Abstract
During spermatogenesis, extensive junction restructuring takes place at the blood-testis barrier (BTB) and the Sertoli cell-spermatid interface known as the apical ectoplasmic specialization (apical ES, a testis-specific adherens junction) in the seminiferous epithelium. However, the mechanism(s) that regulates these critical events in the testis remains unknown. Based on the current concept in the field, changes in the phosphorylation status of integral membrane proteins at these sites can induce alterations in protein endocytosis and recycling, causing junction restructuring. Herein, c-Yes, a non-receptor protein tyrosine kinase, was found to express abundantly at the BTB and apical ES stage-specifically, coinciding with junction restructuring events at these sites during the seminiferous epithelial cycle of spermatogenesis. c-Yes also structurally associated with adhesion proteins at the BTB (e.g., occludin and N-cadherin) and the apical ES (e.g., β1-integrin, laminin β3 and γ3), possibly to regulate phosphorylation status of proteins at these sites. SU6656, a selective c-Yes inhibitor, was shown to perturb the Sertoli cell tight junction-permeability barrier in vitro, which is mediated by changes in the distribution of occludin and N-cadherin at the cell-cell interface, moving from cell surface to cytosol, thereby destabilizing the tight junction-barrier. However, this disruptive effect of SU6656 on the barrier was blocked by testosterone. Furthermore, c-Yes is crucial to maintain the actin filament network in Sertoli cells since a blockade of c-Yes by SU6656 induced actin filament disorganization. In summary, c-Yes regulates BTB and apical ES integrity by maintaining proper distribution of integral membrane proteins and actin filament organization at these sites.
Keywords: Testis, c-Yes, spermatogenesis, blood-testis barrier, apical ectoplasmic specialization, seminiferous epithelial cycle, cell adhesion
Introduction
In mammalian testes, such as in rats, the BTB created by adjacent Sertoli cells near the basement membrane forms an immunological barrier to segregate meiotic and post-meiotic germ cell development from the systemic circulation (Cheng and Mruk, 2010; Meinhardt and Hedger, 2010). At stage VIII of the seminiferous epithelial cycle of spermatogenesis, however, preleptotene spermatocytes must traverse the blood-testis barrier (BTB) to the apical compartment while transforming to leptotene spermatocytes. Following meiosis, spermatids undergo spermiogenesis, which is associated with the appearance of apical ectoplasmic specialization (apical ES), a testis-specific adherens junction (AJ), at the interface between Sertoli cells and step 8–19 spermatids (Cheng and Mruk, 2010; de Kretser, 1990). In short, these events are associated with junction restructuring at the Sertoli-Sertoli cell (e.g., the BTB) and Sertoli cell-spermatid (e.g., apical ES) interface. However, the molecules and mechanism(s) that regulate these events remain unclear (Cheng and Mruk, 2010; Cheng and Mruk, 2009) due to the lack of information on the proteins that regulate the adhesion protein complexes at these sites. While some of the constituent protein complexes at the BTB and the apical ES are known [for reviews, see (Cheng and Mruk, 2002; Cheng and Mruk, 2010; Mruk et al., 2008)], the regulatory molecules that confer cell adhesion function remain largely unexplored. Unlike other blood-tissue barriers (e.g., the blood-brain barrier), the BTB is composed of co-existing tight junction (TJ), basal ES (a testis-specific AJ), desmosome-like junction and gap junction (Mruk et al., 2008); whereas the apical ES is a hybrid AJ composed of proteins usually restricted to AJ (e.g., cadherins), focal adhesion complex (e.g., integrins, laminins), gap junction (e.g., connexin 43), and TJ (e.g., JAM-C, CAR) in other epithelia [for a review, see (Cheng and Mruk, 2010)] illustrating the complexity of cell junctions in the seminiferous epithelium.
c-Yes is a member of the Src non-receptor protein tyrosine kinase family known to regulate cell growth and survival, apoptosis, adhesion, cytoskeletons, and differentiation (Summy et al., 2003; Clump et al., 2005; Boutros et al., 2008). Although c-Yes, similar to c-Src, mediates integrin-based signaling function in different epithelia at the focal adhesion complex (Boutros et al., 2008), few studies were performed to explore its function at the BTB. Of the Src tyrosine kinase family, in which there are currently 11 members in humans and 9 members in rodents (namely c-Src, c-Yes, Fyn, Lyn, Lck, Hck, Blk, Fgr and Yrk) (Meyn and Smithgall, 2009; Manning et al., 2002), c-Src and c-Yes share a high degree of homology in their primary amino acid sequences except for their unique N-terminal domains, and both kinases are ubiquitously expressed in mammalian cells (Summy et al., 2003; Thomas and Brugge, 1997). In rat testes, c-Src expressed stage-specifically (Nishio et al., 1995; Wang et al., 2000). Moreover, c-Src has been linked to forming of regulatory protein complexes at the BTB (Lie et al., 2010a; Li et al., 2009; Wang et al., 2007) and apical ES (Zhang et al., 2005; Wong et al., 2005; Lee and Cheng, 2005) to modulate junction restructuring events at these sites. Yet, little is known regarding the role of c-Yes in the testis. In other epithelia/endothelia and cancer cells, it has been hinted that considerable overlapping functions may be complied by c-Yes and c-Src (Staley et al., 1997; Thomas and Brugge, 1997) because of high homology and tissue co-distribution pattern. However, there is mounting evidence suggesting the differential functions of c-Yes and c-Src (Zhao et al., 1992; Sato et al., 2009; Mariotti et al., 2001; Monteiro, 2006). For instance, c-Yes is monopalmitoylated so that it can be transported from the Golgi pool of caveolin to plasma membrane to affect endocytic vesicle-mediated protein trafficking, whereas c-Src is nonpalmitoylated and it is shuffled between the plasma membrane and late endosomes/lysosomes to regulate protein endocytosis (Sato et al., 2009). Additionally, c-Yes forms a functional complex with occludin in MDCK and human intestinal cell line T84, and a disruption of this complex leads to dephosphorylation of occludin and a disruption of the TJ-barrier (Chen et al., 2002; Nusrat et al., 2000). Herein, we report the role of c-Yes in junction and cytoskeletal dynamics during spermatogenesis.
Materials and methods
Animals
The use of Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) for experiments reported herein was approved by the Institutional Animal Care and Use Committee of The Rockefeller University with Protocol Number 09016. Groups of rats received a single dose of adjudin (50 mg/kg b.w. by gavage) versus controls (untreated rats) (Cheng et al., 2001) with n = 3–6 rats per time point.
Isolation of Sertoli cells and primary Sertoli cell cultures
Sertoli cells were isolated from the testes of 20-day-old male Sprague-Dawley rats as described (Grima et al., 1992). Sertoli cells were plated onto 12-well plates coated with Matrigel™ basement membrane matrix (BD Biosciences, San Jose, CA) at a density of 0.5 × 106 cells/cm2 in F12/DMEM medium supplemented with different factors, and incubated at 35 °C with 95% air and 5% CO2 (v/v) in a humidified atmosphere. To obtain Sertoli cell cultures with ~98% pure, about 48 hours after cell plating, cultures were hypotonically treated with 20 mM Tris, pH 7.4 at 22 °C for 2.5 min to lyse residual germ cells (Galdier et al., 1981) as described (Grima et al., 1997; Lui et al., 2003a). The final cell preparations have negligible contamination of Leydig cells, peritubular myoid cells and/or germ cells (Lee et al., 2004b). To assess the assembly of a functional TJ-permeability barrier, Sertoli cells were cultured on Millicell inserts (Millipore, Billerica, MA) coated with Matrigel™ at a density of 2 × 106 cells/cm2 (Grima et al., 1998). Transepithelial electrical resistance (TER) was recorded using a Millipore Millicell-ERS System as described (Lui et al., 2001; Siu et al., 2009a).
Seminiferous tubule (ST) isolation and co-immunoprecipitation (Co-IP)
Adult rat testes were decapsulated and seminiferous tubules were isolated as described (Zwain and Cheng, 1994). Separated tubules were then pelleted at 700 g for 2 min and lysed in IP lysis buffer. For Co-IP, ~800 μg of total protein derived from the ST lysate was used in each sample tube and incubated with 2 μg of anti-c-Yes monoclonal antibody at 4 °C overnight. Thereafter, 20 μl of Protein A/G plus-agarose (Santa Cruz Biotechnology Inc., Santa Cruz, CA) was added to the immune complex (antigen-antibody complex) and incubated for 5~6 hr at 4°C on a rocking platform. Unbound protein fraction was then removed, and immunoprecipitated complexes were washed with IP lysis buffer and extracted from the Protein A/G plus-agarose using SDS sample buffer (0.125 M Tris, 1% SDS, 1.6% β-mercaptoethanol, 20% glycerol [v/v], pH 6.8 at 22 °C), and analyzed by immunoblotting using specific antibodies (Table 1).
Table 1.
