Abstract
Purpose
To examine predictors of progression of disability in RA, as measured by the Health Assessment Questionnaire disability index (HAQ), and to determine rates of progression during biologic treatment.
Methods
We followed 18,485 RA patients for up to 11 years (mean 3.7 years) in a longitudinal study of RA outcomes. Patients were characterized as having moderate or severe RA versus less severe RA at study entry. Annualized progression rates were determined in multivariable analyses using generalized estimating equations (GEE).
Results
Although all demographic and severity characteristics were associated with baseline differences in HAQ score, progression was only associated with age, comorbidity, initial severity, and treatment. HAQ increased fastest in patients with age > 65, 0.031 (95% CI 0.028, 0.034). HAQ progression was independently associated with the presence of baseline cardiovascular disease, hypertension, diabetes, and the number of comorbid conditions. Annualized progression rates were greater in patients with mild to inactive RA, 0.021 (95% CI 0.019, 0.023), than in moderate to severe RA, 0.003 (0.001, 0.006). The overall progression rate during biologic treatment was 0.008 (95% CI 0.005. 0.011); for patients with moderate to severe RA the rate was 0.001 (95% CI −0.005, 0.003).
Conclusions
Age and comorbidity are important predictors of the rate of loss of functional status, and have a stronger effect on HAQ progression than does biologic treatment. There is little difference in progression rates among biologics. Patients with more severe RA progress less than those with less severe RA, a possible function of regression to the mean.
Keywords: Biologics, progression, HAQ, comorbidity
Introduction
Functional status is the signal predictor of important rheumatoid arthritis (RA) patient outcomes, including mortality (1–3), work disability (4–6), healthcare resource utilization (7, 8), and household income and poverty (9); and the goal of RA therapy is to preserve or improve functional status. The US Food and Drug Administration (FDA) recognizes a treatment indication for improving physical function (10) in adult patients with moderately to severely active rheumatoid arthritis.
Despite its importance, the factors that relate to loss of functional status in RA are not well understood. Functional status deteriorates with age and with presence of comorbidity in all individuals (11). In RA, inflammation and pain together with structural damage and decreased endurance also contribute to functional loss. However, functional status is lost very early in the course of RA (12), and its level is not always directly related to the degree of inflammatory activity.
The level of the Health Assessment Questionnaire disability index (HAQ) serves as one measure of the success or failure of treatment intervention, and its improvement and the maintenance of improvement is accepted as a measure of “improving physical function (10).” In addition, the change in HAQ is regularly used as a surrogate measure for health utility in cost effectiveness evaluation (13, 14).
In the current study, we used the perspective of the patient with established RA and investigated factors that may predict HAQ progression. Additionally, we measured the degree of progression in patients treated with and without biologics, controlling for non-treatment factors.
Methods
We studied 18,485 adult patients with RA who participated in the National Data Bank for Rheumatic Diseases (NDB) longitudinal study of RA outcomes. Participants are volunteers, recruited from the practices of US rheumatologists, who complete mailed or Internet questionnaires about their health at 6-month intervals. They are not compensated for their participation. The diagnosis of RA is made by the patient’s rheumatologist. Patients who were recruited to participate in the NDB as they started a biologic, specifically as part of a biologic safety registry, were excluded from this study because of the possibility of severity bias. The NDB utilizes an open cohort design in which patients are enrolled continuously. The mean duration of patients’ follow-up was 3.7 (SD 3.2, range 0.5–11) years.
Patients were assessed on a semiannual basis between 1998 and 2009. At each assessment we obtained treatment and demographic data by patient self-report. Patients were considered to be on biologic therapy if they used any of the following treatments during the time of the study: etanercept, infliximab, adalimumab, abatacept, certolizumab pegol, or rituximab. However, only 2.4% of patients had received abatacept, certolizumab pegol, or rituximab at the time of study closure, and we elected to restrict biologic analyses to etanercept, infliximab, and adalimumab. Infliximab had been used by 26.9% of patients during 9,893 patients-years; etanercept by 19.4% during 9,504 patient-years, and adalimumab by 7.0% during 2,311 patient years of observation. For this study, Disease modifying anti-rheumatic drugs (DMARDs) refer to non-biologic treatments.
