Abstract
Promoting successful cognitive aging is a topic of major importance to individuals and the field of public health. This review presents a coherent framework not only for evaluating factors, protective activities, and enhancing agents that have already been proposed, but also ones that will be put forward in the future. The promotion of successful cognitive aging involves the dual goals of preventing loss of information processing capacity and cognitive reserve, and enhancing brain capacity and cognitive reserve. Four major lines of evidence are available for evaluating whether a proposed factor promotes successful cognitive aging: 1) epidemiologic/cohort studies; 2) animal/basic science studies; 3) human “proof-of-concept” studies; and 4) human intervention studies. Each line of evidence has advantages and limitations that will be discussed. Through illustrative examples, we trace the ways in which each method informs us about the potential value of several proposed factors. Currently, lines of converging evidence allow the strongest case to be made for physical and cognitively stimulating activities. Although epidemiological data seem to favor the use of statins to lower the risk of dementia, more definitive recommendations await further randomized controlled studies. There is presently no clear evidence that antioxidants or Ginkgo biloba promote successful cognitive aging. The impact of resveratrol, fish oil, and a long list of other proposed agents needs to be determined. Clinicians remain well-positioned to identify and aggressively treat vascular risk factors, diabetes, sleep disorders, and other conditions that may reduce brain capacity, and to encourage activities that can build cognitive reserve.
Keywords: clinical research methods, cognition, cognitive reserve, dementia risk factors, neuroprotection, successful aging
INTRODUCTION
This is a propitious time in which to review the topic of promoting successful cognitive aging. In the face of shifting demographics, heightened awareness of the risk of cognitive impairment and dementia, and strong marketing campaigns promising to slow the aging process and increase cognitive powers, there is a pressing need to be able to separate fact from fiction. Clinicians are increasingly being asked by their middle-aged and older patients what can be done to retain their cognitive abilities and avoid becoming demented. Numerous theories and claims are being promulgated, and the cognitive enhancement business is booming. The goal of this paper is to provide a coherent background for making sense of the major issues associated with the field. It does not aim to complete an exhaustive review of all proposed factors, protective activities, or enhancing agents, as these are very likely to change over time. Rather, it will develop a framework in which to consider not only current but also future possibilities.
DEMOGRAPHIC ISSUES
According to a recent UN report [1], individuals over the age of 60 are the fastest growing age group on earth. They currently account for 700 million people around the world. By 2050, the numbers are expected to grow to 2 billion. In the US alone, there are over 75 million Baby Boomers (the cohort born between 1946 and 1964). In two years, the Boomers will begin turning 65, and by 2030 the US population over 65 will double to more than 70 million. Members of this age group have been particularly concerned about maximizing longevity and quality of life. Studies have suggested more than half of adults over the age of 65 have concerns about their memory [2–4]. Losing one’s mental faculties and independence are among the most feared aspects of getting older. Moreover, caring for older individuals who can no longer manage independently has become a major public health issue [5].
NORMAL, NORMATIVE, AND SUCCESSFUL AGING
“Normal” cognitive aging entails predictable changes in cognition associated with getting older. “Normative” cognitive aging represents a level of neuropsychological functioning that falls within 1.5 or 2 standard deviations of the mean for age. One challenge to this concept of normal cognitive aging is that a large portion of the population over the age of 85 exhibits signs of clinical dementia [6,7]. Does that mean that dementia is a part of normal cognitive aging? An alternative definition of normal cognitive aging suggests that it represents “non-pathological” aging, that is, older individuals without identifiable diseases or conditions (e.g., Alzheimer’s disease (AD), cerebral vascular disease) that negatively impact the central nervous system. However, many would argue that there is a continuum between normal and pathological cognitive aging. For example, a very large percentage of older individuals who are cognitively normal manifest some degree of AD (especially amyloid) pathology [8–10] and cerebrovascular disease [11].
Not surprisingly, there are a variety of definitions of successful cognitive aging. Given the prevalence of dementia, successful aging could be defined as eluding the development of dementia or mild cognitive impairment in the later years of life. A more ‘affirmative’ definition of successful cognitive aging would require that an individual performs in the top tier relative to age-matched controls on cognitive tests. A problem with this definition is that it would exclude individuals who were average performers at midlife and successfully maintained their cognitive status in old age. One of the earliest and most influential conceptualizations was offered by Rowe and Kahn [12] who emphasized the need to recognize that older individuals are a very heterogeneous group, and conceived of successful aging as reflecting minimal or no physiological decline compared to “average” younger adults. By contrast, “super” cognitive aging could be viewed as reflecting performance at a level en par with high functioning young adults.
THEORIES ABOUT THE AGING PROCESS AND COGNITIVE AGING
A simple (minded) definition of aging is that it reflects the process of getting older. Within a more biological framework, aging reflects the process in which a variety of stressors are no longer adequately counteracted by the body’s protective functions [13]. Fundamental components of the aging process involve damage from oxidative stress, diminished ability to detoxify free radicals, decline in mitochondrial function, and accumulation of potentially injurious proteins, all of which can lead to decreased integrity of neuronal membranes, altered metabolic functions, and cell death [14–18].
Some investigators have argued that all components of the nervous system exhibit a similar degree of age-related changes [19–21]. Others have suggested that there is selective vulnerability of specific brain regions and systems, for example, the prefrontal cortex (leading to impaired executive functioning) or subcortical white matter (leading to slowed information processing) [22–26]. Some experts have emphasized the importance of age-related changes in ascending neurotransmitter systems such as dopamine from the ventral tegmental area [27,28] that have widespread effects on information processing.
DISTINGUISHING FACTORS WITHIN AND OUTSIDE OF OUR CONTROL
It has been pointed out that the more that successful aging is due to environmental and not genetic factors, the more control people have to determine their health status and vitality as they grow older [29]. Clearly, individuals do not have control over the genes in which they inherit and intellectual capacities are strongly influenced by genetic factors. Based on twin and other studies, some investigators have estimated that cognitive ability in old age is significantly determined (approximately 50%) by the level of intelligence in childhood [30,31]. In addition, there are major genetic factors that influence the development of age-related biological diseases and processes. One example is the impact of a person’s apolipoprotein E (ApoE) genotype status [30,32–34].
Studies also suggest that environmental factors account for a substantial portion of the variance in successful aging. For example, an investigation of the Seventh-Day Adventists estimated that optimal health-related behaviors can add approximately ten years to the average life expectancy [35]. Below, we will discuss the potential beneficial impact of a range of factors over which some degree of control can be exercised.
MAJOR AIMS OF THE CLINICAL APPROACH TO SUCCESSFUL COGNITIVE AGING
Currently, the promotion of successful cognitive aging is linked to the dual goals of: 1) preventing the loss of information processing capacity and cognitive reserve and 2) enhancing brain capacity and cognitive reserve. Information processing capacity reflects the efficiency and degree to which an individual can manage a range of cognitive, emotional, and functional demands. Many theories about cognitive aging have posited an age-related reduction in overall information processing capacity that reflects a decline in neurophysiological function. Closely linked to the concept of information processing capacity is that of “cognitive reserve” [36], which includes the ability to use brain networks more proficiently in response to task demands, as well as the capacity to utilize alternative cognitive strategies or neural networks in response to cerebral injury or decline.
Numerous conditions can undermine information processing capacity and make the brain more vulnerable to additional insults. A simple model of brain capacity and deterioration suggests that when the number of healthy functioning neurons or their connections diminishes below a critical reserve level or threshold, individuals manifest symptoms of cognitive impairment and eventually dementia [37–39] (see Figure 1). An excellent example of the detrimental impact of cumulative insults to the central nervous system comes from one of the “nun studies” [40], which investigated the occurrence of dementia in participants with and without brain infarcts who met neuropathological criteria for AD. Those with brain infarcts had exhibited much poorer cognitive functioning and a higher prevalence of dementia than those without infarcts. The authors concluded that cerebrovascular disease plays an important role in determining the presence and severity of clinical symptoms of AD.
Figure 1.