Antibodies used for various experiments in this report.
| Antibody | Host species | Vendor | Catalog number | Application(s)/ Dilution(s) |
|---|---|---|---|---|
| c-Yes | Rabbit | Santa Cruz Biotechnology | sc-28883 | IB (1:200) |
| Mouse | Santa Cruz Biotechnology | sc-8403 | IB (1:200) IP (2 μg per reaction tube) IHC (1:75) IF (1:50, in vivo) |
|
| Mouse | BD Transduction laboratories | 610375 | IF (1:100, in vitro) | |
| Laminin β3 | Rabbit | Cheng Lab (Yan and Cheng, 2006) | IF (1:100) | |
| Laminin γ3 | Rabbit | Cheng Lab (Yan and Cheng, 2006) | IF (1:100) | |
| Occludin | Rabbit | Invitrogen | 71–1500 | IB (1:250) |
| IF (1:100) | ||||
| FAK | Rabbit | Millipore | 06–543 | IB (1:1000) |
| CAR | Rabbit | Santa Cruz Biotechnology | sc-15405 | IB (1:200) |
| JAM-A | Rabbit | Invitrogen | 36–1700 | IB (1:300) |
| IB (1:200) | ||||
| N-Cadherin | Rabbit | Santa Cruz Biotechnology | sc-7939 | IF (1:50) |
| β-Catenin | Rabbit | Invitrogen | 71–2700 | IB (1:250) |
| β1-Integrin | Rabbit | Santa Cruz Biotechnology | sc-8978 | IB (1:300) |
| Paxillin | Mouse | Invitrogen | AHO0492 | IB (1:250) |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | IB (1:200) |
| Vimentin | Mouse | Santa Cruz Biotechnology | sc-6260 | IB (1:200) |
| Clathrin | Mouse | BD Transduction Laboratories | 610499 | IB (1:1000) |
| IF (1:100) | ||||
| Androgen receptor | Rabbit | Santa Cruz Biotechnology | sc-816 | IB (1:200) |
IB, immunoblotting; IF, immunofluorescence microscopy; IP, immunoprecipitation.
RT-PCR
RT-PCR was performed essentially as earlier described (Lie et al., 2010a). The primer pair (Gene Link, Hawthorne, NY) used for amplification of c-Yes gene was as follows: sense primer, 5′-GGGCTGCATTAAAAGTAAAG-3′ (nucleotides 3–22) and antisense primer, 5′-TCC-AAAAGGAGTCACCCCAG-3′ (nucleotides 191–210) (GenBank accession no. AB037472). Primers specific to the rat ribosomal protein gene S16 for normalizing was also included: sense primer, 5′-TCCGCTGCAGTCCGTTCAAGTCTT-3′ (nucleotides 15–38) and antisense primer, 5′-GCCAAACTTCTTGGATTCGCAGCG-3′ (nucleotides 379–399) (GenBank accession no. X17665).
Immunoblot analysis
Protein extracts from rat testis, germ and Sertoli cells were prepared in IP (immunoprecipitation) lysis buffer (10 mM Tris, 0.15 M NaCl, 1% NP-40 and 10% glycerol [v/v], pH 7.4 at 22 °C). Protease and phosphatase inhibitor cocktails obtained from Sigma-Aldrich (St. Louis, MO) were added immediately before use at a 1:100 dilution. Total protein concentration was determined by DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Immunoblot analysis was performed using a Fujifilm LAS-4000 mini imaging system as described (Yan and Cheng, 2006; Siu et al., 2009b).
Immunohistochemistry (IHC) and dual-labeled immunofluorescence (IF) analysis
Frozen sections of adult rat testes (7-μm thickness) were obtained with a cryostat at -−20 °C, adhered to poly-L-lysine coated microscope slides (Polysciences, Warrington, PA), and fixed in Bouin’s fixative. For IHC staining, endogenous peroxidase activity was quenched in 3% H2O2 in methanol. Prior to serum blocking step, sections were pretreated in 0.1% Triton-X100. After overnight incubation with the primary antibody (see Table 1) at room temperature, sections were submerged either with (i) biotinylated anti-mouse IgG at a 1:300 dilution (Vector Laboratories, Burlingame, CA) and signal was generated using 3-amino-9-ethylcarbazole (AEC, red precipitate) as the chromogenic substrates for IHC staining or with (ii) Alexa Fluor® secondary antibodies (1:200 dilution) and mounted with DAPI (Invitrogen, Eugene, OR) for immunofluorescence microscopy. For immunofluorescence cell staining, Sertoli cells cultured at ~0.025–0.05 × 106 cells/cm2 on Matrigel-coated coverslips or glass slides were fixed with methanol at −20 °C for 5 min. For F-actin staining, sections or cells were fixed with 4% paraformaldehyde (w/v) in PBS (10 mM NaH2PO4 and 0.15 M NaCl, pH 7.4 at 22 °C) at room temperature for 10 min, to be followed by an incubation with rhodamine phalloidin (Invitrogen, Eugene, OR) and with the secondary antibody conjugated to Alexa Fluor® dye. Images were obtained using an Olympus BX61 fluorescence microscope (Olympus America, Melville, NY, USA), and TIFF images were acquired with the Olympus MicroSuite Five software package (Version 1224), and analyzed (such as image overlays) using Adobe PhotoShop.
Assessment of changes in protein distribution at the Sertoli-Sertoli cell interface following treatment with TGF-β3, testosterone (T), or SU6656
Sertoli cells were isolated and plated at time 0 as describe above. On day 4 when an intact cell epithelium was formed with a TJ-permeability barrier and ultrastructures of TJ and basal ES were detected by electron microscopy (Siu et al., 2005), Sertoli cells were treated with TGF-β3 at a concentration of 3 ng/ml which was selected as earlier described (Lui et al., 2001). Vehicle control (4 mM HCl/0.1% BSA) and cells without any treatment were served as controls. To examine the role of c-Yes in Sertoli cell TJ-permeability barrier function, Sertoli cells were exposed to T (Sigma-Aldrich, St. Louis, MO) at 2 × 10−7 M, SU6656 [2-oxo-3-(4, 5, 6, 7-tetrahydro-1H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid dimethylamide] (Calbiochem, San Diego, CA) at 2 × 10−8 M or T (2 × 10−7 M) + SU6656 (2 × 10−8 M) on day 3 and these concentrations were selected based on earlier reports (Chung and Cheng, 2001; Blake et al., 2000); controls included 0.1% ethanol/0.02% DMSO (vehicle control) and cells receiving no treatment. It is noted that SU6656 is a selective Yes inhibitor with an IC50 at 20 nM versus Src, Fyn and Lyn with an IC50 at 280 nM, 170 nM and 130 nM, respectively (Blake et al., 2000; Bowman et al., 2001), such that at 20 nM, only c-Yes was selectively inhibited. Cells were terminated at specified time points in IP lysis buffer for lysate preparation. For assessment of the effects of SU6656 on the Sertoli cell TJ- barrier, TER across the Sertoli cell epithelium was quantified up to 7 days.
Statistical analysis
Comparisons between treatment groups, such as those in Fig. 5B and Fig. 6B, were performed by one-way ANOVA and post hoc Dunnett’s test. Comparisons between adjudin-treated groups versus controls (such as at time 0 hr in Fig. 3B, 4B) or control group (such as in Fig. 7B) with the blots in controls arbitrarily set at 1.0 were performed using Student’s t-test. All statistical analyses were performed using GB-STAT (V7.0, Dynamic Microsystems, Silver Spring, MD). For in vitro experiments, a minimum of 3 independent experiments using different batches of Sertoli cell cultures (with triplicates for each time point) were performed, and at least n = 3 to 5 rats for each time point in in vivo experiments.
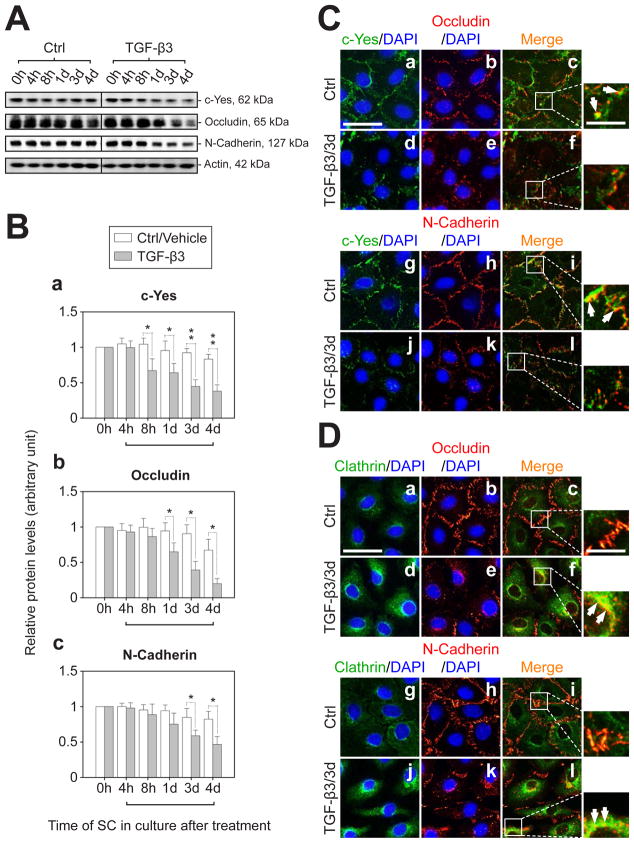
Fig. 5. Changes in the steady-state protein level of c-Yes and its co-localization with BTB-associated proteins following treatment of Sertoli cell epithelium with TGF-β3.