To measure functional status, we used the Health Assessment Questionnaire disability index (HAQ) (15). The HAQ has 41 questions, including 20 activities of daily living items and 21 items about aids and devices. Patient’s self-reported individual comorbid conditions (16), and we computed a comorbidity score based on the presence of pulmonary disorders, myocardial infarction, other cardiovascular disorders, stroke, hypertension, diabetes, spine/hip/leg fracture, depression, GI ulcer, other GI disorders, and cancer, as previously described (17). Visual analog scales (VAS) for pain and patient global were also collected with a range of 0 through 10.
To identify patients with moderate to severe RA, we first asked 7, 389 patients to rate the current severity of their RA (no symptoms, mild, moderate and severe severity). We then determined by logistic regression that the same patients simultaneous Patient Activity Scale (PAS) (18) score had a receiver operating characteristic (ROC) curve value of 0.90 for the classification of moderate to severe RA versus mild to inactive RA at a PAS value of 3.7. We used this cut point to classify all RA patients in this study. The PAS is an index comprised of HAQ, VAS pain, and VAS patient global.
Statistical methods
Missing data
Missing data occurred through two mechanisms in this study: 1) when patients did not complete or validly complete a questionnaire item (mechanism 1) and 2) when the item was not part of the assessment questionnaire (mechanism 2). The NDB made use of 3 types of questionnaires. All participants completed at least once a comprehensive 28-page questionnaire that included all study questions. Over the course of the study, this questionnaire was completed at 92.7% of observations, and had 0.4% missing data for HAQ (mechanism 1). A short and an even shorter (brief) questionnaire was completed by 6.5% and 1.0% of patients respectively; these questionnaires did not include the HAQ (mechanism 2). Patients completing shorter questionnaires are older and have lower health status compared with patients completing the comprehensive questionnaire. Considering all questionnaires, the overall missing data for HAQ was 8.1%. Because of the possibility that excluding data from short and brief questions would introduce unacceptable bias, we elected to impute missing variables. To replace missing HAQ values, we used multiple imputation by chained equations (ICE) to create 5 multiple imputed datasets for analyses (19), and we combined data according to Rubin’s rules (20). Variables used in ICE included: questionnaire type, enrollment year, number of observations, SF-36 PCS, SF-36 MCS, disease duration, comorbidity index, calendar year, age, sex, education, and income.
Specific analyses
In preliminary analyses we evaluated the possibility that HAQ progression might be non-linear with respect to time using graphic inspection and multivariable regression splines (21). We did not find evidence for nonlinearity of HAQ progression and, therefore, used a linear functional form in our analyses.
To determine rates of progression we used general estimating equations (GEE) with semi-robust standard errors. In the first series of analyses we regressed HAQ on age categories, sex, and the specific covariate of interest (Table 1), and we repeated this analysis separately for each covariate. In the multivariable models of Table 2, all variables were included, but in order to determine rates of HAQ change over time per covariate, we changed the functional form of each covariate to interact with the study duration variable (time) in separate analysis for each covariate. Analyses included 135,738 observations from the 18,485 patients. The analyses determine the rate of HAQ progression when patients are receiving specific biologics compared to when they are not receiving them. A patient can contribute time to multiple treatments. Analyses comparing overall biologic use versus no biologic use were previously reported (22).
Table 1.