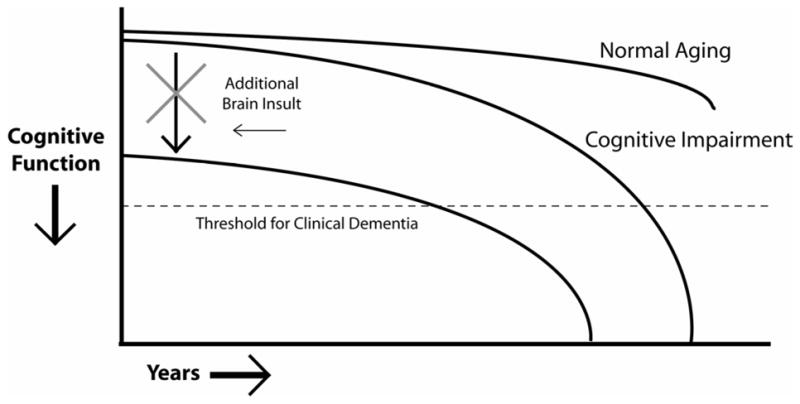
Illustration of the impact on cognitive functioning of processes injurious to the brain. The cognitive functioning curve is shifted downward, leading patients to cross the threshold for clinical dementia earlier. Clinicians should aggressively treat conditions that have the potential to reduce cognitive reserve and information processing capacity, such as hypertension, diabetes, sleep disorders, and side-effects from medication.
There have been many other studies which validate the notion that systemic diseases or conditions that impair cerebral function increase the likelihood that brain pathology will manifest as cognitive impairment or frank dementia [41]. For example, obstructive sleep apnea (OSA) may exacerbate cognitive dysfunction in patients with dementia [42]. Treatment of the OSA appears to improve some aspects of cognitive functioning in non-demented older adults as well as those with dementia [42,43]. Of note, sleep-related breathing disorders are very common in the elderly, with reports of prevalence rates between 24 and 42% [44].
Clinicians are in an excellent position to consider the presence of medical conditions that may erode brain/cognitive reserve. For example, a recent publication from the Rush Religious Orders Study reported that use of drugs with anticholinergic activity was associated with a more rapid decline in cognitive performance in older individuals who were studied over an average of 7.8 years [45]. Since many older patients are on a long list of medications, it is appropriate to carefully review their potential cognitive side-effects. Other conditions that can be addressed with patients include hypertension, diabetes, obesity, sleep disorders, endocrine dysfunction, mood disorders, and potentially toxic substances.
Cognitive reserve is partially a reflection of intellectual capacity (IQ) and thus has a strong genetic component. However, there is evidence that education, occupational experience, and participation in ongoing intellectually stimulating activities help to determine reserve [46–53]. Higher pre-morbid intelligence and more years of education have been associated with a reduced risk of cognitive decline and dementia [47,49,50,54,55]. A study of 130 Catholic clergy [56] during life and at autopsy suggested that individuals with more education required more pathology (neuritic plaques) to reach any given level of cognitive impairment. Moreover, education modified the degree to which AD pathology had a deleterious effect on cognition (i.e., the association of pathology with cognitive function differed as a function of years of education). Thus, education (and perhaps cognitive reserve in general) may not simply increase the threshold for how much cerebral injury can be tolerated before developing symptoms, but also mitigate the functional effects of brain injury, perhaps by compensatory mechanisms such as functional reorganization or the utilization of alternative networks. The beneficial effects of cognitive reserve do not only apply to neurodegenerative disorders. For instance, a recent study that examined predictors of late cognitive decline after penetrating head injury [57] found that pre-injury intellectual capacity was the most consistent predictor of cognitive outcome across all phases of recovery and decline after such injuries.
In the last decade, there has been growing support for the concept of cognitive reserve through the use of functional imaging studies. Investigation of patients with dementia at the same level of severity has revealed that those with more years of education tend to exhibit much more severe reductions in cerebral blood flow to parietotemporal regions [51] and greater amyloid burden, as measured by PiB imaging [58,59], than matched patients with fewer years of education. These findings are consistent with the notion that patients with more years of education have built enough cognitive reserve to be able to manage more severe pathology. Other research has yielded analogous findings for occupational level and the degree to which an individual had participated in intellectually stimulating life activities [52,60]. Such findings serve as examples of “proof-of-concept” human studies regarding the power of cognitive reserve.
AVAILABLE LINES OF EVIDENCE
There are a number of different ways in which to examine whether a proposed factor may have an impact on the promotion of successful cognitive aging. Four major lines of evidence will be reviewed: 1) epidemiologic/cohort studies, 2) animal/basic science studies, 3) human proof-of-concept studies, and 4) human intervention studies. For each line of evidence, we will discuss advantages and limitations (Table 1) and provide several illustrative examples.
Table 1.
Advantages and limitations of different lines of evidence
| Lines of Evidence | Advantages | Limitations | Examples |
|---|---|---|---|
| Epidemiological/Cohort Studies |
|
|
|
| Basic Science/Animal Studies |
|
|
|
| Human “Proof- of-Concept” Studies |
|
|
|
| Human Intervention Studies |
|
|
|
Key: DHA= docosahexaenoic acid
Epidemiological/cohort studies
The first major line of investigation involves epidemiological studies in which, for example, a cohort of subjects is followed longitudinally and the potential impact of specified risk factors on outcomes are determined, after trying to control for the influence of other potentially relevant factors. The advantages of such studies include their large number of subjects and their acquisition of information about many factors. Particularly valuable to the study of successful cognitive aging is the ability of epidemiological studies to follow subjects over years to decades. A major limitation to this line of investigation is that findings can only establish the presence of an association between a designated factor and a clinical outcome, which is not proof of causality. Uncertainty remains about the potential influence of unidentified factors and whether an intervention directed at a particular factor would necessarily change the outcome in clinically significant ways. For example, although prospective cohort studies have indicated that treatment with estrogen or NSAIDs reduces the risk of cognitive decline and dementia, these results have not been confirmed by randomized controlled intervention studies [61–66].
Physical activity
There have been a growing number of longitudinal cohort studies that have suggested that individuals who participate to a greater degree in physical activities are at lower risk for developing cognitive impairment and dementia [67–69,69–74]. For example, the Nurses’ Health Study, which examined over 18,000 women aged 70 to 81 years old, found that higher levels of physical activity were associated with better cognitive performance on tests of general cognition, verbal memory, category fluency, and attention [71]. The Honolulu-Asia Aging Study investigated the distance walked per day in more than 2,000 physically able men aged 71 to 93 years. After adjusting for age, men who walked the least (<0.25 miles/day) experienced a 1.8-fold excess risk of dementia compared with those who walked more than 2 miles/day [75]. Rovio and colleagues [76] from the Karolinska Institute reported that vigorous leisure-time physical activity at midlife of at least twice a week (each lasting 20–30 minutes) was associated with a reduced risk of dementia and AD an average of 21 years later in a sample of over 1,400 adults, even after adjustments for age, sex, education, follow-up time, locomotor disorders, ApoE genotype, vascular disorders, smoking, and alcohol consumption. Exercise also has been shown to reduce the risk of cerebrovascular events [77,78] which can undermine cognitive function.
Intellectually stimulating activity
Many studies that have indicated that as people age, participation in intellectually stimulating activities may sustain cognitive functioning, create a buffer against mental decline (presumably increasing cognitive reserve), and even promote longevity [79–83]. Individuals who participate in cognitively stimulating activities (e.g., reading newspapers, playing games like checkers, chess, cards, or crossword puzzles) appear to be at lower risk for developing probable AD [84,85] and mild cognitive impairment [86]. The causal direction of this association is difficult to prove. Perhaps, participation in fewer activities is due to the early effects of an underlying neurodegenerative process. A potential challenge to this hypothesis comes from studies like the Swedish Kungsholmen Project that have baseline data on the leisure activities of subjects an average of 6 years prior to the assessment of neuropsychological and functional status [87]. Even more convincing are studies that have found that increased participation in cognitively stimulating leisure activities at mid-life is associated with decreased risk of developing clinical dementia many decades later [76,88,89]. Interestingly, analogous results have been reported indicating that the quality and extent of an individual’s social connections are predictive of cognitive outcomes [90–92].
Vascular disease risk factors
Many longitudinal cohort studies have suggested that individuals with higher levels of cardiovascular risk factors have a decreased likelihood of manifesting successful aging, including successful cognitive aging. For example, the Honolulu Asia Aging Study followed almost 6,000 Japanese-American middle-age men up to 40 years. It suggested that the following factors measured at mid-life were associated with successful aging: high grip strength, avoidance of hyperglycemia, hypertriglyceridemia, hypertension, smoking, excessive alcohol consumption, and being overweight, and attainment of higher years of education [93]. The “Study of Osteoporotic Fractures” included almost 10,000 women whose mean age was 72 at baseline and 85 at follow-up [94]. Those individuals who maintained their cognitive status were more likely to lack diabetes, hypertension, smoking, and a limited social network, and to consume a moderate amount of alcohol.