(A) Sertoli cells were cultured at a density of 0.5×106 cells/cm2 for 4-d to allow the establishment of a functional TJ-barrier. Thereafter, the Sertoli cell epithelium was submitted to TGF-β3 treatment (3 ng/ml), with F12/DMEM replaced daily which also contained the desired amount of TGF-β3. Controls were Sertoli cells cultured with vehicle. Cell lysates were obtained for immunoblot analysis. Actin served as a protein loading control. (B) Results of the immunoblotting study, such as those shown in (A) were summarized in this histogram. The protein levels of c-Yes (a), occludin (b) and N-cadherin (c) at 0 h (hour) with or without TGF-β3 treatment were arbitrarily set at 1. Each bar is the mean ± SD of three independent experiments. Herein, the protein level after TGF-β3 treatment versus its corresponding control was compared (*, P<0.05; **, P<0.01). h, hour(s); d, day(s). (C) IF staining of Sertoli cells treated with TGF-β3 for 3-d to evaluate the cytokine effects on the distribution of c-Yes (green, FITC), occludin (red, Cy3, a-f) and N-cadherin (red, Cy3, g-l) and the changes in their co-localization pattern. Besides a TGF-β3-induced decline in the levels of c-Yes, occludin and N-cadherin, treatment of cells with TGF-β3 also resulted in a loss of interactions between c-Yes and the integral membrane proteins at the BTB, because of the result of protein internalization, as indicated in smaller panels on the right-hand column, which are magnified views of the boxed area in c, f, i, and l (see f vs. c and l vs. i). White arrows illustrate the explicit co-localization of corresponding proteins in the same row. (D) Since the BTB marker proteins occludin and N-cadherin appeared to become internalized following TGF-β3 treatment, an endocytic vesicle marker clathrin (green, FITC) was used in this dual-labeled IF analysis to examine if the changes noted in C was mediated via endocytosis, and indeed occludin (red, Cy3, a-f) and N-cadherin (red, Cy3, g-l) were found to localize to this endocytic component more extensive after TGF-β3 treatment (see white arrows in the right panels in D). Bar in a (also applies to b-l in C-D) = 55 μm, wherein bar = 20 μm for the magnified box area in c, f, i and l in C and D, which applies to all micrographs in this column on the right panel.
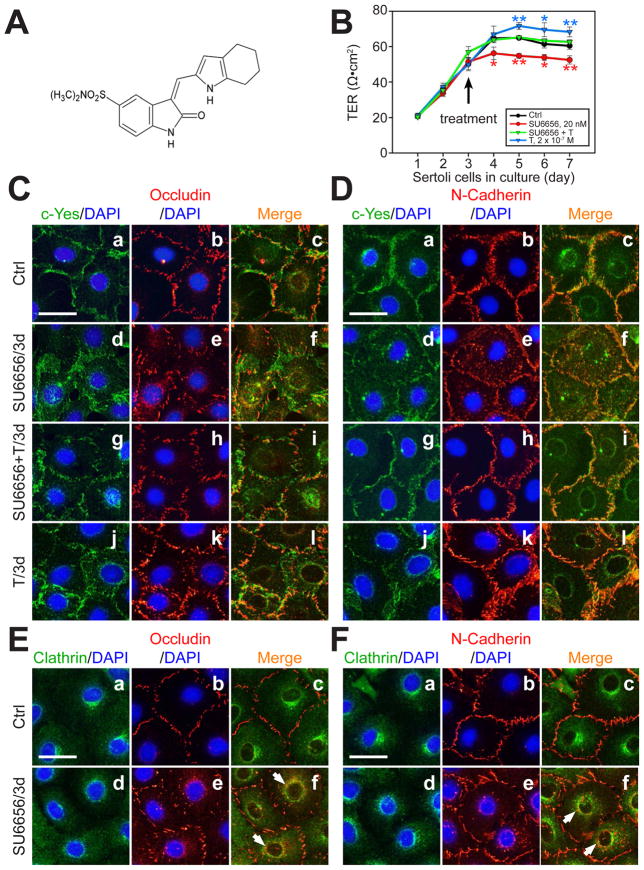
Fig. 6. A study to assess the effects of SU6656 on the Sertoli cell TJ-permeability barrier and protein distribution at the Sertoli-Sertoli cell interface.
(A) Structural formula of SU6656. (B) Sertoli cells were cultured at a density of 2×106 cells/cm2 on Matrigel-coated bicameral units and the TJ barrier function was monitored by quantifying the TER across the Sertoli cell epithelium. On day 3, Sertoli cells in the bicameral units were treated with either SU6656 (2×10−8 M), testosterone (T, 2×10−7 M), or SU6656 + T (see arrow). After TER reading, media were replaced with fresh F12/DMEM containing the desired amount of either SU6656 or T. Each data point is a mean ± SD of quadruplicates of a representative experiment, and this experiment was repeated three times using different batches of Sertoli cells and yielded similar results (*, P<0.05; **, P<0.01). (C, D) Dual-labeled IF analysis to assess changes on the protein distribution of either occludin (C) or N-cadherin (D) at the cell-cell interface using Sertoli cells cultured at 0.05 × 106 cells/cm2 on Matrigel-coated coverslips and cells were subjected to the same treatments as shown in B. Bar in a (also applies to b-l) = 45 μm. (E, F) Dual-labeled IF analysis was used to confirm the internalization of BTB proteins occludin and N-cadherin by their increase in partial co-localization with clathrin after SU6656 treatment. Bar in a (applies to b-f) = 45 μm. White arrows illustrate co-localization of either occludin or N-cadherin with clathrin.
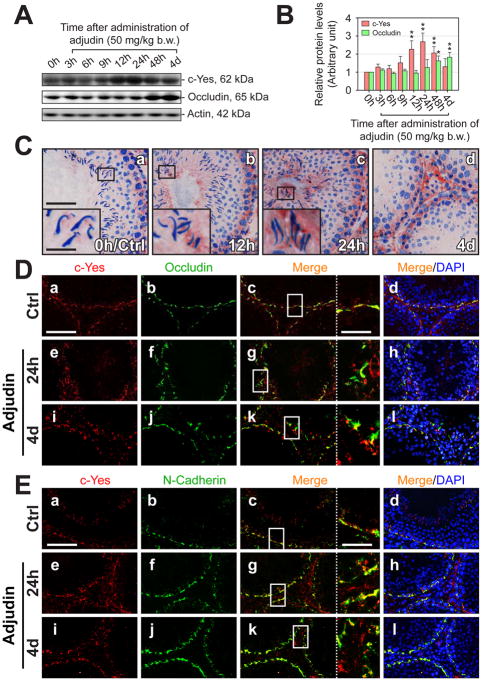
Fig. 3. Changes in the steady-state level of c-Yes and its association with TJ and basal ES proteins at the BTB during adjudin-induced anchoring junction restructuring and germ cell depletion.
(A) Testis lysates (50 μg protein) were used for immunoblotting to assess changes in the steady-state protein level of c-Yes, with occludin served as a positive control and actin a loading control. An induction of c-Yes expression was observed during the 12- to 48-h period after treatment. (B) A histogram with each bar shown as a mean ± SD of n = 3 rats using immunoblots such as those shown in (A). The protein levels of c-Yes and occludin at 0 hour were arbitrarily set at 1 and values were normalized and plotted against actin (*, P<0.05; **, P<0.01). (C) IHC staining of c-Yes obtained from the same rats after adjudin treatment used for immunoblot analysis. Within 12-h of treatment, some elongating/elongated spermatids were found to be misoriented and were depleting from the epithelium, even in a stage VII (C, b) tubule, and a considerable increase in c-Yes expression at the apical ES was detected in “prematurely” departing elongating spermatids in a stage VI (C, c) tubule (a-c, inset, magnified view of boxed area in the same panel). By 4 days following treatment, when virtually all tubules were devoid of elongating/elongated spermatids, c-Yes staining persisted at the BTB site. Bar in a (also applies to b-d) = 80 μm. Bar in inset in a = 20 μm, also applies insets in b and c. (D-E) IF staining showing c-Yes (red, Cy3) and BTB proteins occludin (green, FITC in D) and N-cadherin (green, FITC in E), and changes in their colocalization. Bar in a (also applies to b-l) = 120 μm. The staining patterns of c-Yes became “puffy” and less “compact” in the treatment groups versus control (normal) rats, and as a result, the association of c-Yes with these two BTB markers appeared to be considerably reduced, as shown in c, g, and k (inset at the right-hand partition with dotted lines is magnified view of boxed area in the same panel; bar = 40 μm). h, hour(s); d, day(s).