Patient entry characteristics with associated mean (SD) HAQ scores and multivariable rates of HAQ progression.
| Patient characteristics | ||||
|---|---|---|---|---|
| Predictor | Percent | HAQ mean (SD) | Annual rate of HAQ Increase or decrease (−)# | Difference |
| Overall | 100 | 1.06 (0.73) | 0.014 (0.012, 0.015) | |
| Age group | ||||
| < 40 years† | 7.0 | 0.87 (0.69) | −0.006 (−0.014, 0.002) | |
| 40–64 years | 55.7 | 1.05 (0.71) | 0.006 (0.004, 0.007) | 0.012 (0.004, 0.020)* |
| ≥ 65 years | 37.3 | 1.12 (0.75) | 0.031 (0.028, 0.034) | 0.037 (0.029. 0.046)* |
| Male gender | 23.3 | 0.79 (0.69) | 0.015 (0.011, 0.019) | 0.001 (−0.003, 0.005) |
| Female gender† | 76.7 | 1.14 (0.72) | 0.014 (0.012, 0.057) | |
| College education (+) | 26.3 | 0.83 (0.69) | 0.012 (0.009, 0.015) | −0.002 (−0.006, 0.001) |
| College education (−)† | 73.7 | 1.14 (0.72) | 0.015 (0.004, 0.066) | |
| Smoking category | ||||
| Never smoked † | 47.2 | 1.03 (0.73) | 0.013 (0.011, 0.016) | |
| Past smoker | 38.7 | 1.06 (0.72) | 0.016 (0.013, 0.018) | 0.002 (−0.001, 0.006) |
| Present smoker | 14.1 | 1.18 (0.70) | 0.011 (0.005, 0.016) | −0.003 (−0.008. 0.003) |
| Body mass index > 30 | 28.7 | 1.22 (0.70) | 0.014 (0.011, 0.018) | 0.001 (−0.003, 0.005) |
| Body mass index ≤ 30† | 71.3 | 0.99 (0.72) | 0.014 (0.012, 0.016) | |
| Comorbidity | ||||
| Heart disease (+) | 8.1 | 1.30 (0.73) | 0.024 (0.017, 0.032) | 0.009 (0.005, 0.013)* |
| Heart disease (−)† | 1.04 (0.72) | 0.013 (0.011, 0.015) | ||
| Hypertension (+) | 31.3 | 1.12 (0.72) | 0.021 (0.018, 0.024) | 0.010 (0.006, 0.014)* |
| Hypertension (−)† | 1.00 (0.72) | 0.011 (0.009, 0.013) | ||
| Lung disease (+) | 11.5 | 1.32 (0.71) | 0.016 (0.010, 0.023) | 0.004 (−.004, 0.012) |
| Lung disease (−)† | 1.03 (0.72) | 0.014 (0.012, 0.015) | ||
| Psychiatric illness (+) | 14.8 | 1.40 (0.69) | 0.011 (0.005, 0.016) | −0.004 (−0.010, 0.002) |
| Psychiatric illness (−)† | 1.00 (0.72) | 0.014 (0.012, 0.016) | ||
| GI illness (+) | 15.7 | 1.34 (0.70) | 0.012 (0.007, 0.016) | −0.001 (−0.006, 0.004) |
| GI illness (−)† | 1.01 (0.72) | 0.014 (0.012, 0.016) | ||
| Endocrine (+) | 19.3 | 1.22 (0.72) | 0.019 (0.015, 0.023) | 0.006 (0.001, 0.011)* |
| Endocrine (−)† | 1.02 (0.72) | 0.013 (0.011, 0.015) | ||
| Comorbid conditions | ||||
| 0† | 27.2 | 0.81 (0.69) | 0.009 (0.006, 0.011) | |
| 1 | 27.1 | 0.98 (0.69) | 0.012 (0.009, 0.015) | 0.003 (−0.001, 0.007) |
| 2 | 21.6 | 1.13 (0.71) | 0.019 (0.015, 0.023) | 0.010 (0.006, 0.015)* |
| 3 | 12.8 | 1.27 (0.70) | 0.023 (0.017, 0.029) | 0.014 (0.008, 0.021)* |
| 4 or more | 11.5 | 1.50 (0.69) | 0.021 (0.015, 0.028) | 0.012 (0.005, 0.020)* |
| Year of RA onset | ||||
| Quartile 1 (<1975) | 28.7 | 1.28 (0.74) | 0.017 (0.015, 0.020) | 0.007 (0.001, 0.014)* |
| Quartile 2 (1975–1989) | 25.1 | 1.10 (0.70) | 0.012 (0.009, 0.016) | 0.002 (−0.004, 0.009) |
| Quartile 3 (1990–1995) | 27.0 | 0.94 (0.69) | 0.012 (0.009, 0.015) | 0.003 (−0.004, 0.009) |
| Quartile 4 (>1996)† | 19.2 | 0.86 (0.69) | 0.010 (0.004, 0.016) | |
| Moderate or severe RA (+) | 52.2 | 1.54 (0.58) | 0.003 (0.001, 0.006) | −0.017 (−0.021, −0.014)* |
| Moderate or severe RA (−)† | 47.8 | 0.62 (0.54) | 0.021 (0.019, 0.023) | |
Comparison group
Statistically significant (p<0.05)
Each non-“Overall” predictor was adjusted for baseline HAQ score, age, sex, education, smoking, BMI, comorbidity, RA onset and RA severity.