Several large prospective cohort studies have provided powerful epidemiological evidence that untreated hypertension at mid-life increases the risk of subsequent dementia [95,96]. Moreover, a recently reported investigation of over 6,000 subjects suggested that use of antihypertensive medications was associated with a reduced risk of dementia, especially in subjects ≥75 years old (8% risk reduction per year of medication use) [97]. Analogous kinds of data exist for the association between increased risk of developing dementia and high body mass index or the metabolic syndrome [98–102]. For example, a recent study indicated that a high body mass index at mid-life was a strong predictor of developing probable AD or vascular dementia, independent of diabetes and other cardiovascular co-morbidities [101,102]. In general, the greater the number of cardiovascular risk factors at mid-life (e.g., smoking, hypertension, high cholesterol, diabetes) the higher the risk of suffering from dementia in late life [103].
Many epidemiological studies have reported that high cholesterol increases the risk of developing dementia [104–106]. For instance, one study [107] followed over 1,400 middle-age adults (mean age 50) for an average of 21 years and found that elevated total cholesterol levels (≥6.5 mmol/L (251 mg/dL)) increased the odds of developing probable AD (odds ratio 2.8), after adjusting for ApoE status, other midlife vascular risk factors, and other confounders. However, other investigations, such as the Framingham Study [108], have found no relationship between total cholesterol and the risk of incident AD. Recently, three large cohort investigations concluded that statin use reduces the risk of the development of dementia [109–111]. For example, the Rotterdam Study [110] followed close to 7,000 individuals prospectively over an average of 9 years. They reported a decreased risk of AD (hazard ratio 0.57) among participants who used statins. Hazard ratios were similar for lipophilic and hydrophilic statins. Non-statin cholesterol-lowering drugs did not reduce the risk of incident AD. Sparks [111] reported a similar advantage for individuals who took statins ‘electively’ as for those being treated for elevated cholesterol levels. However, the beneficial impact of statins has not been a universal finding [112–114]. For example, in the Religious Orders Study of over 900 older Catholic clergy [112], statin use at baseline did not affect the risk of developing dementia in up to 12 years of follow-up; nor was it associated with global cognitive status or performance in five separate cognitive domains. Other studies using a time-dependent proportional hazards model, such as the Adult Change in Thought study [113], have drawn similar conclusions. The cause of these discrepant findings is not clear.
Diet/Nutrition
Although many studies have suggested that the nature of a person’s diet is likely to have an impact on the risk of developing dementia, it has been very difficult to determine which specific nutritional factors lower the risk of dementia or increase the likelihood of successful cognitive aging. For example, reports have been inconsistent regarding whether a high intake of vitamins E, C, B6, B12, folate, fish, or unsaturated fats is linked to a lower risk of AD [115–119]. An alternative approach has been to examine whether certain kinds of diets reduce the likelihood of developing a dementing illness without trying to determine which specific dietary factors are most important. For example, a recent study of over 2,000 community-dwelling non-demented individuals examined the relationship between the degree to which individuals adhered to a Mediterranean diet and their risk of developing probable AD [120]. The Mediterranean diet includes a high intake of fruits and vegetables; monounsaturated fatty acids such as those found in olive oil; fish; nuts and cereals; and relatively little consumption of red meat. Over the course of a 4 year follow-up, higher adherence to the Mediterranean diet was linked to a lower risk of probable AD, after adjusting for age, sex, ethnicity, education, ApoE genotype, caloric intake, smoking, body mass index, and medical comorbidity. A follow-up study suggested that the benefit of the Mediterranean diet was not simply due to greater physical activity among individuals who consume healthier food, but represented an independent factor [73]. Whether adherence to the Mediterranean diet works through reducing the risk of vascular disease, altering the neurobiological cascade associated with AD, or some other mechanism remains to be determined [121]. In a subsequent study of patients diagnosed with mild cognitive impairment, those who most closely adhered to a Mediterranean diet were less likely to progress to a clinical dementia over the course of 4.5 years [122].
Alcohol consumption
The Rotterdam study [123], which followed close to 8,000 older adults, reported that light to moderate alcohol consumption (i.e., 1–3 drinks/day) was associated with a reduced risk of developing dementia. These results are consistent with a recent meta-analysis of 22 longitudinal studies of alcohol use and incipient dementia/cognitive decline that suggested that small amounts of alcohol may be protective against dementia [124]. The definition of light to moderate alcohol consumption varied across studies (from <1 to up to 28 glasses per week). Another recent study found that patients with mild cognitive impairment who consumed moderate amounts of alcohol had a lower rate of progression to clinical dementia than non-drinkers [125]. Many of these studies have not differentiated between consumption of wine, beer, or hard liquor. There is some evidence to suggest that wine may have a particularly salutary effect on brain and cardiovascular health, and lower the risk of developing cognitive decline and dementia [126–130]. Differences between red and white wine have not been systematically studied [124]. Potential mechanisms may include its antioxidant, antithrombotic, vasodilating, and anti-amyloid effects, which may in part be mediated by resveratrol, one of the active ingredients found in red wine (see below) [129,131–133].
Basic Science/Animal Studies
The second major line of investigation involves animal and basic science research. This work provides an opportunity to investigate questions under more highly controlled conditions. For example, researchers can “enroll” animals in an intervention study, measure the behavioral impact, and then sacrifice the animals to examine their brains. Potentially pertinent changes in brain structure, synaptic connectivity, neurotransmitters and their receptors, hormones, growth factors, and inflammatory markers can be measured. Cellular and molecular effects can be investigated in the service of identifying underlying mechanisms. There are important limitations to this approach. In-vitro systems tend to reduce complex issues into simpler ones in order to study them. Findings from basic science or animal models need to be translated to the study of humans. There are numerous examples of research on drug development for human disorders in which interventions that had worked in animals or in-vitro systems failed to do so in humans. There are very prudent reasons why governmental regulatory agencies do not approve drugs based on in-vitro and animal models alone, but require large-scale studies of human beings.
Physical activity
Studies of animals have indicated that exercise promotes neurogenesis and synaptogenesis. Exercise upregulates the production of brain derived neurotrophic factor (BDNF) [134–136]. BDNF is believed to help induce neurogenesis and promote survival and/or neuronal differentiation. It has also been linked to long-term potentiation necessary for memory formation. In addition, aerobic exercise in animals has been shown to release two molecules, vascular endothelial growth factor and insulin-like growth factors, which stimulate angiogenesis [134].
Cognitively stimulating/Enriched environment
There have been numerous studies comparing animals placed in “enriched” versus standard environments. An enriched environment often means a cage filled with potentially interesting junk to explore. The opportunity of aging animals to interact with a more complex environment appears to influence neurogenesis and dendritic complexity, and promote cognitive abilities and the capacity to compensate for injury [137–141]. A study of transgenic AD mice exposed to an enriched environment exhibited a marked reduction in cerebral Aβ levels and amyloid deposits compared to animals reared under standard conditions [142]. The animals in the enriched environment exhibited an increase in the activity of the Aβ-degrading protease, neprilysin, and altered processing of the amyloid β-protein precursor (AβPP), reducing amyloid burden. DNA microarray analysis revealed an upregulation in levels of transcripts encoded by genes associated with learning, memory, vasculogenesis, neurogenesis, and cell survival. In another recent study [143] using a mouse model of circumscribed neuronal injury, animals placed in an enriched environment were able to re-establish learning behavior and access long-term memories even after significant brain atrophy and hippocampal neuronal loss. The environmental enrichment correlated with chromatin modifications (increased histone acetylation) implicated in transcriptional regulation of gene expression. Inhibitors of histone deacetylases reproduced the histone acetylation effects of environmental enrichment and were associated with dendritic sprouting, increased synaptic number, and the reestablishment of learning behavior.
Pharmacological agents
A myriad of medications, supplements, herbs, and vitamins have been subjected to in vitro and animal studies to examine their potential to protect the brain or enhance its cognitive functions. Below we limit our comments to just a few representative examples, including statins, fish oil, resveratrol, and Ginkgo biloba. In part, these were chosen because pertinent information is also available on them from epidemiologic studies, human intervention studies of normal older adults, or both.
Statins (HM-CoA reductase inhibitors) have been shown to have pleiotropic effects independent of their impact on cholesterol [144]. These effects include improvement of endothelial function, inhibition of platelet activation, reduction of blood coagulability, and suppression of inflammatory reactions [144–147]. In addition, there is in vivo evidence that statins may modulate the metabolism of AβPP, reducing the production of Aβ [144]. Docosahexaenoic acid (DHA), a key component of fish oil, and its derivatives (DHA-derived neuroprotectin D1) appear to have neurotrophic, antiapoptotic, and anti-inflammatory signaling properties that affect neuronal survival and repair [148,149]. Animal models suggest that DHA and its derivatives can diminish inflammatory responses associated with colitis, peritonitis, and ischemic stroke [150].