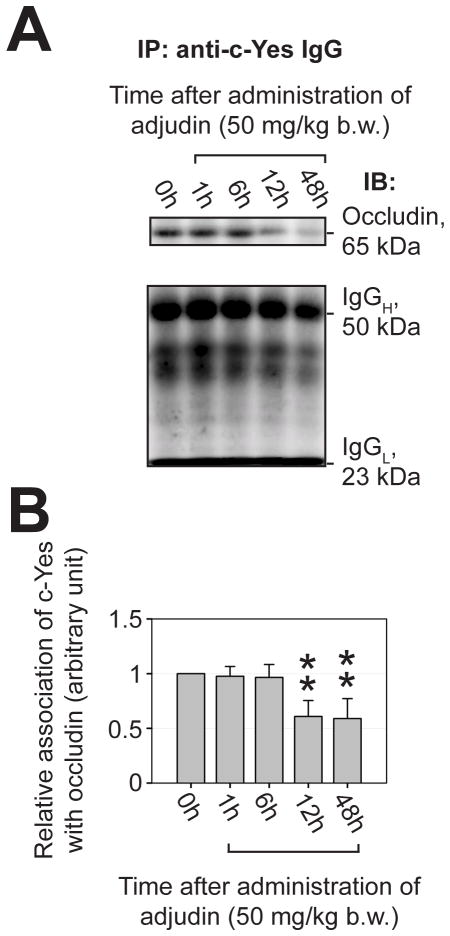
Fig. 4. A loss of association between c-Yes and integral membrane protein occludin at the BTB after administration of adjudin to adult rats was examined by co-immunoprecipitation (Co-IP).
(A) c-Yes was first immunoprecipitated using an anti-c-Yes antibody with ~800 μg protein from corresponding tubule lysates obtained from rats treated with adjudin, and the changes in its interaction with occludin was quantified using Co-IP (see Materials and Methods) (upper panel). The lower panel shows the heavy (H) and the light (L) chains of IgG following Co-IP from the same blots shown on the upper panel to illustrate equal protein loading and uniform protein transfer. (B) Results of Co-IP experiments shown in (A) were summarized in this histogram. Each bar is a mean ± SD of three independent experiments. The relative association of c-Yes with occludin at 0 hour was arbitrarily set at 1 (*, P<0.05; **, P<0.01).
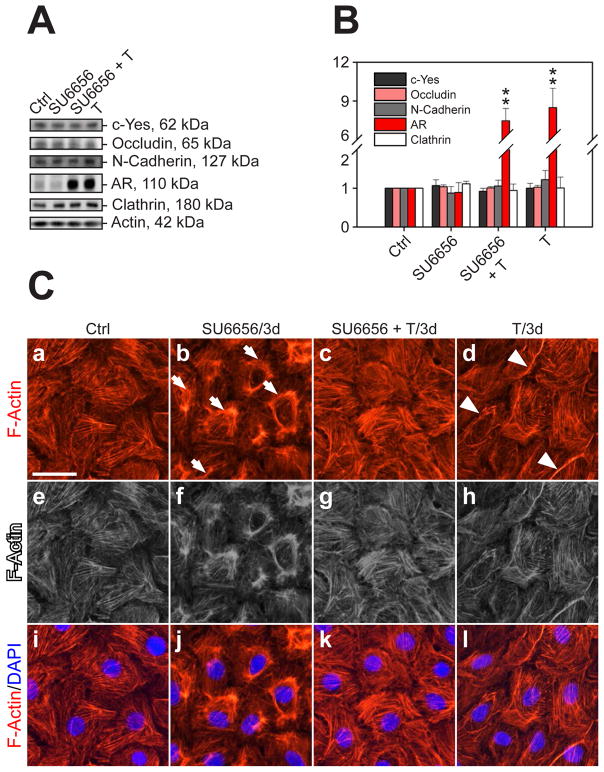
Fig. 7. A study to assess the effects of SU6656 on the steady-state protein levels of c-Yes and other BTB-associated proteins and cellular redistribution of F-actin in the Sertoli cell epithelium.
(A) Immunoblot analysis to assess changes in the protein levels of c-Yes, occludin, N-cadherin, androgen receptor (AR) and clathrin after treatment of SU6656, T or SU6656 + T. (B) Histogram of the immunoblot analysis from three independent experiments normalized against actin (**, P<0.01). (C) Sertoli cells were cultured at 0.025–0.04 × 106 cells/cm2 on Matrigel-coated coverslips for 1.5~2 days, forming an intact epithelium with ultrastructures of TJ and basal ES (Siu et al., 2005), before treatment. After 3-day incubation with SU6656, T or SU6656 + T, cells were stained with rhodamine phalloidin to visualize F-actin (red, Cy3). In comparison with controls, F-actin filaments in cells treated with SU6656 became considerably disorganized, and withdrew from the cell cytosol and more concentrated toward the nuclei (arrows in b) (see b and j vs. a and i, blue, DPAI). Gray scale images shown in e-h were used to better illustrate the changes shown in a-d vs. i-l. However, the presence of T was shown to block the disruptive effects of SU6656 on the actin filament network in Sertoli cells when compared to control cells (see c and k vs. b and j, a and i). In cells treated with T alone, the F-actin filament network was similar to control cells but the network appeared to be fortified at the cell-cell interface (see white arrowheads in d), apparently to promote the Sertoli cell TJ-permeability barrier function as shown in Fig. 6.
Results
c-Yes expressed stage-specifically in the seminiferous epithelium of adult rat testis
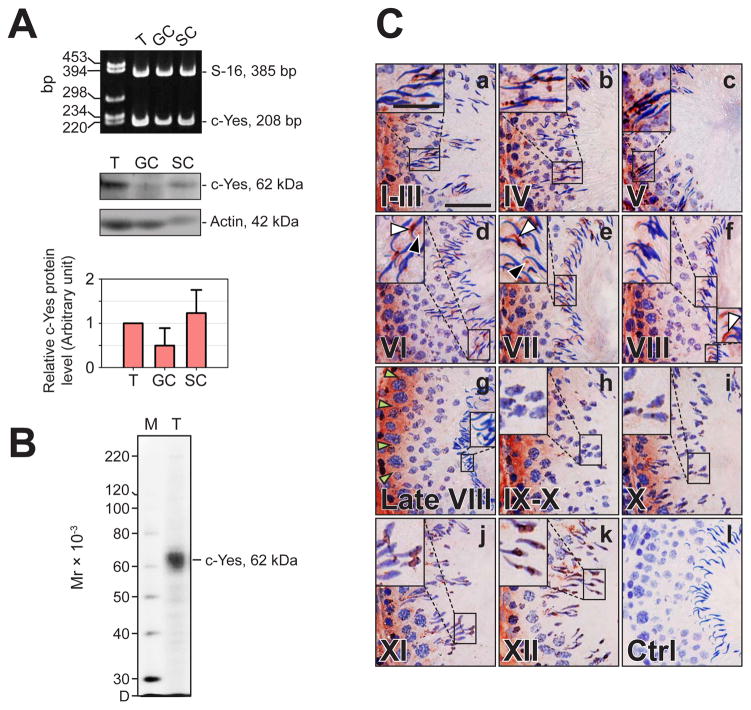
The presence of c-Yes in the rat testis was examined by RT-PCR (Fig. 1A). To confirm this finding, immunoblot analysis using an antibody specific for c-Yes (Table 1) was performed, a similar distribution pattern as of RT-PCR was revealed by using lysates of rat testes, germ and Sertoli cells (Fig. 1A). The specificity of an anti-c-Yes monoclonal antibody was illustrated by immunoblotting shown in Fig. 1B, which was used for IHC to study the cellular localization of c-Yes in the seminiferous epithelium of adult rat testes (Fig. 1C, a-k). Immunoreactive c-Yes was concentrated at the BTB in almost all stages of the epithelial cycle, with the highest intensity of staining occurring at stages VIII-XI (Fig. 1C, f-h), coinciding with the restructuring of the BTB to accommodate the transit of primary preleptotene spermatocytes at the site. c-Yes was also detected at the Sertoli cell-elongating/elongated spermatid interface consistent with its localization at the apical ES except the rapid disappearance of c-Yes immunoreactivity at late stage VIII shortly before spermiation when apical ES undergoing disintegration. It is noted that at stages VI-VIII prior to sperm release, obvious specific staining (Fig. 1C, d-f) was observed at both the apical ES (white arrowheads) and the future tubulobulbar complex (TBC, black arrowheads) which is a disintegrating ultrastructure of apical ES (Cheng and Mruk, 2010). No positive staining was visualized in control sections utilizing mouse (non-immune) IgG or merely PBS instead of the anti-c-Yes IgG (Fig. 1C, l vs. a-k). Thus, c-Yes is a constituent element at the BTB and the apical ES.
Fig. 1. Cellular localization and stage-specific expression of c-Yes in the seminiferous epithelium of adult rat testes during the epithelial cycle of spermatogenesis.
(A) Expression of c-Yes in testes (T) versus Sertoli (SC) and germ cells (GC) was assessed by RT-PCR (upper panel) and immunoblot analysis (middle panel) using either S16 or actin as a loading control. The relative c-Yes protein level in germ and Sertoli cells was compared with that in testes (50 μg protein per lane), of which the protein level was arbitrarily set at 1. Result is a mean ± SD of three independent experiments (lower panel). (B) An immunoblot demonstrating the specificity of an anti-c-Yes monoclonal antibody (mAb) (see Table 1) using lysate of rat testes (25 μg protein). Mr, Molecular weight; M, MagicMark™ XP Western Protein Standard (Invitrogen); D, dye front. (C) Frozen sections (7-μm thickness) of testes were used for immunohistochemistry (IHC) with an anti-c-Yes mAb shown in B, and representative tubules sorted by stages (I-XII) were shown in (a-k). The specificity of the c-Yes staining shown in (a-k) by IHC was validated and shown in (l) in which mouse IgG was used in place of the anti-c-Yes IgG. The expression of c-Yes is stage-specific, with the most intense staining in the epithelium near the basement membrane consistent with its localization at the BTB at stage VIII-IX (green arrowheads in g indicates the BTB site). c-Yes distribution in the seminiferous epithelium can also be discerned partly bordering the heads of elongating/elongated spermatids consistent with its localization at the apical ES except at late stage VIII when its expression diminished considerably (as magnified views from the boxed area in a-k), and c-Yes was found both at the convex (white arrowheads, d-f) and the concave (black arrowheads, d-e) sides at stages VI-VIII prior to spermiation. c-Yes was also found in the interstitial tissue (as the immune reaction toward the interstitium). c-Yes is a ubiquitously expressed tyrosine kinase, thus its staining pattern is diffused. Bar in a (also applies to b-l) = 55 μm; bar in insert in a (also applies to inserts in b-k) = 25 μm.