Table 2.
Annual rates of progression of HAQ by treatment*
| Treatment | HAQ Rate/year | P-value† |
|---|---|---|
| All Patients | ||
| Infliximab All | 0.006 (0.001, 0.010) | 0.012 |
| Infliximab + MTX | 0.007 (0.002, 0.012) | 0.003 |
| Infliximab − MTX | 0.011 (0.006, 0.016) | <0.001 |
| Etanercept All | 0.007 (0.003, 0.010) | <0.001 |
| Etanercept + MTX | 0.004 (−0.000, 0.009) | 0.062 |
| Etanercept − MTX | 0.009 (0.003, 0.014) | 0.001 |
| Adalimumab All | 0.010 (0.003, 0.017) | 0.003 |
| Adalimumab + MTX | 0.006 (−0.003, 0.015) | 0.186 |
| Adalimumab − MTX | 0.015 (0.005, 0.024) | 0.002 |
| No biologic, DMARD (+/−) | 0.018 (0.016, 0.020) | <0.001 |
| No biologic, DMARD (+) | 0.018 (0.016, 0.021) | <0.001 |
| Patients with Moderate to Severe RA | ||
| Infliximab All | −0.003 (−0.009, 0.003) | 0.361 |
| Infliximab + MTX | −0.001 (−0.008, 0.006) | 0.740 |
| Infliximab − MTX | −0.000 (−0.008, 0.007) | 0.937 |
| Etanercept All | −0.004 (−0.009, 0.001) | 0.130 |
| Etanercept + MTX | −0.005 (−0.012, 0.001) | 0.110 |
| Etanercept − MTX | −0.003 (−0.010, 0.005) | 0.495 |
| Adalimumab All | 0.006 (−0.004, 0.015) | 0.262 |
| Adalimumab + MTX | −0.003 (−0.018, 0.011) | 0.671 |
| Adalimumab − MTX | 0.012 (−0.002, 0.025) | 0.093 |
| No biologic, DMARD (+/−) | 0.008 (0.005, 0.012) | <0.001 |
| No biologic, DMARD (+) | 0.010 (0.005, 0.014) | <0.001 |
Adjusted for baseline HAQ score, age, sex, education, smoking, BMI, comorbidity, and RA onset.
P-value is for the null hypothesis that the HAQ rate per year is 0.
DMARD (+/−) = with or without DMARD use, DMARD (+) = with DMARD use
All data were analyzed using Stata 11.0 (Stata Corporation, College Station, TX). Statistical significance was set at the 0.05 level.
The study was carried out in compliance with the Helsinki Declaration, and was approved by the Institutional Review Board of the St. Francis Regional Medical Center, Wichita, KS. All patients signed an informed consent.
KM and FW drafted the manuscript, and participated in the conception, design, data collection, and data analysis of the study. GW participated in the conception, design, collection and review of the manuscript. All authors read and approved the final version of the manuscript.
Results
At entry to the study, the mean (SD) age of patients was ~60 years, with a duration of RA of ~12 years. Twenty-three (23.3) percent were men, and 25.0% had completed college. The patient global at entry was 3.6 (2.5), and the mean number of DMARDs/biologics used through the time of entry was 2.7 (1.9).