Resveratrol, a polyphenol found in grape skins and red wine, is believed to mimic caloric restriction by stimulating sirtuins (SIRT1, Sir2) [151,152]. SIRT1 is deacetylase enzyme that regulates the activity of transcriptional factors and proteins. It may activate intracellular pathways critical for antioxidant activities, cell cycle regulation, mitochondrial energy production, vascular tone, and oncogene suppression [132,133,152]. A seminal paper in Nature [153] demonstrated that resveratrol extended the life span of yeast. Subsequent studies found similar effects (prolongation of lifespan) in the worm, C. elegans, in the fruit fly, and in aging fish (Nothobranchius furzeri). In mouse and rat experiments, anti-cancer, anti-inflammatory, and glucose lowering effects have been demonstrated. Resveratrol reportedly improved endurance in mice fed this compound for 15 weeks. There also is evidence that resveratrol may interfere with amyloid plaque deposition and enhance Aβ clearance [152,154].
Ginkgo biloba, which contains flavonoids, reportedly exhibits antioxidant activity as a free radical scavenger and suppressor of active oxygen and nitrogen species [155–157]. Reportedly, it improves blood flow by influencing vasomotor function and inhibiting the activation of platelets and smooth muscle cells, and has a modulatory effect on neurotransmitters and their receptors. There also are reports suggesting that Ginkgo biloba may inhibit the aggregation of Aβ [158].
Human Proof-of-Concept Studies
The third line of investigation involves what may be labeled human proof-of-concept studies that examine markers of plasticity, reserve, efficiency, and neural compensation. For example, differences in information processing among adults who vary in terms of degree of successful cognitive aging are measured. Although the number of subjects studied is limited, they often are investigated using the tools of modern cognitive neuroscience that can elucidate potential underlying mechanisms. The results of such studies provide an opportunity for further hypothesis generation and testing. At times, they have provided clinicians with a rationale for trying certain interventions, even in the absence of randomized controlled trials. The disadvantages are similar to research derived from basic science and animal studies. Such studies may highlight links between certain factors and changes in brain size or activity, but do not necessarily translate into improved function, clinical status, or performance in daily activities.
Structural neuroimaging
Aerobic fitness is associated with greater white matter integrity after controlling for age and gender [159]. This is an important finding, given the growing evidence that age-related changes in white matter are associated with declines in information processing speed and frontal-executive function [26,160–162]. Morphometric MRI studies have provided evidence that cognitive activity or training alters brain morphology of relevant structures, leading to an increase in cortical thickness. For example, comparisons of the MRIs of individuals learning to juggle versus those of control subjects reveal an increased size of bilateral mid-temporal grey matter and left posterior intra-parietal sulcus, structures associated with the visual processing of movement [163]. Similarly, comparisons of the MRIs of licensed London taxi drivers versus control subjects reveal increased volume of the posterior hippocampus believed to help mediate spatial memory [164].
Cognitively high and average performing older individuals exhibit differences in cortical thickness. For example, older individuals who perform better on tests of fluid intelligence have been shown to have a thicker cortex, especially within the posterior cingulate of the right hemisphere, and the superior and middle frontal gyri, and gyrus rectus bilaterally [165]. The authors hypothesized that age-related declines in efficiency of more specialized cognitive processes may be compensated by an increase in monitoring and control in high performing old adults. They argued that this cognitive activity itself may alter the brain morphology of relevant structures, leading to an increase in cortical thickness in pertinent areas.
Functional imaging
Positron emission tomography (PET) can image neurotransmitter binding activity. Recently, there was a report in Science [166] that demonstrated that 14 hours of working memory training in young adult subjects over 5 weeks was associated with decreases in prefrontal and parietal dopamine D1 receptor binding potential (larger decreases in D1 binding potential correlated with larger improvements in working memory). This was interpreted as indicating that the dopamine receptor system is plastic and can be influenced by structured mental activity.
Functional magnetic resonance imaging (fMRI) has been used to examine the impact of cognitive training on older adults. For example, Erickson and colleagues [167] conducted a randomized, longitudinal dual-task training study in older subjects. They found that training improved task performance and was associated with an increase in asymmetric hemispheric activation (increase in left and decrease in right hemisphere activity within the ventral lateral prefrontal cortex (PFC), and a decrease in age-related differences in the pattern of prefrontal activation). Logan and colleagues [168] found that training which emphasized semantic elaboration during a memory encoding task led to improved performance of older subjects. This was associated with increased activity of the PFC (especially the left ventral region), which, compared to young subjects, had been under-recruited at baseline, prior to training. Both studies suggest that the aging brain can exhibit plasticity that may be influenced by training and intervention programs.
Many functional imaging studies using glucose metabolism or cerebral blood flow have reported age-related increases in frontal lobe activity (and unlike young subjects, the recruitment of both hemispheres) in response to a number of task demands. In some cases, these age-associated increases in neural activity and more widely distributed responses have been interpreted as reflecting compensatory activity (in response to age-related declines in physiological functioning). In other cases, they have been viewed as reflecting reduced processing efficiency (i.e., diminished selective recruitment of specialized neural processes [169]). To help sort out functional significance of age-related changes in neural activity, investigators are increasingly dividing their subjects into groups based on task or neuropsychological performance and then examining the differences between groups on measures of neural function used to carry out the task.
A number of functional imaging studies have shown that older subjects who perform comparably to young subjects on working memory, source memory, or episodic memory tasks recruit more brain activity than young subjects and than old subjects who perform worse [170–173]. However, this pattern is not associated with all cognitive functions (e.g., inhibitory control) [174,175] and some investigators would argue that older individuals who perform the best often exhibit a pattern of activity on functional imaging closest to that of younger adults [167,168]
Event-related potentials
Young adults with higher aerobic fitness tend to generate larger amplitude and shorter latency P3 event-related potentials than less-fit subjects [134,176]. This has been interpreted as consistent with the idea that greater physical fitness or activity facilitates functions associated with the allocation of neural resources and with more rapid information processing speed [134]. A more complex story emerges when considering the interaction between fitness and age-related changes in the P3 component. McDowell and colleagues [177] reported that low-active elderly participants generated larger P3 amplitude than observed in high-active old or either high- or low-active young subjects. The authors concluded that higher levels of physical activity in the elderly may be associated with a reduction in the neural resources needed to be allocated in response to simple cognitive challenge.
Our lab has shown, using a subject-controlled visual novelty oddball paradigm, that cognitively high performing old subjects generate a larger novelty P3 response than cognitively high performing middle age and young subjects and than cognitively average performing old subjects [80,82,178]. These electrophysiological results occur in the context of behavioral findings that cognitively high performing old subjects are as engaged by novelty as their cognitively high performing younger counterparts and more engaged by novelty than cognitively average performing old adults. Across the older participants, the better the performance on tests of frontal-executive function, the larger the P3 amplitude in response to novel stimuli (and the more time they spent viewing novel stimuli). These results provide a strong basis for arguing that the larger P3 response to novel stimuli observed in cognitively high performing old adults does not simply represent less efficient processing, but a successful compensatory mechanism, presumably in response to other age-related physiological changes.
Human Intervention Studies
The final line of investigation involves intervention studies in human subjects. Ideally, baseline measures are obtained, individuals are randomized to treatment or placebo groups, and outcomes are measured to establish treatment effects. The potential variables investigated can include cognitive performance and biological or neuroimaging markers. Many would consider these kinds of studies to be the “gold standard” for proof of a factor’s impact on successful cognitive aging. However, there are challenges and limitations to such an approach. For example, to what extent can the observed effects be generalized? How long do the effects endure and to what extent are they clinically relevant? How feasible is it to translate such interventions into practice outside of a controlled study? Also, some of the most pertinent kinds of studies (e.g., impact on developing dementia late in life of antihypertensive treatment versus placebo in middle-aged subjects with hypertension) often are not feasible for financial, logistical, or ethical reasons.
Exercise/Physical training
A Cochrane Database System Review [179] noted that 8 out of 11 randomized controlled exercise intervention studies reported that structured aerobic exercise interventions resulted in increased cardiorespiratory fitness of the intervention group, with an average improvement of approximately 14%. Most studies involved approximately 1 hour of exercise 3 times per week. This improvement was associated with better performance in cognitive functions, including auditory attention (effect size 0.52), speed of information processing (effect size 0.26), and visual attention (effect size 0.26). A recent review article by Hillman, Erickson, and Kramer [134] concluded that physical activity improved cognition in both normal older adults and patients with early AD. Although benefits were seen for a range of cognitive functions, there appeared to be disproportionate improvement in executive control (including planning, working memory, and multi-tasking).