Is c-Yes a putative component of adhesion protein complexes at the BTB and the apical ES?
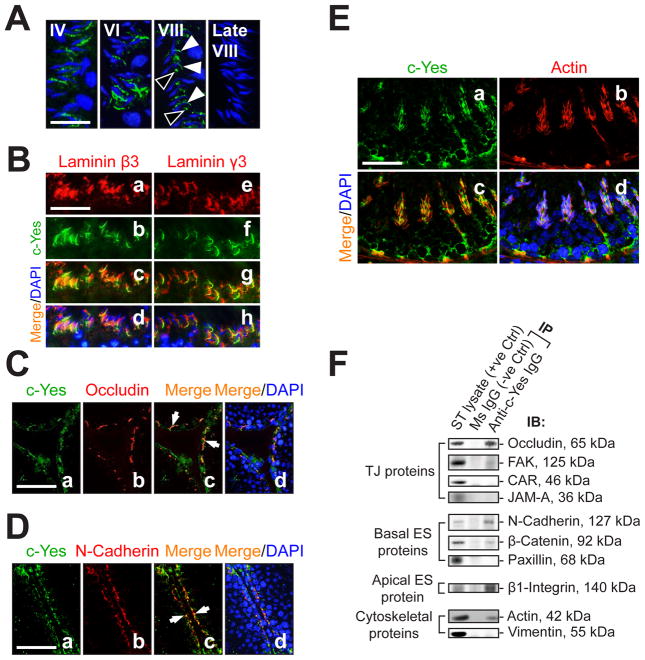
We first attempted to confirm if c-Yes is an integrated component of the apical ES in the seminiferous epithelium by immunofluorescence (IF) microscopy (Fig. 2A). c-Yes was found to localize around the heads of developing spermatids (Fig. 2A, stages IV and VI), gradually came into sight at the TBC of the late, elongated spermatids (Fig. 2A, stage VIII), and disappeared just shortly before spermiation (Fig. 2A, late stage VIII). To further confirm these observations, dual-labeled IF analysis was performed using two putative apical ES proteins, laminin β3 and γ3, which were known to reside exclusively on elongating/elongated spermatids that form bona fide complex with α6β1-integrin restricted to Sertoli cells at the apical ES (Yan and Cheng, 2006). c-Yes was shown to co-localize with these two apical ES-proteins in the seminiferous epithelium (Fig. 2B, c, d vs. a, b and g, h vs. e, f). In like manner, the associations of c-Yes with occludin (a TJ-protein) and N-cadherin (a basal ES-protein) at the BTB were examined, and c-Yes was also found to co-localize with these BTB proteins (Fig. 2C-D). Additionally, the co-localization of c-Yes with F-actin filaments in the seminiferous epithelium was examined (Fig. 2E) and these results also corroborate data shown in Fig. 2A-D, because F-actin is known to be abundant at the BTB and the apical ES and serves as the attachment site for adhesion protein complexes in these ultrastructures (Lie et al., 2010b). To extend the IF observations, Co-IP was carried out to determine the interactions between c-Yes and its binding partners at the BTB and the apical ES. It was found that c-Yes structurally interacted with TJ proteins occludin and FAK, but not CAR or JAM-A; with basal ES proteins N-cadherin and β-catenin, but not paxillin; with apical ES protein β1-integrin; and with cytoskeletal proteins such as actin, but not vimentin.
Fig. 2. c-Yes is an integrated component of the BTB and the apical ES as demonstrated by dual- labeled immunofluorescence (IF) analysis and co-immunoprecipitation (Co-IP).
(A) Fluorescence microscopy was used to illustrate stage-specific localization of c-Yes (green, FITC) at the apical ES. Cell nuclei were visualized with DAPI (blue). c-Yes staining was detected at the apical ES surrounding the heads of the developing spermatids throughout the epithelial cycle until shortly before spermiation when it vanished completely (at late stage VIII). It is noted that c-Yes was found to be both at the convex (white arrowheads) and the concave (black arrowheads) sides in early stage VIII, consistent with IHC data shown in Fig. 1. Bar in first panel (also applies to the other three) = 30 μm. (B) Dual-labeled IF analysis confirmed c-Yes occurrence at the apical ES by its co-localization with laminin β3 (a-d) and γ3 (e-h) (red, Cy3), which are known apical ES components expressed exclusively by elongating/elongated spermatids (Yan and Cheng, 2006). Bar in a (also applies to b-h) = 60 μm. (C-D) Presence of c-Yes at the BTB was supported by moderate co-localization (white arrows) with TJ (occludin) (C) and basal ES (N-cadherin) (D) proteins. Bar in a (also applies to b-d) = 100 μm. (E) c-Yes presence at the BTB and the apical ES was further corroborated by its co-localization with F-actin, suggesting it is likely a regulatory component of the structural scaffolding of actin filaments. Bar in a (also applies to b-d) = 40 μm. (F) A study using Co-IP to assess the structural interactions between c-Yes and selected component proteins of the BTB and the apical ES using ~800 μg protein of seminiferous tubule (ST) lysates. Mouse (MS) IgG instead of anti-c-Yes IgG served as a negative control.
An increase in the steady-state protein level of c-Yes and changes in c-Yes association with other proteins during adjudin-mediated AJ restructuring and germ cell loss
Adjudin is known to exert a selective action restricted to Sertoli-germ cell adhesion in particular at the apical ES, which results in AJ restructuring and germ cell loss from the seminiferous epithelium (Mruk et al., 2006; Mruk et al., 2008). The steady-state protein level of c-Yes in adult rat testes following administration of a single oral dose of adjudin at 50 mg/kg b.w. was investigated by immunoblotting (Fig. 3A), and a significant up-regulation of c-Yes was observed beginning at ~12 hours after treatment (Fig. 3B). By 4 days after treatment, the level of c-Yes seemed to regress mildly but remained higher than rats at time 0. The level of occludin was also stimulated by 48 hr post treatment consistent with an earlier report (Lie et al., 2006), which apparently was used to reinforce the BTB from the adjudin-induced damage to the seminiferous epithelium. Since these changes in c-Yes level when assessed by immunoblotting may be the result of alterations of cellular compositions in the samples being analyzed since both Sertoli and germ cells expressed c-Yes (see Fig. 1), the surge in c-Yes expression in the epithelium after adjudin treatment was further examined by IHC (Fig. 3C). c-Yes was shown to be induced in the seminiferous epithelium at the BTB when examined by IHC by 24-hr through 4-day (Fig. 3C, b-d vs. a), also, more c-Yes appeared to associate with mis-oriented and “prematurely” departing elongating spermatids by 24-hr (Fig. 3C, c vs. a). When virtually all elongating/elongated spermatids were depleted from the epithelium by 4 days following treatment, intense c-Yes staining persisted at the BTB (Fig. 3C, d vs. a). Dual-labeled IF analysis was next used to examine changes in co-localization of c-Yes and occludin (a TJ protein) (Fig. 3D) and N-cadherin (a basal ES protein) (Fig. 3E). In controls, c-Yes co-localized with occludin (Fig. 3D, c vs. a) and N-cadherin (Fig. 3E, c. vs. a). However, following treatment of rats with adjudin, c-Yes was seen to become mis-localized in the epithelium (Fig. 3D, e, i vs. a; Fig. 3E, e, i. vs. a), moving away from the BTB site, such that it no longer co-localized precisely with occludin and N-cadherin (Fig. 3D, g, k vs. c; Fig. 3E, g, k vs. c), suggesting a loss of association between c-Yes and occludin/N-cadherin. This observation was further corroborated and validated by a Co-IP study showing the loss of association between c-Yes and occludin after administration of adjudin (Fig. 4).