We examined the association of demographic and comorbidity variables on HAQ scores at entry in Table 1. The HAQ score in moderate to severe RA was 1.54 compared with 0.62 for mild or inactive RA, and the overall HAQ score was 1.06. In addition, HAQ scores differed among categories for all variables. The greatest HAQ scores were noted in comorbidity, including heart problems (1.30), pulmonary disorders (1.32), psychiatric disorders (1.40), and multiple comorbid conditions (1.27–1.50).
In Table 1 we also examined the multivariable effect of the variables at baseline on the annual rate of HAQ progression. HAQ progressed fastest in patients with age > 65 years. Among comorbid conditions, significant HAQ progression rates were: cardiovascular disease 0.009, hypertension 0.010, diabetes 0.006, and multiple comorbidities (point estimate range 0.010 to 0.014). Rates were greater in patients with mild to inactive RA, 0.021 whose mean (SD) HAQ score was 0.6 (0.5), than in moderate to severe RA, 0.003 where the mean (SD) HAQ score was 1.5 (0.6). To further examine a possible regression to the mean effect, HAQ annual progression rates for patients whose baseline HAQ was <1 were 0.025 (95% CI 0.023 to 0.028), ≥1 and <2 were 0.007 (95% CI 0.005 to 0.010) and ≥ 2 and ≤ 3 were −0.016 (95% CI −0.022 to −0.010) with multivariable adjustment of all predictors listed in Table 1.
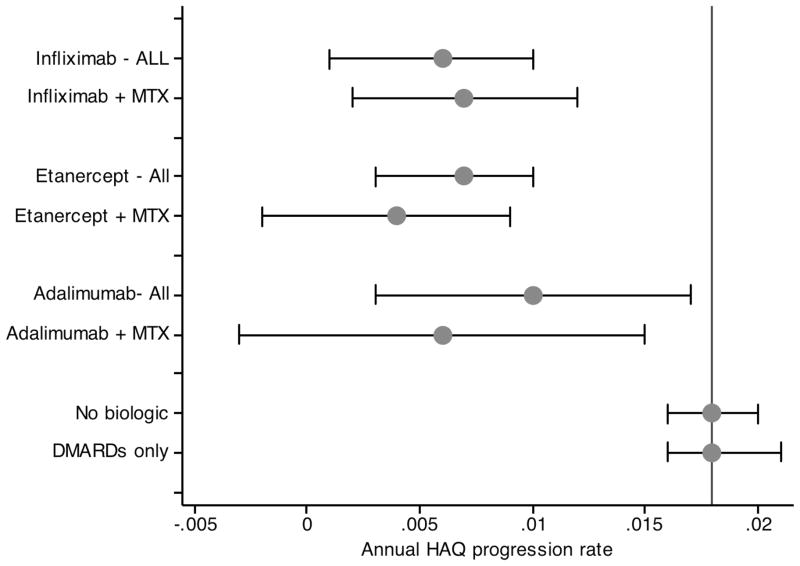
In Table 2 and Figures 1 and 2 we studied the effect of biologic therapy on HAQ progression in all patients and separately in the group of patients with moderate to severe RA. Presented with baseline differences between biologic non-use and use, the analyses were adjusted for baseline HAQ (1.05 vs. 1.10, p<0.001), age (60.3 vs. 58.3, p<0.001), male sex (23.5% vs. 22.5%, p=0.239), college education (25.7% vs. 28.8%, p<0.001), BMI (27.9 vs. 32.2, p<0.001), RA onset year (1989 vs. 1989, p=172), smoking status (53% vs 52% ever smoked, p=118), and the number of comorbid conditions (1.58 vs. 1.48, p<0.001). Among all patients the rate of HAQ progression without biologics was 0.018 (95% CI 0.016, 0.020), and was not lower in the absence of DMARD use. The rate of progression on biologics was 0.008 (95% CI 0.005, 0.011), and the rate of progression was similar among all of the biologics (Table 2 and Figure 1).
Figure 1.
