI. Experimental subendocardial ischemia during left ventricular hypertension. Surg Forum. 1971;22:124–125. [PubMed] [Google Scholar]
- 37.Hermans MM, Henry R, Dekker JM, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007 Jun;18(6):1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 38.Cheng LT, Tang LJ, Cheng L, Huang HY, Wang T. Limitation of the augmentation index for evaluating arterial stiffness. Hypertens Res. 2007 Aug;30(8):713–722. doi: 10.1291/hypres.30.713. [DOI] [PubMed] [Google Scholar]
- 39.Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing. 2007 Jan;36(1):43–48. doi: 10.1093/ageing/afl115. [DOI] [PubMed] [Google Scholar]
- 40.Ronnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH. Altered age-related blood pressure pattern in type 1 diabetes. Circulation. 2004 Aug 31;110(9):1076–1082. doi: 10.1161/01.CIR.0000139903.29522.8D. [DOI] [PubMed] [Google Scholar]
- 41.Hall WD. Abnormalities of kidney function as a cause and a consequence of cardiovascular disease. Am J Med Sci. 1999 Mar;317(3):176–182. doi: 10.1097/00000441-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Soedamah-Muthu SS, Chaturvedi N, Toeller M, et al. Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care. 2004 Feb;27(2):530–537. doi: 10.2337/diacare.27.2.530. [DOI] [PubMed] [Google Scholar]
- 43.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torffvit O, Lovestam-Adrian M, Agardh E, Agardh CD. Nephropathy, but not retinopathy, is associated with the development of heart disease in Type 1 diabetes: a 12-year observation study of 462 patients. Diabet Med. 2005 Jun;22(6):723–729. doi: 10.1111/j.1464-5491.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 45.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002 Aug;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 46.Buysschaert M, Joudi I, Wallemacq P, Hermans MP. Comparative performance of serum cystatin-c versus serum creatinine in diabetic subjects. Diabetes Metab. 2003 Sep;29(4 Pt 1):377–383. doi: 10.1016/s1262-3636(07)70048-4. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida M, Tomiyama H, Yamada J, et al. Relationships among renal function loss within the normal to mildly impaired range, arterial stiffness, inflammation, and oxidative stress. Clin J Am Soc Nephrol. 2007 Nov;2(6):1118–1124. doi: 10.2215/CJN.01880507. [DOI] [PubMed] [Google Scholar]
- 48.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney international. 2006 Jan;69(2):266–271. doi: 10.1038/sj.ki.5000031. [DOI] [PubMed] [Google Scholar]