Treatment of Sertoli cells with TGF-β3 led to down-regulation of the protein levels of c-Yes and integral membrane proteins at the BTB which is mediated via clathrin-mediated endocytosis
Since cytokines (e.g., TGF-β3) are known to regulate BTB dynamics by perturbing the Sertoli cell TJ-permeability barrier function (Xia et al., 2009; Lui et al., 2001) and c-Yes is an integrated component of the occludin-based protein complex at the BTB, we sought to understand the role of c-Yes in TGF-β3-mediated effects on BTB. Sertoli cells cultured alone for 4 days to form an intact epithelium with an established TJ-permeability barrier were treated with TGF-β3 (3 ng/ml) for up to 4 days and immunoblotting was performed to assess the steady-state levels of c-Yes, occludin and N-cadherin (Fig. 5A). Compared with untreated/vehicle control cells, the steady-state levels of the three proteins in Sertoli cells treated with TGF-β3 all exhibited a time-dependent declining trend and the rapid down-regulation of c-Yes occurred preceding the TGF-β3-induced TJ-protein reduction (Fig. 5B, a vs. b, c). This finding was corroborated by dual-labeled IF analysis on co-localization of c-Yes with occludin and N-cadherin at the BTB and shown in Fig. 5C. After TGF-β3 treatment, it was noted that both c-Yes and occludin (Fig. 5C, a-c vs. d-f) as well as c-Yes and N-cadherin (Fig. 5C, g-i vs. j-l) became mis-localized, redistributed from the cell-cell interface to cell cytosol even though some of these internalized c-Yes/occludin and c-Yes/N-cadherin remained co-localized (Fig. 5C, see enlarged images in c, f, i & l). It has been reported that TGF-β3 perturbs BTB dynamics by accelerating protein endocytosis via clathrin pathway (Yan et al., 2008; Xia et al., 2009) and the endocytosed proteins were targeted to endosome-mediated degradation (Yan et al., 2008; Su et al., 2010), thereby destabilizing the BTB. We thus sought to obtain evidence to suggest that the time-dependent decline of the steady-state protein levels of c-Yes and BTB markers was due to the clathrin-derived endocytic vesicle internalization at the BTB (Fig. 5D). Indeed, better co-localization of occludin/N-cadherin with clathrin was observed after TGF-β3 treatment. It is to note that a mild but significant decline of occludin (Fig. 5A-B) in control Sertoli cells cultured for 8 days is consistent with an earlier report (Xia et al., 2009). The c-Yes staining pattern in Sertoli cells with its membrane and perinuclear location is also consistent with findings in COS-1 cells (Sato et al., 2009).
SU6656, a c-Yes inhibitor, impairs Sertoli cell BTB integrity, which is mediated by changes in protein distribution at the Sertoli-Sertoli cell interface
SU6656 (Fig. 6A) is a small-molecule selective inhibitor for Src family kinases, with an IC50 of 2 × 10−8 M (20 nM) for c-Yes but 280 nM, 170 nM and 130 nM for c-Src, Fyn and Lyn, respectively (Blake et al., 2000), illustrating it is a selective inhibitor of c-Yes, and targeted primarily at c-Yes versus other Src kinase members (Meyn et al., 2005). SU6656 blocks the kinase activity of c-Yes by inserting a methylphenyl group into an hydrophobic pocket of the kinase catalytic site (Talmor-Cohen et al., 2004). This inhibitor was used to assess its effects on the Sertoli cell TJ-barrier function (Fig. 6B) and protein distribution at the cell-cell interface (Fig. 6C-D). Sertoli cells cultured on bicameral units at a density of 2×106 cells/cm2 were incubated for 4 days (beginning on day 3) with 2 × 10−8 M SU6656, 2 × 10−7 M T, or SU6656 + T, which are present in both the apical and basal compartments, and the TER was quantified daily (Fig. 6B). T alone elicited a rapid increase in TER (Fig. 6B), in agreement with earlier reports that T promotes BTB integrity (Meng et al., 2005; Wang et al., 2006). SU6656 alone induced a disruption of the Sertoli cell TJ-barrier (Fig. 6B). Interestingly, when applied together with T, the disruptive effect of SU6656 was blocked. In addition, dual-labeled IF analysis showed that, after application of SU6656 for 3 days (Fig. 6C-D), BTB proteins occludin and N-cadherin became mis-localized, moving from cell surface to cell cytosol (Fig. 6C-D), which could be prevented by T. This c-Yes inhibitor appeared to exert its disruptive effects via the clathrin-mediated endocytic pathway since the endocytosed occludin and N-cadherin associated more with clathrin following treatment with SU6656 (Fig. 6E-F).
Treatment of Sertoli cells with SU6656 does not perturb the steady-state levels of c-Yes and selected BTB-associated proteins, but it perturbs the actin filament network
Sertoli cells were cultured at 0.5 × 106 cells/cm2 on Matrigel-coated 12-well dishes for ~3 days before treated with SU6656 (20 nM), T (2 × 10−7M) or SU6656 (20 nM) + T (2 × 10−7M) and terminated 3 days thereafter. There was no statistically significant change in the steady-state levels of c-Yes, occludin, N-cadherin and clathrin versus controls (Fig 7A-B), except that a significant surge in AR level was detected in Sertoli cells treated with T alone or SU6656 + T. However, when the actin filament network in these cells was examined, treatment of SU6656 induced a disorganized actin network (Fig. 7C, b, j vs. a, i), moving away from the cell periphery, however the presence of T blocked the SU6656-induced actin network disruption (Fig. 7C, c, k vs. b, j). It was also noted that the presence of T appeared to strengthen the actin network in the cell border at the Sertoli-Sertoli cell interface (Fig. 7C, d, l vs. a, i). The panel in e-h are gray scale images of a-d to better illustrate changes when compared to i-l. These findings thus support the observations reported in Fig. 6B that the presence of SU6656 perturbed the Sertoli cell TJ-barrier function, and this disruptive effect of SU6656 could be blocked by T; also, T was shown to promote the Sertoli cell TJ-barrier function.
Discussion
c-Yes is involved in BTB and apical ES restructuring during spermatogenesis
Studies by IHC and dual-labeled immunofluorescence analysis have shown that c-Yes is localized at the BTB and apical ES. It is noted that ES is a testis-specific atypical AJ type (Wong et al., 2008), typified by the presence of hexagonally packed actin filament bundles lying parallel to the plasma membrane and sandwiched between the plasma membrane and the cisternae of endoplasmic reticulum (Cheng and Mruk, 2002; Mruk and Cheng, 2004; Vogl et al., 2008) and is found at the interface of (i) Sertoli-Sertoli cells at the BTB known as the basal ES, or (ii) Sertoli cell-spermatid (step 8 and beyond until step 19 spermatids) known as the apical ES (Cheng and Mruk, 2010). Once apical ES appears during spermiogenesis, it is the only anchoring device for the developing spermatids in the epithelium. Interestingly, c-Yes was expressed stage-specifically with its staining being highest at the apical ES and the BTB at stage VII and VIII, respectively. However, c-Yes at the apical ES diminished rapidly almost to a non-detectable level by late stage VIII when examined by IHC and fluorescence microscopy, but it is most intensive at the BTB as reported herein. This pattern of restricted spatial and temporal expression and localization of c-Yes in the seminiferous epithelium of adult rat testes, however, is very different from c-Src since c-Src immunostaining is the highest in both stage VII-VIII at the apical ES (Lee and Cheng, 2005; Wang et al., 2000) and while c-Src is also detected at the BTB, its expression at this site remained relatively unaltered throughout the epithelial cycle (Wang et al., 2000; Lee and Cheng, 2005). These findings further support the notion that c-Yes and c-Src, while they are two closely related members of the Src tyrosine kinase family and have some overlapping physiological functions, are having distinct functions in the testis during spermatogenesis, similar in other epithelia in which they are having different cellular roles (Zhao et al., 1992; Sato et al., 2009; Mariotti et al., 2001; Monteiro, 2006).
Based on this stage-specific, and highly restricted temporal and spatial expression pattern of c-Yes, plus other data reported herein, we hypothesize that c-Yes is uniquely important to regulate junction restructuring at the apical ES and BTB based on the following rationales. First, at stage VII to early stage VIII when c-Yes expression is high at the apical ES, this anchoring device, beginning from the concave side of the spermatid head, undergoes restructuring as manifested by extensive invaginations of the plasma membrane, mimicking “giant” endocytic vesicles (Young et al., 2009b), and this ultrastructure was designated as TBC (tubulobulbar complex) (Russell, 1979; Cheng and Mruk, 2010; Vogl et al., 2008). Recent studies have shown that protein crucial to protein endocytic-vesicle trafficking, such as clathrin, N-WASP and cortactin are indeed integrated components of the TBC (Young et al., 2009a). At this time, the hexagonally packed actin filament bundles also undergo restructuring to be replaced by branched actin network, thereby facilitating the migration of elongating spermatids to the luminal edge of the tubule to prepare for spermiation. We have recently shown that this actin restructuring is induced by the concomitant loss of Eps8 (an actin bundling protein) (Lie et al., 2009) but the intensive expression of Arp3 (an actin branching/nucleation protein) (Lie et al., 2010b) at the apical ES at stage VII to early stage VIII, promoting the formation of branched actin network at the disrupted apical ES (= TBC) beginning from the concave side of the spermatid head which gradually spread to the remaining apical ES until spermiation takes place (Cheng and Mruk, 2010). The fact that c-Yes closely mimics the pattern of stage-specific and temporal/spatial expression of Arp3 illustrate that this protein kinase may work in conjunction with the Arp2/3 and N-WASP protein complex, such as via protein phosphorylation, to regulate actin dynamics at the apical ES. Additionally, c-Yes was found to structurally associate with β1-integrin and co-localized with β3- and γ3-laminin chains at the apical ES, which are the constituent components of the α6β1-integrin-laminin-α3β3γ3 adhesion complex at the apical ES (Yan and Cheng, 2006; Salanova et al., 1995; Siu and Cheng, 2004), thus, we hypothesize that c-Yes induces endocytosis of these adhesion protein complexes, thereby destabilizing the apical ES to facilitate spermiation at stage VIII of the epithelial cycle. It is also noted that at stage VII of the epithelial cycle, the BTB remains relatively quiescent compared to BTB at stage VIII, thus, an elevated c-Yes expression at the BTB is not necessary. At late stage VIII, when spermiation takes place, further actin branching activity is no longer needed, the expression of c-Yes [and also Arp3 (Lie et al., 2010b)] thus diminished considerably at the apical ES to a virtually undetectable level. The concept that c-Yes is intimately related to apical ES restructuring is further supported by the adjudin model when apical ES was primarily affected, a surge in c-Yes expression was associated with mis-oriented and prematurely “departing” elongating spermatids. Such an increase in c-Yes expression at the apical ES is possibly being used to induce unwanted phosphorylation of apical ES proteins (e.g., N-cadherin, β1-integrin) to elicit protein endocytosis, thereby destabilizing the apical ES.