Annual rate of progression according to treatment status in all patients. “All” indicates a biologic treatment regardless of the use of MTX, while “+ MTX” indicates a biologic treatment together with the use of MTX. Vertical lines represent non-biologic treatment with or without DMARD treatment (0.018) and no biologic treatment with DMARD treatment (0.018).
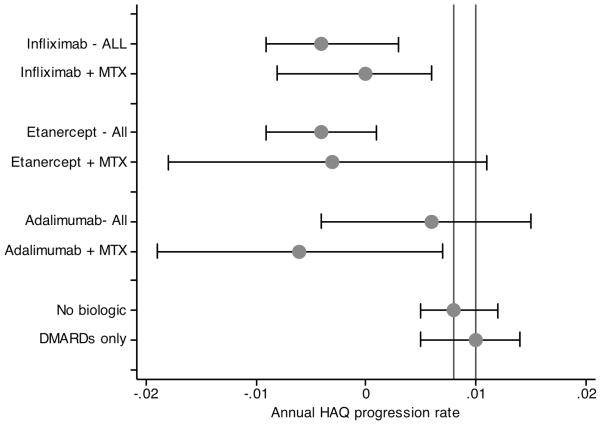
Figure 2.
Annual rate of progression according to treatment status in patients entering cohort with moderate to severe RA. “All” indicates a biologic treatment regardless of the use of MTX, while “+ MTX” indicates a biologic treatment together with the use of MTX. Vertical lines represent non-biologic treatment with or without DMARD treatment (0.008) and no biologic treatment with DMARD treatment (0.010).
Among patients with moderate or severe RA at baseline, HAQ progression rates were lower (Table 2 and Figure 2). While not on any biologic and on a DMARD, the rate of progression was 0.010 (95% CI 0.005, 0.014), and on any biologic the rate was −0.001 (95% CI −0.005, 0.003). Once again, rates among the individual biologics were similar.
Discussion
The aim of this study was not, as is most often the case, to investigate levels of disability and disability-associated clinical predictors and outcomes. Instead, it was to start with the current level of disability and ask what are the factors that predict the rate of progression of disability, and to describe that rate. This is a germane question, as patients in studies and in clinical practice do not represent a tabula rasa.
Among the important results of this study was the observation that moderate to severe RA at entry was associated with lower rates of HAQ progression than found in patients with mild or inactive RA. The simple and sufficient cause for this result is regression toward the mean (23, 24). The more severe the RA, the higher is the HAQ score, and the less likely is progression. As a corollary, it may be said that patients with more severe RA did not have greater HAQ progression — they already had greater progression that led to their current higher HAQ scores overall. The distinction between the level of the HAQ score and its rate of change is important and should always be kept in mind. It follows from these data that it will always be easier to control HAQ progression with treatment when the patients receiving the treatment are more severe rather than less severe. As shown in Table 1, the rate of HAQ progression is 0.017 (0014, 0.0212) units per year less in moderate to severe RA than in less severe RA.
To our knowledge, previous studies in RA have not examined non-treatment factors that may contribute to HAQ progression rate. It is important to note that non-treatment factors had greater effect on HAQ progression than did treatment factors. For example, the rate of progression for those >65 years of age was 0.031 (0.028, 0.034) while the rate of progression in etanercept patients on MTX (the best case) was 0.004 (−0.000, 0.009) compared with the no biologic treatment rate of 0.018 (0.016, 0.020), a difference of approximately 0.014 units per year. Such results are important because they underscore the point that HAQ rate is substantially contributed to by non-RA factors.
In addition to age, and its infirmities, we demonstrated that the presence of comorbidities at baseline contributed independently to the rate of HAQ progression (Table 1). These data indicate that we should be careful in attributing all changes in HAQ to treatment effect. Our data suggest that in studies, including randomized clinical trials, there may be greater treatment response in younger and healthier patients, though we should not extrapolate the observational community data presented here to clinical trials.