Particularly intriguing are the exercise intervention studies that have looked at effects on not only cognition but also underlying brain structure and activity. For instance, in a study reported by Colcombe and colleagues [180], older individuals who participated in an aerobic training group for 6 months (i.e., ~1 hour, 3 times per week) exhibited significant increases in the size of components of the prefrontal cortex and superior temporal lobes. In another study by the same researchers [181], more physically fit older subjects as well as those randomly assigned to participate in the aerobic fitness training group (~45 minutes of walking, 3 times per week) for 6 months performed better on an attention task (more efficient response to conflicting cues on a flanker task). Moreover, fMRI revealed that they exhibited greater neural activity in regions associated with attentional control (middle and superior frontal gyrus, superior parietal lobule) and less activity in the anterior cingulate cortex.
Cognitive training
The potential impact of cognitive training on older adults has been a topic of increasing interest [182–186]. One of the greatest challenges is trying to demonstrate that the training results in changes outside of the laboratory that are applicable to everyday life. To date, there have been no reports to suggest that cognitive training programs of older adults reduce the risk of developing a clinical dementia. Rather, the emphasis has been on establishing that cognitive training can improve measures of intellectual performance and daily activities. For example, in a study by Ball and colleagues [182], 2,832 older individuals (65–94 years old) were randomly assigned to one of three training groups for memory (verbal episodic memory), reasoning (problem-solving that follows a serial pattern), or speed of processing (visual search and identification), or to a control group. The initial intervention improved performance relative to baseline, however, the improvement was limited to the cognitive realm that had been targeted. Improvements lasted through the 2 years of follow-up. Fifty percent of the random sample was given booster training (four 75 minute sessions over 2–3 weeks) 11 months after the initial training period. Booster training increased the training improvements in speed and reasoning, which were maintained at the 2-year follow-up. Training was not shown to have a measurable impact on everyday functioning at 2 years. In a 5-year follow-up study [183], the investigators reported that each intervention had maintained effects on the targeted cognitive ability. On a self-report survey of instrumental activities of daily living, all three intervention groups indicated less difficulty than the control group, which only reached significance for the reasoning group. Also, only the booster training for speed of processing showed an effect on performance-based functional measures of everyday processing speed.
There are a growing number of cognitive training programs that are being marketed to older adults. Many provide little, if any, data to support the claims being made about the particular program being promoted. Moreover, to date, there is no clear evidence to suggest that formal cognitive training programs are more effective than traditional forms of intellectually stimulating activities (e.g., crossword puzzles, challenging reading, card games, etc.); nor have there been published head-to-head studies comparing one commercially-available program to another. Several other important issues have not yet been addressed, including the potential impact of a training program that titrates difficulty to a participant’s current level of success, the game-like, competitive features of a program, and the effect of a participant’s desire to get a good return-on-investment for the cost of purchasing the software.
Pharmacologic agents
Numerous agents have been proposed to provide neuroprotection (by counteracting hypothesized underlying injurious age-related processes), cerebral enhancement (by counteracting deleterious consequences of the aging process through boosting neurotransmitters, hormones, or cerebral blood flow), or both. Table 2 provides a partial list of such agents. A selected few will be reviewed here.
Table 2.
Proposed cerebral enhancing/protective agents
| Proposed Cerebral Enhancing Agents | Proposed Cerebral Protective Agents | Proposed agents with both cerebral enhancing and protective properties |
|---|---|---|
| Ampakines | Antioxidants | Acetyl-L-carnitine |
| Cholinesterase Inhibitors | Omega-3 fatty acids (fish oil; EPA-DHA) | B vitamins (B6, B12, Folate) |
| CREB | Resveratrol | CDP- Choline |
| Hydergine | Statins | Cerebrolysin |
| Modafinil | Gamma- aminobuteyric acid | |
| Piracetam | Ginkgo biloba | |
| Stimulant medications | Ginseng | |
| Hormonal replacement/supplementation | ||
| Pentoxifylline | ||
| Phosphatidylserine | ||
| Phospolipids | ||
| Selegeline | ||
| Vinpocetine |
Key: CREB= cAMP response element binding; EPA-DHA= eicosapentaenoic acid and docosahexaenoic acid
Anti-hypertensive drug treatment
As noted before, there is strong epidemiological evidence that hypertension increases the risk of dementia. Recently, there have been four randomized placebo-controlled studies investigating the effects of anti-hypertensive agents on the incidence of dementia in older individuals [187,188]. The systolic hypertension in Europe (SYST-EUR) study [189] suggested that active treatment with nitrendipine, enalapril, and/or HCTZ reduced the rate of dementia by approximately 50%. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) [190,191] found that treatment with perindopril and indapamide was associated with a reduction in cognitive decline compared to placebo (relative risk 19%). In contrast, two other studies did not demonstrate an advantage to treatment with anti-hypertensive medication. The Systolic Hypertension in the Elderly Program (SHEP) subjects received chlorthalidone, atenolol, or reserpine (versus placebo) [192,193] and the Study on Cognition and Prognosis in the Elderly (SCOPE) [194] (candesartan versus placebo) did not demonstrate a significant difference between treatment and placebo groups in change in MMSE score or the incidence of dementia. Of note, there were problems with the analysis of data because of the number of patients on placebo who were given active treatment (e.g., 84% in the SCOPE trial). In addition the trials were not very long (2–4.5 years) and the hypertensive subjects treated were not middle-aged, but older. It is very plausible that the most effective way for antihypertensive treatment to reduce the risk of dementia will be to intervene early (e.g., in middle-age).
Statins
As mentioned previously, statins are reported to have multiple effects independent of their impact on cholesterol, including suppression of inflammatory reactions, inhibition of platelet activation, reduction of blood coagulability, and modulation of the metabolism of AβPP. In addition, several cohort investigations have found that individuals who take statins have a lower risk of becoming demented [109–111]. There have been two very large prospective intervention studies, the PROSPER study [195] that included almost 6,000 participants randomized to pravastatin or placebo, and the Heart Protection Study Collaborative Group [196] that followed over 20,000 adults randomly assigned to simvastain or placebo. The main purpose of these studies was to understand the relationship between statin use and the risk of coronary artery disease and other vascular events. However, both reported on the cognitive effects and found no clear cognitive benefit from being on statins. For example, the PROSPER study compared baseline cognitive performance to last on-treatment cognitive performance an average of 3.2 years later. No significant group differences (treated versus placebo) were found in changes in MMSE score or performance on tests of attention and memory.
A small study (n = 97) conducted by Parale et al. [197] aimed to assess the cognitive impact of a statin by comparing subjects on atorvastatin versus placebo who were matched for age, gender, education, and the presence of hypertension and diabetes. Changes between baseline and six months were measured in five cognitive domains. Subjects treated with the statin scored significantly better than the placebo group on tests of psychomotor speed, mental flexibility, working memory, and memory retrieval. Clearly more research is needed to determine the extent to which treatment with statins reduces the likelihood of developing dementia and helps to preserve cognitive function, especially if used over many years.
Antioxidants
As discussed earlier, one of the major theories about the aging process posits a central role for cellular injury due to oxidative stress. Thus, it is not surprising that many agents with potential antioxidant properties have been considered (including vitamin E, vitamin C, beta carotene, folic extract, curcumin, and green tea). The results of most trials to date have been disappointing [198]. Table 3 summarizes major studies that have investigated the impact of antioxidants on cognitive status in healthy older adults or those with mild cognitive impairment, none of which reported beneficial results. Thus, despite its strong intuitive appeal, we do not yet have convincing evidence to support the use of antioxidant supplementation to augment or maintain cognitive status in cognitively normal adults or those diagnosed with mild cognitive impairment.
Table 3.