Second, c-Yes expression is the highest at the BTB at stage VIII when it is undergoing extensive restructuring to accommodate the transit of preleptotene spermatocytes at the site. It is known that “new” TJ-fibrils are first being assembled behind the spermatocytes in transit via actions of testosterone and/or TNFα prior to the disruption of the “old” BTB site above these cells mediated by cytokines (e.g., TGF-β3, TNFα) and that “old” integral membrane proteins (e.g., occludin, claudins, JAMs, cadherins) can be recycled from the “old” to the “new” BTB site via protein recycling and/or transcytosis [for a review, see (Cheng and Mruk, 2010)]. We hypothesize that c-Yes participates in these events in particular the assembly of the “new” TJ-fibrils for the establishment of the new BTB behind the preleptotene spermatocytes in transit. This postulate was supported by the study in which an inhibition of c-Yes by a selective inhibitor SU6656 would lead to a disruption of the Sertoli cell TJ-barrier, as manifested by a significant disruption of the Sertoli cell TJ-barrier. Interestingly, this inhibitory effect of SU6656 on c-Yes function could be blocked by testosterone, illustrating both c-Yes and testosterone may promote the TJ-barrier function at the BTB.
c-Yes participates in the cytokine-induced BTB disruption via changes in protein endocytosis and endosome-mediated intracellular protein degradation
Recent studies have shown that cytokines (e.g., TGF-β2, TGF-β3, TNFα) regulates BTB dynamics via their disruptive effects on the Sertoli cell TJ-permeability barrier (Lui et al., 2001; Siu et al., 2003; Lui et al., 2003b), which is mediated by enhancing the kinetics of protein endocytosis (Xia et al., 2009; Yan et al., 2008) and endosome-mediated degradation (Su et al., 2010; Yan et al., 2008). These events likely take place above the preleptotene spermatocytes in transit at the BTB [for reviews, see (Cheng and Mruk, 2009; Cheng and Mruk, 2010)]. Herein, c-Yes was found to co-localize with occludin (a TJ-protein) and N-cadherin (a basal ES protein) at the BTB near the Sertoli-Sertoli cell interface, and treatment of these cells with TGF-β3 was shown to induce redistribution of these proteins, moving from the cell surface to cell cytosol, and became more tightly associated with clathrin (a endocytic vesicle marker). While c-Yes became less associated with occludin and/or N-cadherin at the cell-cell interface because of the increases in protein endocytosis, c-Yes, however, remained associated with most of the internalized occludin and/or N-cadherin, suggesting that c-Yes may take part in protein endocytosis and the subsequent protein trafficking events, such as recycling, transcytosis and/or endosome/ubiquitin-mediated protein degradation. It was reported that when c-Yes is monopalmitoylated in COS-1 cells, it is biosynthetically transported from the Golgi pool of caveolin to the plasma membrane (Sato et al., 2009), and caveolin is known to take part in protein transcytosis (Frank et al., 2009; Hansen and Nichols, 2010) in tissues including the testis at the BTB (Su et al., 2010). Based on these findings, we hypothesize that c-Yes plays a crucial role in mediating the TGF-β3-induced BTB restructuring events by facilitating endocytic vesicle-mediated events, such as recycling and transcytosis, to maintain the immunological barrier during the transit of spermatocytes (Cheng and Mruk, 2010). This concept also explains the relative high level of expression of c-Yes at the BTB throughout the epithelial cycle.
Regulation of actin-based cytoskeleton by c-Yes
Herein, we have shown that a blockade of c-Yes function by SU6656 would lead to a disintegration of the actin cytoskeleton in Sertoli cell epithelium, and this observation also explains the loss of Sertoli cell TJ-barrier function is possibly due to the loss of actin-based scaffolding function. It is known that Src tyrosine kinases are crucial to maintain the cytoskeleton network (Thomas et al., 1995). For instance, it was reported that c-Yes is crucial to maintain the actin cytoskeleton in pancreatic acinar cells in a study using PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidi-ne) (Lynch et al., 2004), a selective Src tyrosine kinase inhibitor (Lee et al., 2004a), and c-Yes appears to exert this actin regulatory effect together with the focal adhesion protein Pyk2 (Lynch et al., 2004). In short, our observations are consistent with the earlier reports that c-Yes is an actin regulator (Lynch et al., 2004; Mathew et al., 2008). However, it is noted that the presence of testosterone can block the disruptive effects of SU6656 on the actin cytoskeleton analogous to its effects to block the SU6656-induced Sertoli cell TJ-barrier disruption. We speculate that c-Yes, being a non-receptor protein tyrosine kinase, regulates BTB and apical ES adhesion via its effects on the phosphorylation status of the integral membrane proteins at these sites, a blockade of c-Yes function by SU6656 thus compromises their proper phosphoprotein status. On the other hand, androgen, being capable of promoting BTB integrity (Meng et al., 2005) and germ cell adhesion (Wang et al., 2006) in the testis, likely regulates protein phosphorylation via non-classical action in the testis since it was shown that testosterone induced activation of c-Src and MAPK in the testis [for a review, see (Walker, 2010)]. However, it remains to be determined if: (i) c-Yes indeed regulates phosphorylation status of proteins at the BTB and the apical ES and (ii) testosterone mediates alteration of protein phosphorylation at these sites in the presence or absence (e.g., via RNAi of c-Yes) of c-Yes in future studies. Based on findings reported here, we hypothesize that c-Yes and testosterone are crucial regulators to promote the assembly of the “new” TJ network behind the preleptotene spermatocytes in transit at the BTB.
Summary
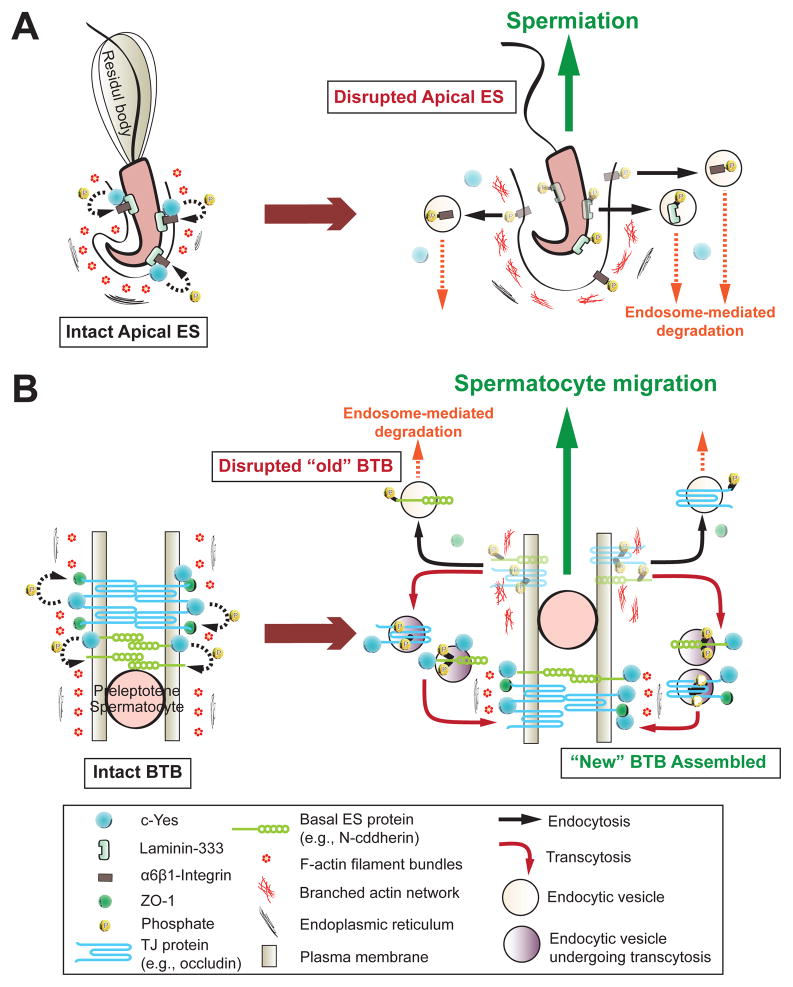
In short, c-Yes is a regulator of apical ES and BTB dynamics, which is mediated via restricted temporal and spatial expression of c-Yes at these sites during the epithelial cycle of spermatogenesis. c-Yes is probably involved in the junction restructuring events at the apical ES and the BTB via its effects on endocytic vesicle-mediated protein trafficking events as the result of changes in phosphorylation status of the integral membrane proteins and actin cytoskeleton, which must be vigorously investigated in future studies. The hypothetical action of c-Yes in mediating the changes in cell adhesion in the seminiferous epithelium based on findings in this report is summarized in Fig. 8.