With respect to biologic treatment, progression rates were similar among treatments, and there was no clear difference according to MTX status (Table 2). The width of the confidence intervals reflect the proportion of patients treated with the different biologics in the study: infliximab 27%, etanercept 19%, and adalimumab 7%. The progression rate during biologic treatment was −0.001 (95% CI −0.005, 0.003) for those with moderate to severe RA and was 0.008 (95% CI 0.005. 0.011) when patients were not subset by entry severity.
The results of this report with respect to HAQ progression differ from those reported by Symmons et al. in 466 patients with established RA (duration >5 yr) who were on stable therapy for at least 6 months (25). Their patients had a baseline HAQ of ~1.28 and an estimated average HAQ increase of 0.051 (95% CI 0.037, 0.065) units per year compared with an average progression rate of 0.014 (0.012, 0.015) noted in the current study. Both groups were similar with respect to age (~60 years) and RA duration (~13 years). However, the lifetime DMARD count at baseline in the current study was 2.7 (1.9) compared with 1.4 (0.7) in the UK study. In addition, we have reported elsewhere that 847 RA patients with 6,444 observations during ordinary care in a US clinical rheumatology practice had an annualized rate of HAQ-II progression of 0.018 (95% CI 0.001, 0.036) units per year (12). The HAQ-II is a surrogate for the HAQ with almost the same psychometric characteristics (26). In addition, the patient global was 4.6 (2.6) in this clinical cohort, 3.6 (2.5) in the current study, and ~6.3 (~1.8) in the UK report. These differences in results suggest that progression results may be related to demographic, severity, and treatment opportunities in different study cohorts.
One of the limitations of this report is that survey participants generally have higher socioeconomic levels and more education (27), factors that are associated with better health. We believe that patients in this cohort have, on average, received close to optimum specialist care. In a setting of a stable cohort, such as is seen here, the effect of biologic therapy may be less than seen in randomized clinical trials and in settings where treatment is restricted by severity criteria because there is less room for improvement. It should be noted, however, that our study did not measure HAQ at clinical start of therapy, so this study was not intended to show a HAQ treatment effect, but rather the impact on progression of disability in biologic treated patients. One advantage of our regular semi-annual assessments is that they are less responsive to short-term HAQ fluctuations that may be noted when assessments are caused by clinical exigencies.
Although we classified patients by severity, this classification was based on patient report, as physician data were not available. It is possible that study results in moderate to severe RA patients might have been different if the cohort was classified according to DAS (Disease Activity Scale) measures (28). However, we have recently reported that rates of progression noted here are similar in a contemporary cohort of clinic patients (12).
In summary, in a cohort of RA patients participating in a questionnaire-based, longitudinal RA outcome study, age and comorbidity have a stronger effect on HAQ progression than biologic treatment. There is little difference between individual biologics. Patients with more severe RA progress less than those with less severe RA, a possible function of regression to the mean.
Acknowledgments
This study was partially supported by Pfizer, Inc. Kaleb Michaud received partial funding for this study from the Arthritis Foundation’s New Investigator Award and NIH ARRA grant #1RC1AR058601-01.
