Abstract
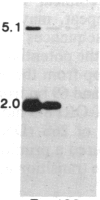
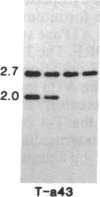
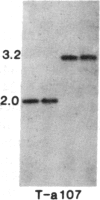
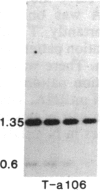
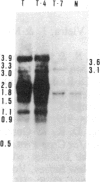
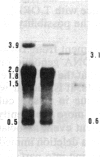
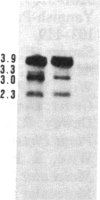
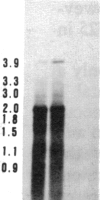
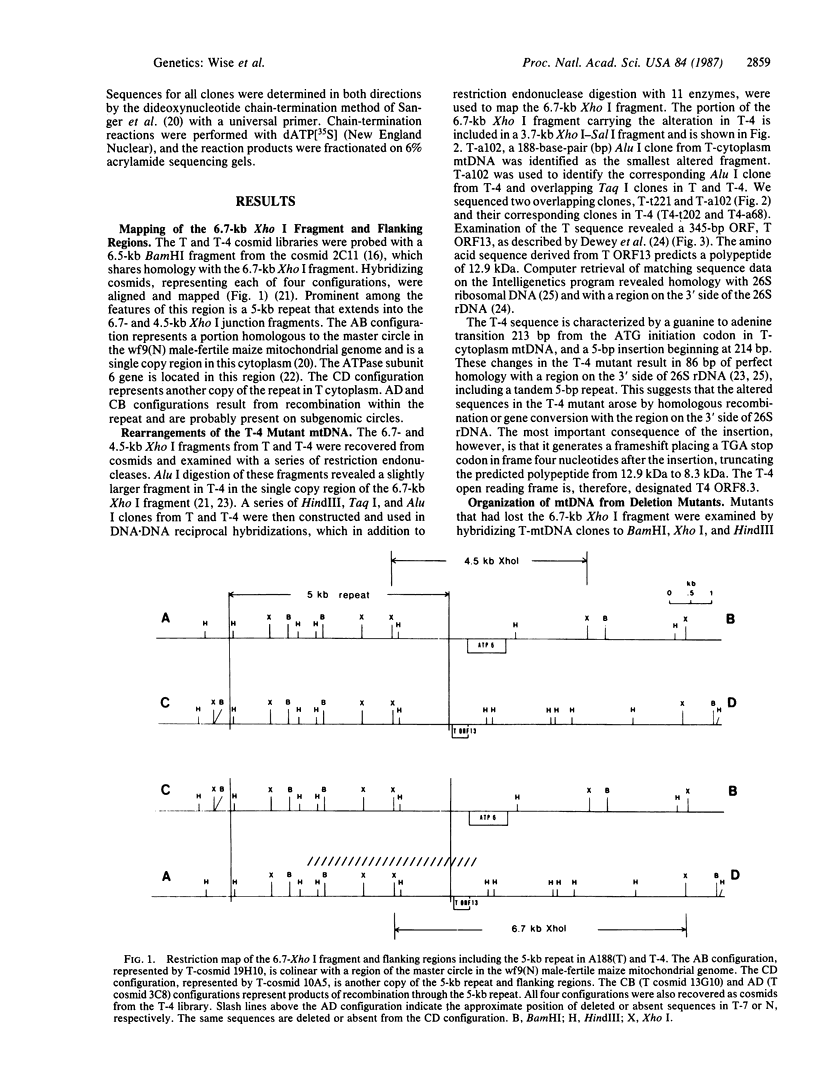
Tissue culture-derived mutants of male-sterile and disease toxin-sensitive Texas (T)-cytoplasm maize that exhibit male fertility and toxin insensitivity carry numerous alterations in mitochondrial DNA. In these mutants, a 6.7-kilobase Xho I fragment characteristic of parental T cytoplasm has been rearranged. In the mutant T-4, the parental 6.7-kilobase Xho I fragment contains a guanine to adenine transition adjacent to a 5-base-pair insertion not found in T cytoplasm. The insertion, internal to a 345-base-pair open reading frame (T ORF13), generates a frameshift, resulting in a premature stop codon that terminates the open reading frame at base pair 222. In other mutants, the 345-base-pair ORF is part of a 3-kilobase deletion, which extends into a 5-kilobase repeat characteristic of mtDNA from T but not N male-fertile cytoplasm. Clones specific to T ORF13 hybridize to eight transcripts in T and T-4, yet only hybridize to three transcripts in T-7, a deletion mutant. Transcription of the T ORF13 region appears not to be altered in T-4, but the frameshift mutation in the T ORF13 reading frame indicates that a biologically inactive gene product could be associated with the mutational events. The results suggest that cytoplasmic male sterility and disease toxin sensitivity may be associated with presence of T ORF13 in T-cytoplasm maize.
Keywords: Cochliobolus heterostrophus toxin, cytoplasmic male sterility, tissue culture
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dale R. M., Mendu N., Ginsburg H., Kridl J. C. Sequence analysis of the maize mitochondrial 26 S rRNA gene and flanking regions. Plasmid. 1984 Mar;11(2):141–150. doi: 10.1016/0147-619x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., Timothy D. H. Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol. 1985 Nov;79(3):914–919. doi: 10.1104/pp.79.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3841–3845. doi: 10.1073/pnas.75.8.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbach B. G., Green C. E., Donovan C. M. Inheritance of selected pathotoxin resistance in maize plants regenerated from cell cultures. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5113–5117. doi: 10.1073/pnas.74.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughnan J. R., Gabay-Laughnan S. Cytoplasmic male sterility in maize. Annu Rev Genet. 1983;17:27–48. doi: 10.1146/annurev.ge.17.120183.000331. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J., Koeppe D. E. Southern corn leaf blight: susceptible and resistant mitochondria. Science. 1971 Jul 2;173(3991):67–69. doi: 10.1126/science.173.3991.67. [DOI] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S. Heterogeneity of Maize Cytoplasmic Genomes among Male-Sterile Cytoplasms. Genetics. 1978 May;89(1):121–136. doi: 10.1093/genetics/89.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]