Randomized controlled studies of antioxidants, fish oil, and Ginkgo biloba
| Study | Agent(s) | Subjects | Duration | Major Findings |
|---|---|---|---|---|
| Petersen et al., 2005 | Vitamin E 1000 IU 2×/day |
769 older adults (55–90) with MCI | 3 years | No impact on progression to dementia |
| Heart Protection Study Group (2002) | Vitamin E 600 IU/day Vitamin C 250 mg/day Beta carotene 20 mg |
20,536 older adults (40–80) | 5 years | No impact on cognitive status |
| Yaffe et al., 2004 (AREDS) | Vitamin E 400 IU/day Vitamin C 500 mg/day Beta carotene 15 mg/day |
2,166 older adults (61–87) | 6.9 years | No effect on cognitive performance |
| Kang et al. 2006 (Women’s Health Study) | Vitamin E 600 IU on alternate days | 13,807 older women (70–81) | 9 years | No effect on cognitive performance |
| van de Rest, 2008 | EPA-DHA 1,800 mg/day or 400 mg/day |
302 older adults (65+) | 26 weeks | No effect on cognitive performance |
| DeKosky et al. (2008) | Ginkgo biloba 120 mg 2×/day | 2,069 older adults with normal cognition or MCI (75+) | 6.1 years | No impact on progression to dementia |
| Solomon et al. (2002) | Ginkgo biloba 40 mg 3×/day | 230 older adults (>60) | 6 weeks | No effect on cognitive performance |
Key: IU= international units; AREDS= Age-Related Eye Disease Study Research Group; EPA-DHA= eicosapentaenoic acid and docosahexaenoic acid; MCI= mild cognitive impairment
A similar conclusion can be drawn about omega-3 fatty acids (fish oil), which, as reviewed earlier, theoretically can reduce oxidative damage, inflammation, and risk of vascular injury. A Cochrane Database review in 2006 [199] suggested that up to that point there had been no randomized, placebo-controlled, double-blind studies that lasted a minimum of 6 months in persons 60 years and older without pre-existing dementia and established cognitive endpoints. However, recently there was a double-blind placebo-controlled trial [200] in which 302 cognitively healthy older individuals were assigned to 1,800 mg or 400 mg per day of eicosapentaenoic acid and docosahexaenoic acid (EPA-DHA) versus placebo for 6 months (Table 3). There was no evidence of an effect on cognitive performance in the treatment compared to the placebo groups. It remains to be determined if cognitive benefits are derived from taking omega-3 fatty acids for more than 6 months.
There has been an escalating interest in resveratrol, a compound found in red wine that has been shown to extend the life span of yeast, fruit flies, and other animals. As mentioned previously, there have been many provocative in vivo and animal studies. However, there have been no published controlled studies of the impact of resveratrol on human neurodegenerative diseases, on age-related cognitive changes, nor on its ability to increase physical endurance in humans. It remains to be determined if the promise of resveratrol will be fulfilled. Of note, despite the limited data on humans, there has been little hesitation to market resveratrol as a ticket to the “fountain of youth” [201–203].
Ginkgo biloba
Ginkgo biloba is purported to have a number of potentially useful cognitive enhancing and cerebral protective properties, including mild stimulant effects, antioxidant properties, increased cerebral blood flow, and inhibition of platelet activating factor. A recently reported study by DeKosky and colleagues [204] examined 2,069 older community volunteers (>75 years old) with either normal cognition or mild cognitive impairment (Table 3). Treatment with Gingko biloba was not found to be effective in reducing either the overall rate of developing dementia or AD in older individuals with normal cognition or those with mild cognitive impairment over a mean follow-up of 6.1 years.
The potential impact of Ginkgo biloba on healthy, cognitively normal adults under age 60 also has been investigated. A systematic review done by Canter and Ernst [205] evaluated 15 randomized clinical trials, of which 7 were single dose and 8 were longer term studies, ranging from days to weeks. The authors found no convincing evidence for a robust positive effect of Ginkgo biloba on any aspect of cognition in healthy younger adults. Solomon and colleagues [206] investigated healthy older community-dwelling men and women who were randomized Ginkgo biloba or placebo (Table 3). The investigators purposefully chose the dose of Ginkgo and the duration of study to match the manufacturer’s recommendations that promised to improve attention, memory, and related cognitive functions within as little as 4 weeks. However, no benefits were found in any outcome measure, which included tests of verbal and nonverbal learning and memory, verbal fluency, language, and attention, participants’ self-report on a memory questionnaire, and global rating by spouses, friends, or relatives. In summary, barring new evidence, one cannot recommend the use of Ginkgo biloba to enhance cognition in older individuals.
DISCUSSION
Promoting successful cognitive aging is a topic of profound importance to both individuals and the field of public health. Different patterns of cognitive aging have been identified and there is a growing need to better understand what influences these trajectories. Although genetic factors play an important role in determining intellectual capacity and in predisposing an individual to a variety of age-related disorders, there are numerous factors over which we can exercise some degree of control. Clinicians can play an important role in identifying and treating conditions that reduce cognitive reserve and intellectual capacity, including vascular risk factors, sleep disorders, metabolic dysfunction, mood disorders, and side-effects from medication. They can serve as advocates for and educators about brain health and well-being. In order to accomplish this, clinicians need a framework for evaluating claims made about current and future cognitive enhancing activities and agents.
This paper reviewed different approaches to the investigation of whether a proposed factor actually promotes successful cognitive aging. In many cases, it is essential to acknowledge that the currently available evidence is insufficient and that further investigation is required. Pertinent data may be obtained from epidemiological/cohort studies, basic science and animal investigations, human proof-of-concept studies, and randomized controlled trials in humans. These approaches provide different perspectives upon which to evaluate the factors under consideration. Each approach has its advantages and limitations. Often, the most convincing case in favor of a factor’s impact on successful cognitive aging will be made through lines of converging evidence. Table 4 summarizes the relative progress that has been made in the evaluation of a few selected factors that can serve as examples.
Table 4.
Relative progress along 4 major lines of evidence of selected factors proposed to promote successful cognitive aging
| Factor | Epidemiological Cohort Studies | Animal/Basic Science Studies | Human Proof of Concept | RCTs |
|---|---|---|---|---|
| Exercise | + + + + | + + + + | + + + | + + |
| Intellectual Stimulation | + + + | + + | + + + | + |
| Cholesterol/Statins | + + − | + + | N/A | − − + |
| Ginkgo biloba | N/A | + + | N/A | − − |
| Resveratrol | + | + + + | N/A | N/A |
| DHA | + | + + | N/A | − |
| Antioxidants | + + − | + + | N/A | − − |
| Diet/Nutrition | + + | + | N/A | N/A |
Key: RCT= randomized controlled trials; DHA = docosahexaenoic acid; N/A= Not applicable, data not yet available
Currently, the strongest case can be made for physical and cognitively stimulating activities, both of which appear to be able to enhance cognitive performance and reduce the likelihood of developing dementia. Epidemiologic studies suggest that regular exercise and participation in cognitively stimulating activities diminish the risk of dementia. Animal studies suggest that exercise is associated with the upregulation of relevant neural growth factors such as BDNF, and that environmental enrichment promotes neurogenesis, increases the capacity to compensate for cerebral insults, and augments cognitive function. Proof-of-concept studies highlight the plasticity of the human brain, even in older adults. For example, compared to their age-matched counterparts, better-fit and more cognitively competent individuals exhibit differences in brain structure, neural activity, and electrophysiology that appear to be more adaptive. Cognitive experience or mental practice influences the size of pertinent brain regions and modulates neurotransmitter activity. Randomized controlled intervention studies suggest that aerobic exercise and cognitive training programs are associated with improved cognitive performance and can affect relevant biological markers.
There are several barriers to translating knowledge about factors that may promote successful cognitive aging into real world practice. First, more research is needed to move from general principles derived from scientific studies to concrete clinical recommendations. Clinicians tend to be a practical group who wants to know the “bottom line” regarding recommendations for patient care. For example, in addition to appreciating that exercise is good for brain health, there is an interest in determining the minimal amount of exercise a person can do to be of benefit. Secondly, more research is needed to determine the most effective strategies and interventions for encouraging individuals to adopt healthier lifestyles, especially if such changes are perceived as effortful or burdensome. Specifically, how can we influence individuals or groups of individuals to sustain engagement in activities that promote cognitive well-being?
Appealing solely to the advancement of one’s personal health may motivate a relatively small, self-selected group and not serve as a foundation for public policy or clinical care [207]. It seems intuitively obvious (but in need of scientific verification) that activities that are experienced as being intrinsically enjoyable or meaningful are the ones in which people are most likely to participate and remain engaged over time. For instance, it seems much more probable that individuals would continue to play bridge or cards over the course of their adult lives because they enjoyed them than because these activities are theoretically good for their cognitive health. Many leisure activities represent a combination of mental, physical, and social components [208]. For example, bridge or card playing can be both intellectually and socially stimulating. Participation in sports can provide an opportunity for both aerobic exercise and social interactions. There is epidemiological support for the notion that the most beneficial effects may be derived from participating in a range of activities that involve a mixture of intellectual, physical, and social components [208]. Moreover, activities that reflect a combination of attributes may provide a powerful motivational hook for maintaining participation. Another promising strategy has been to harness the desire of older individuals to feel generative, have an impact on others, and remain socially active [72,74]. The Baltimore Experience Corp is a novel, real-world intervention research program in which 149 older subjects were randomly assigned to a wait-list control group or to one that helps elementary school children with reading achievement, library support, and classroom behavior for 15 hours per week during the academic year. The participants included a disproportionate number of African Americans with relatively low income and education. Pilot data suggest that participants remained actively engaged with the program over many months and tended to show improvements in executive functions and memory, especially those individuals who were the least cognitively competent at baseline.