Contributor Information
Kaleb Michaud, University of Nebraska Medical Center, Omaha, Nebraska, National Data Bank for Rheumatic Diseases, Wichita, Kansas
Gene Wallenstein, Pfizer Global Research and Development, New London, Connecticut
Frederick Wolfe, National Data Bank for Rheumatic Diseases, Wichita, Kansas, University of Kansas School of Medicine, Wichita, Kansas
References
- 1.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1530–1542. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.Farragher TM, Lunt M, Bunn DK, Silman AJ, Symmons DP. Early functional disability predicts both all-cause and cardiovascular mortality in people with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2007;66(4):486–492. doi: 10.1136/ard.2006.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Croon EM, Sluiter JK, Nijssen TF, Dijkmans BA, Lankhorst GJ, Frings-Dresen MH. Predictive factors of work disability in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2004;63(11):1362–1367. doi: 10.1136/ard.2003.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allaire S, Wolfe F, Niu J, LaValley MP, Zhang B, Reisine S. Current risk factors for work disability associated with rheumatoid arthritis: recent data from a US national cohort. Arthritis Rheum. 2009;61(3):321–8. doi: 10.1002/art.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker N, Michaud K, Wolfe F. Work limitations among working persons with rheumatoid arthritis: results, reliability, and validity of the work limitations questionnaire in 836 patients. J Rheumatol. 2005;32(6):1006–1012. [PubMed] [Google Scholar]
- 7.Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum. 2003;48(10):2750–62. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- 8.Yelin E, Wanke LA. An assessment of the annual and long-term direct costs of rheumatoid arthritis: the impact of poor function and functional decline. Arthritis Rheum. 1999;42(6):1209–1218. doi: 10.1002/1529-0131(199906)42:6<1209::AID-ANR18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K, Choi HK, Williams R. Household income and earnings losses among 6,396 persons with rheumatoid arthritis. J Rheumatol. 2005;32(10):1875–1883. [PubMed] [Google Scholar]
- 10.Research USDoHaHSFCfDEa. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med. 1998;338(15):1035–1041. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Michaud K. The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marra CA, Esdaile JM, Guh D, Kopec JA, Brazier JE, Koehler BE, et al. A comparison of four indirect methods of assessing utility values in rheumatoid arthritis. Med Care. 2004;42(11):1125–1131. doi: 10.1097/00005650-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Bansback N, Marra C, Tsuchiya A, Anis A, Guh D, Hammond T, et al. Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57(6):963–71. doi: 10.1002/art.22885. [DOI] [PubMed] [Google Scholar]
- 15.Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 16.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Practice & Research Clinical Rheumatology. 2007;21(5):885–906. doi: 10.1016/j.berh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Michaud K, Li T, Katz RS. Chronic Conditions and Health Problems in Rheumatic Diseases: Comparisons with RA, Non-inflammatory Rheumatic Disorders, Systemic Lupus Erythematosus and Fibromyalgia. J Rheumatol. 2010 doi: 10.3899/jrheum.090781. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Michaud K, Pincus T. A Composite Disease Activity Scale for Clinical Practice, Observational Studies and Clinical Trials: The Patient Activity Scale (PAS/PAS-II) J Rheumatol. 2005;32(12):2410–2415. [PubMed] [Google Scholar]
- 19.Royston P. Multiple imputation of missing values. Stata Journal. 2004;4(3):227–241. [Google Scholar]
- 20.Rubin DB. Multiple imputation for non-response in surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 21.Royston P, Sauerbrei W. Multivariable modeling with cubic regression splines: A principled approach. Stata Journal. 2007;7:45–70. [Google Scholar]
- 22.Wolfe F, Michaud K. The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study. Arthritis Res Ther. 2010;12(2):R35. doi: 10.1186/ar2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Some examples of regression towards the mean. BMJ. 1994;309(6957):780. doi: 10.1136/bmj.309.6957.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney CW, Von Korff M. Regression to the mean in treated versus untreated chronic pain. Pain. 1992;50(3):281–5. doi: 10.1016/0304-3959(92)90032-7. [DOI] [PubMed] [Google Scholar]
- 25.Symmons D, Tricker K, Harrison M, Roberts C, Davis M, Dawes P, et al. Patients with stable long-standing rheumatoid arthritis continue to deteriorate despite intensified treatment with traditional disease modifying anti-rheumatic drugs--results of the British Rheumatoid Outcome Study Group randomized controlled clinical trial. Rheumatology (Oxford) 2006;45(5):558–565. doi: 10.1093/rheumatology/kei169. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Michaud K, Pincus T. Development and validation of the health assessment questionnaire II: a revised version of the health assessment questionnaire. Arthritis Rheum. 2004;50(10):3296–3305. doi: 10.1002/art.20549. [DOI] [PubMed] [Google Scholar]
- 27.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–53. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 28.van der Heijde DMFM, van ‘t Hof M, van Riel PLCM, van de Putte LBA. Development of a Disease Activity Score Based on Judgment in Clinical Practice by Rheumatologists. J Rheumatol. 1993;20:579–581. [PubMed] [Google Scholar]