Fig. 8. A schematic drawing illustrating the underlying mechanism by which c-Yes regulates apical ES and BTB restructuring during the seminiferous epithelial cycle of spermatogenesis.
On the left panels of (A) and (B) are the corresponding intact apical ES and BTB (e.g., stage VII) in the epithelium of adult rat testes, with c-Yes highly expressed and tightly associated with the corresponding cell adhesion protein complexes at these sites, such as apical ES proteins: β1-integin; and BTB proteins: occludin and N-cadherin. When apical ES undergoes disruption to prepare for spermiation at stage VIII of the epithelial cycle (right panel in A), c-Yes likely induces protein phosphorylation, causing protein endocytosis to destabilize the apical ES to facilitate spermiation. Similar events take place at the “old” BTB site above the preleptotene spermatocytes in transit in which c-Yes induces phosphorylation of occludin and N-cadherin, which in turn become internalized via endocytic vesicle-mediated pathway likely mediated by cytokines (e.g., TGF-β2, TNFα), thereby destabilizing the “old” BTB site to facilitate the transit of spermatocytes. However, c-Yes is possibly working in concert with androgen which is known to promote BTB integrity (such as via de novo synthesis of “new” TJ proteins to be assembled to the “new” TJ-fibrils below the preleptotene spermatocytes in transit to assemble a “new” BTB) to establish a “new” BTB below the spermatocytes (right panel in B, see also text for details). Additionally, other proteins (e.g., 14-3-3) (Wong et al., 2009) and testosterone (Yan et al., 2008) promote protein transcytosis, most likely from the “old” to the “new” BTB site (see right panel in B). In short, this provides a unique mechanism to maintain the immunological barrier and seminiferous epithelial integrity during the epithelial cycle of spermatogenesis.
Abbreviations
- BTB
blood-testis barrier
- c-Yes also known as YES1 or v-Yes-1
Yamaguchi sarcoma viral oncogene homolog 1, a non-receptor tyrosine protein kinase and a member of the Src family tyrosine protein kinases
- c-Src
the transforming (sarcoma-inducing) gene of Rous sarcoma virus, this is the first member of the Src family of tyrosine protein kinases, all members have the characteristic src-homology (SH) domain structure, such as kinase domain (SH1), SH2 and SH3 domains, and SH4 domain for membrane localization
Footnotes
This work was supported in part by grants from the National Institutes of Health (NICHD R01 HD056034 and R01 HD056034-02S1 to CYC; HD029990 Project 5 to CYC; R03 HD061401 to DDM).
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–27. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, et al. Stat3-mediated Myc expression is required for Src transformation and PDFG-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–24. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227–37. doi: 10.1091/mbc.01-08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–74. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–63. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–61. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Chung NP, Cheng CY. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–88. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- Clump DA, Qazi IH, Sudol M, Flynn DC. c-Yes response to growth factor activation. Growth Factors. 2005;23:263–72. doi: 10.1080/08977190500199360. [DOI] [PubMed] [Google Scholar]
- de Kretser D. Germ cell-Sertoli cell interactions. Reprod Fertil Dev. 1990;2:225–35. doi: 10.1071/rd9900225. [DOI] [PubMed] [Google Scholar]
- Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: Role in atherosclerosis. Cell Tissue Res. 2009;335:41–7. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- Galdier M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: A new model for the study of somatic-germ cell interactions. J Androl. 1981;5:249–59. [Google Scholar]
- Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, alpha 2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–40. doi: 10.1016/0303-7207(92)90219-v. [DOI] [PubMed] [Google Scholar]
- Grima J, Wong CC, Zhu LJ, Zong SD, Cheng CY. Testin secreted by Sertoli cells is associated with the cell surface, and its expression correlates with the disruption of Sertoli-germ cell junctions but not the inter-Sertoli tight junction. J Biol Chem. 1998;273:21040–53. doi: 10.1074/jbc.273.33.21040. [DOI] [PubMed] [Google Scholar]
- Grima J, Zhu L, Cheng CY. Testin is tightly associated with testicular cell membrane upon its secretion by sertoli cells whose steady-state mRNA level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. J Biol Chem. 1997;272:6499–509. doi: 10.1074/jbc.272.10.6499. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20:177–86. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim JY, Anderson WB. Src tyrosine kinase inhibitor PP2 markedly enhances Ras-independent activation of Raf-1 protein kinase by phorbol myristate acetate and H2O2. J Biol Chem. 2004a;279:48692–701. doi: 10.1074/jbc.M403132200. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–60. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- Lee NP, Mruk DD, Conway AW, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are regulators that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004b;25:200–15. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009;106:10213–8. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010a;42:975–86. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010b;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Xia W, Wong CQ, Mruk DD, Yan HHN, Wong CH, et al. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood-testis barrier in adult rat testes. J Endocrinol. 2006;191:571–86. doi: 10.1677/joe.1.06996. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–77. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003a;68:2189–206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003b;68:1597–612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kohler S, Leser J, Beil M, Garcia-Marin LJ, Lutz MP. The tyrosine kinase Yes regulates actin structure and secretion during pancreatic acinar cell damage in rats. Pfugers Arch Eur J Physiol. 2004;447:445–51. doi: 10.1007/s00424-003-1188-7. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–58. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, George SP, Wang Y, Siddiqui MR, Srinivasan K, Tan L, et al. Potential molecular mechanism for c-Src kinase-mediated regulation of intestinal cell migration. J Biol Chem. 2008;283:22709–22. doi: 10.1074/jbc.M801319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. doi: 10.1016/j.mce.2010.03.022) 2010. in press. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–70. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn MA, 3rd, Schreiner SJ, Dumitrescu TP, Nau GJ, Smithgall TE. SRC family kinase activity is required for murine embryonic stem cell growth and differentiation. Mol Pharmacol. 2005;68:1320–30. doi: 10.1124/mol.104.010231. [DOI] [PubMed] [Google Scholar]
- Meyn MA, 3rd, Smithgall TE. Chemical genetics identifies c-Src as an activator of primitive ectoderm formation in murine embryonic stem cells. Sci Signal. 2009;2:ra64. doi: 10.1126/scisignal.2000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro AN. Involvement of the SH3 domain in Ca2+-mediated regulation of Src family kinases. Biochimie. 2006;88:905–11. doi: 10.1016/j.biochi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–80. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–8. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- Nishio H, Tokuda M, Itano T, Matsui H, Takeuchi Y, Hatase O. pp60c-src expression in rat spermatogenesis. Biochem Biophys Res Commun. 1995;206:502–10. doi: 10.1006/bbrc.1995.1072. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, et al. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816–22. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat Rec. 1979;194:233–46. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, et al. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–75. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci U S A. 2009a;106:9298–303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EW, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009b;150:3336–44. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–64. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–87. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Staley CA, Parikh NU, Gallick GE. Decreased tumorigenicity of a human colon adenocarcinoma cell line by an antisense expression vector specific for c-Src. Cell Growth Differ. 1997;8:269–74. [PubMed] [Google Scholar]
- Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGF-beta3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–60. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summy JM, Sudol M, Eck MJ, Monteiro AN, Gatesman A, Flynn DC. Specificity in signaling by c-Yes. Front Biosci. 2003;8:s185–205. doi: 10.2741/1011. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Eliyahu E, Shapiro R, Shalgi R. Are Src family kinases involved in cell cycle resumption in rat eggs? Reproduction. 2004;127:455–63. doi: 10.1530/rep.1.00104. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267–71. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience and Springer Science + Business Media; 2008. pp. 186–211. [Google Scholar]
- Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1557–69. doi: 10.1098/rstb.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–92. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junction complex formation in mouse testes. Endocrinology. 2006;147:5624–33. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Wang W, Wine RN, Chapin RE. Rat testicular Src: normal distribution and involvement in ethylene glycol monomethyl ether-induced apoptosis. Toxicol Appl Pharmacol. 2000;163:125–34. doi: 10.1006/taap.1999.8870. [DOI] [PubMed] [Google Scholar]
- Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, Cheng CY. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14–3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–23. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–59. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009a;80:153–61. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009b;80:162–74. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wong CH, Xia W, Mruk DD, Lee NP, Lee WM, et al. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-beta-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–84. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sudol M, Hanafusa H, Krueger J. Increased tyrosine kinase activity of c-Src during calcium- induced keratinocyte differentiation. Proc Natl Acad Sci U S A. 1992;89:8298–302. doi: 10.1073/pnas.89.17.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, Cheng CY. Rat seminiferous tubular culture medium contains a biological factor that inhibits Leydig cell steroidogenesis: its purification and mechanism of action. Mol Cell Endocrinol. 1994;104:213–27. doi: 10.1016/0303-7207(94)90124-4. [DOI] [PubMed] [Google Scholar]