Converging lines of evidence, as illustrated by research on physical and cognitively stimulating activities, may be most convincing and provide a means of addressing the limitations associated with any single avenue of study. However, in many cases it is unrealistic to expect that all of the different research approaches will provide pertinent input. What should clinicians do while they wait for the results from randomized controlled trials that may never take place or at least not occur for many years? Should clinicians provide advice based on available evidence derived from epidemiological investigations, basic science and animal work, and/or human proof-of-concept studies? Like many issues in clinical medicine, it is necessary to weigh potential risks and benefits, be aware of the limits of current data, and modify suggestions as new information becomes available. Here are some provisional recommendations. Despite the absence of randomized clinical trials, available epidemiological data and knowledge about the relationships between untreated hypertension, cerebrovascular disease, and cognitive decline provide a powerful justification to advocate for aggressive treatment of hypertension and other vascular risk factors by middle-age or earlier. Although epidemiological evidence seems to favor the use of statins to lower the risk of dementia, more definitive recommendations await randomized controlled studies that ideally would be conducted over many years. Currently, there is no clear evidence that antioxidants or Ginkgo biloba promote successful cognitive aging. The impact of resveratrol, DHA, and a long list of other proposed agents (Table 2) remains to be determined.
Major efforts should be made to ensure transparency about what is known versus what is speculative, and to translate this information into language that is accessible to the public that lacks expertise or professional training in this field. Unfortunately, these kinds of distinctions are very rarely made explicit by advertising campaigns that market nutraceutical agents. Rather, they tend to promulgate the most favorable bits of information while disregarding salient limitations of available data. Despite an interest in enhancing cognitive performance and avoiding dementia, many aging individuals may not ardently pursue physical or intellectual activities because of diminished motivation or related issues, but rather opt for the hypothetical and often hyped benefits of pills, medications, or supplements that are being promoted. Such individuals are at risk for becoming targets of very strong marketing campaigns from the nutraceutical industry. The worldwide nutraceutical industry is flourishing. Currently, it is estimated that the nutraceutical industry is a $120 billion a year business (evenly distributed between supplements, foods, and beverages) [209]. It is very likely that this industry will continue to grow in the future. It is currently legal to market products as “brain boosters” or “memory enhancers” without proof of efficacy, if no claims are made about being effective in the cure or treatment of illness [210]. Although a number of agents seem promising, for many of them (e.g., Ginkgo biloba, resveratrol) the current claims are not substantiated by research in humans. Clearly, there is a need for many more well-designed studies to adequately address the outstanding questions of this field.
There are numerous challenges to such a research endeavor. Who will fund these studies, especially if the nutraceutical industry can market its products without being required to substantiate claims through rigorous scientific investigation? Does a study’s failure to demonstrate efficacy “prove” that the agent is not beneficial? To what extent might the negative outcome of a study have been different if the investigators had used an alternative dose of the agent for a different duration of time in a different population of subjects? Did the study have adequate statistical power? Even if the results are statistically significant, on what grounds do we determine whether they are clinically relevant? Of course, these issues are the same ones faced by any clinical trial attempting to investigate the efficacy of a medication. Despite the fact that the risks of many proposed cognitive enhancers are claimed to be low and are directed at healthy individuals, not patients, serious consideration should be given to holding these agents to the same high standards of proof as other kinds of drugs in the field of medicine. In conclusion, given the persistent yearning of individuals not only to live longer, but also to age successfully, health care professionals need to be in a position to understand the pertinent issues, provide thoughtful recommendations, and help to educate and protect the public.
Acknowledgments
This research was funded in part by the National Institute on Aging [R01 AGO17935-07] and by generous support from D. Wimberly and S. Muss. The author would like to thank Dr. Barry Fogel and Dr. Martin Samuels for their very thoughtful comments, and Katie Gartner for her excellent administrative assistance.
Footnotes
The author’s disclosure is available online (http://www.j-alz.com/disclosures/view.php?id=166).
References
- 1.Department of Economic and Social Affairs PD. World Population Ageing. United Nations Publications; New York: 2007. [Google Scholar]
- 2.Commissaris CJ, Ponds RW, Jolles J. Subjective forgetfulness in a normal Dutch population: possibilities for health education and other interventions. Patient Educ Couns. 1998;34:25–32. doi: 10.1016/s0738-3991(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 3.Ponds RW, Commissaris KJ, Jolles J. Prevalence and covariates of subjective forgetfulness in a normal population in The Netherlands. Int J Aging Hum Dev. 1997;45:207–221. doi: 10.2190/MVQ1-WB58-875H-Y4X0. [DOI] [PubMed] [Google Scholar]
- 4.Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: a community study. J Geriatr Psychiatry Neurol. 1993;6:105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. [Accessed on August 31, 2009];Alzheimer’s disease facts and figures. 2007 http://www.alz.org/national/documents/Report_2007FactsAndFigures.pdf. Last updated 2007.
- 6.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 7.Hy LX, Keller DM. Prevalence of AD among whites: a summary by levels of severity. Neurology. 2000;55:198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 9.Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67:1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- 10.Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004;2004:e26. doi: 10.1126/sageke.2004.23.pe26. [DOI] [PubMed] [Google Scholar]
- 11.Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 13.Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Hipkiss AR. Accumulation of altered proteins and ageing: causes and effects. Exp Gerontol. 2006;41:464–473. doi: 10.1016/j.exger.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 16.Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 17.Terman A, Gustafsson B, Brunk UT. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol Aspects Med. 2006;27:471–482. doi: 10.1016/j.mam.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Tosato M, Zamboni V, Ferrini A, Cesari M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging. 2007;2:401–412. [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 20.Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: not due to sensory acuity reductions operating during cognitive assessment. Psychol Aging. 2001;16:196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- 21.Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- 22.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 23.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 24.Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- 26.Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, Hitzemann R, Smith G, Fields SD, Gur R. Dopamine transporters decrease with age. J Nucl Med. 1996;37:554–559. [PubMed] [Google Scholar]
- 28.Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding Y-S, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 29.Perls T. Dementia-free centenarians. Exp Gerontol. 2004;39:1587–1593. doi: 10.1016/j.exger.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 31.McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- 32.Mahley RW, Huang Y, Weisgraber KH. Detrimental effects of apolipoprotein E4: potential therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:537–540. doi: 10.2174/156720507783018334. [DOI] [PubMed] [Google Scholar]
- 33.Espeseth T, Greenwood PM, Reinvang I, Fjell AM, Walhovd KB, Westlye LT, Wehling E, Lundervold A, Rootwelt H, Parasuraman R. Interactive effects of APOE and CHRNA4 on attention and white matter volume in healthy middle-aged and older adults. Cogn Affect Behav Neurosci. 2006;6:31–43. doi: 10.3758/cabn.6.1.31. [DOI] [PubMed] [Google Scholar]
- 34.Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- 35.Fraser GE, Shavlik DJ. Ten years of life: Is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- 36.Stern Y. Cognitive Reserve Theory and Application. Psychology Press; New York: 2006. [Google Scholar]
- 37.Bertoni-Freddari C, Fattoretti P, Casoli T, Di Stefano G, Giorgetti B, Balietti M. Brain aging: The zinc connection. Exp Gerontol. 2008;43:389–393. doi: 10.1016/j.exger.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- 39.Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- 40.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 41.Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer’s disease. Expert Rev Neurother. 2008;8:743–750. doi: 10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- 42.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev. 2007;11:99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14:95–103. doi: 10.1097/01.JGP.0000196627.12010.d1. [DOI] [PubMed] [Google Scholar]
- 45.Tsao J, Shah R, Leurgens S, Wilson R, Janos A, Wei P, Bennett D, Heilman K. Impaired cognition in normal individuals using medications with anticholinergic activity occurs following several years. 2008 AAN Meeting Program A. 2008:418. [Google Scholar]
- 46.Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Kawachi I, Berkman LF, Grodstein F. Education, other socioeconomic indicators, and cognitive function. Am J Epidemiol. 2003;157:712–720. doi: 10.1093/aje/kwg042. [DOI] [PubMed] [Google Scholar]
- 48.Scarmeas N, Zarahn E, Anderson KE, Hilton J, Flynn J, Van Heertum RL, Sackeim HA, Stern Y. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects. NeuroImage. 2003;19:1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychol Med. 1997;27:1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- 50.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 51.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 52.Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, Mayeux R. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer’s disease pathology. Neurology. 1995;45:55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- 53.Potter GG, Helms MJ, Plassman BL. Associations of job demands and intelligence with cognitive performance among men in late life. Neurology. 2008;70:1803–1808. doi: 10.1212/01.wnl.0000295506.58497.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngandu T, von Strauss E, Helkala EL, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Education and dementia: what lies behind the association? Neurology. 2007;69:1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- 55.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 56.Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 57.Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain. 2008;131:543–558. doi: 10.1093/brain/awm300. [DOI] [PubMed] [Google Scholar]
- 58.Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63:112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 59.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Marder KS, Bell KL, Sackeim HA, Van Heertum RL, Moeller JR, Stern Y. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, Bastian LA, Mehta KM, Breitner JC. Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology. 2001;57:2210–2216. doi: 10.1212/wnl.57.12.2210. [DOI] [PubMed] [Google Scholar]
- 62.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 63.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 64.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 65.Kang JH, Weuve J, Grodstein F. Postmenopausal hormone therapy and risk of cognitive decline in community-dwelling aging women. Neurology. 2004;63:101–107. doi: 10.1212/01.wnl.0000132522.13574.67. [DOI] [PubMed] [Google Scholar]
- 66.Scharf JM, Daffner KR. NSAIDs in the prevention of dementia: a Cache-22? Neurology. 2007;69:235–236. doi: 10.1212/01.wnl.0000271092.02245.6d. [DOI] [PubMed] [Google Scholar]
- 67.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 68.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 69.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 70.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 71.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 72.Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 73.Scarmeas N, Luchsinger J, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer’s disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, Harris TB. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 76.Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 77.Abbott RD, Rodriguez BL, Burchfiel CM, Curb JD. Physical activity in older middle-aged men and reduced risk of stroke: the Honolulu Heart Program. Am J Epidemiol. 1994;139:881–893. doi: 10.1093/oxfordjournals.aje.a117094. [DOI] [PubMed] [Google Scholar]
- 78.Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. Am J Geriatr Pharmacother. 2008;6:100–118. doi: 10.1016/j.amjopharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Scinto LFM, Holcomb PJ. Age-related differences in novelty and target processing among cognitively high performing adults. Neurobiol Aging. 2005;26:1123–1295. doi: 10.1016/j.neurobiolaging.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Increased Responsiveness to Novelty is Associated with Successful Cognitive Aging. J Cogn Neurosci. 2006;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- 81.Daffner KR, Chong H, Riis J, Rentz DM, Wolk DA, Budson AE, Holcomb PJ. Cognitive status impacts age-related changes in attention to novel and target events in normal adults. Neuropsychology. 2007;21:291–300. doi: 10.1037/0894-4105.21.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR. Compensatory neural activity distinguishes different patterns of normal cognitive aging. NeuroImage. 2008;39:441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swan GE, Carmelli D. Curiosity and mortality in aging adults: a 5-year follow-up of the Western Collaborative Group Study. Psychol Aging. 1996;11:449–453. doi: 10.1037//0882-7974.11.3.449. [DOI] [PubMed] [Google Scholar]
- 84.Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes de Leon CF, Morris MC, Schneider JA, Evans DA. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 85.Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 86.Verghese J, Levalley A, Derby C, Kuslansky G, Katz M, Hall C, Buschke H, Lipton RB. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 88.Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH, Petot GJ, Debanne SM. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proc Natl Acad Sci U S A. 2001;98:3440–3445. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 91.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 92.Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. Am J Public Health. 2008;98:1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, Donlon TA, Willcox DC, Curb JD. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 94.Barnes DE, Cauley JA, Lui LY, Fink HA, McCulloch C, Stone KL, Yaffe K. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007;55:259–264. doi: 10.1111/j.1532-5415.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 95.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 96.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 97.Haag MD, Hofman A, Koudstaal PJ, Breteler MM, Stricker BH. Duration of antihypertensive drug use and risk of dementia: A prospective cohort study. Neurology. 2009;72:1727–1734. doi: 10.1212/01.wnl.0000345062.86148.3f. [DOI] [PubMed] [Google Scholar]
- 98.Dik MG, Jonker C, Comijs HC, Deeg DJ, Kok A, Yaffe K, Penninx BW. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30:2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- 99.Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 100.Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res. 2007;4:123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- 101.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 102.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 103.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 104.Dufouil C, Richard F, Fievet N, Dartigues JF, Ritchie K, Tzourio C, Amouyel P, Alperovitch A. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–1538. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 105.Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 106.Mainous AG, Eschenbach SL, Wells BJ, Everett CJ, Gill JM. Cholesterol, transferrin saturation, and the development of dementia and Alzheimer’s disease: results from an 18-year population-based cohort. Fam Med. 2005;37:36–42. [PubMed] [Google Scholar]
- 107.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 108.Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, D’Agostino RB, Wolf PA. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 109.Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–350. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 111.Sparks DL, Kryscio RJ, Sabbagh MN, Connor DJ, Sparks LM, Liebsack C. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–421. doi: 10.2174/156720508785132316. [DOI] [PubMed] [Google Scholar]
- 112.Arvanitakis Z, Schneider JA, Wilson RS, Bienias JL, Kelly JF, Evans DA, Bennett DA. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 113.Li G, Higdon R, Kukull WA, Peskind E, Van Valen MK, Tsuang D, van Belle G, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 114.Zandi PP, Sparks DL, Khachaturian AS, Tschanz J, Norton M, Steinberg M, Welsh-Bohmer KA, Breitner JC. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 115.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 116.Balk E, Chung M, Raman G, Tatsioni A, Chew P, Ip S, DeVine D, Lau J. B vitamins and berries and age-related neurodegenerative disorders. Evid Rep Technol Assess (Full Rep ) 2006:1–161. [PMC free article] [PubMed] [Google Scholar]
- 117.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roman B, Carta L, Martinez-Gonzalez MA, Serra-Majem L. Effectiveness of the Mediterranean diet in the elderly. Clin Interv Aging. 2008;3:97–109. doi: 10.2147/cia.s1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Dyk K, Sano M. The impact of nutrition on cognition in the elderly. Neurochem Res. 2007;32:893–904. doi: 10.1007/s11064-006-9241-5. [DOI] [PubMed] [Google Scholar]
- 120.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Knopman DS. Mediterranean diet and late-life cognitive impairment. JAMA. 2009;302:686–687. doi: 10.1001/jama.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, Breteler MM. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 124.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- 125.Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Baldassarre G, Scapicchio P, Scafato E, Amodio M, Capurso A, Panza F. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- 126.Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–192. [PubMed] [Google Scholar]
- 127.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 128.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 129.Broustet JP. Wine and health. Heart. 1999;81:459–460. doi: 10.1136/hrt.81.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Letenneur L. Risk of dementia and alcohol and wine consumption: a review of recent results. Biol Res. 2004;37:189–193. doi: 10.4067/s0716-97602004000200003. [DOI] [PubMed] [Google Scholar]
- 131.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev. 2008;66:591–596. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 132.Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 133.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 134.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 135.Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 136.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 137.Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 138.Albert MS. Changing the trajectory of cognitive decline? N Engl J Med. 2007;357:502–503. doi: 10.1056/NEJMcibr073273. [DOI] [PubMed] [Google Scholar]
- 139.Connor JR, Melone JH, Yuen AR, Diamond MC. Dendritic length in aged rats’ occipital cortex: an environmentally induced response. Exp Neurol. 1981;73:827–830. doi: 10.1016/0014-4886(81)90216-8. [DOI] [PubMed] [Google Scholar]
- 140.Kempermann G, Kuhn HG, Gage FH. More hippocampus neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 141.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 142.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 143.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 144.Miida T, Takahashi A, Ikeuchi T. Prevention of stroke and dementia by statin therapy: experimental and clinical evidence of their pleiotropic effects. Pharmacol Ther. 2007;113:378–393. doi: 10.1016/j.pharmthera.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 145.Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208–213. doi: 10.1177/1073858406297121. [DOI] [PubMed] [Google Scholar]
- 146.Schmeer C, Kretz A, Isenmann S. Statin-mediated protective effects in the central nervous system: general mechanisms and putative role of stress proteins. Restor Neurol Neurosci. 2006;24:79–95. [PubMed] [Google Scholar]
- 147.Menge T, Hartung HP, Stuve O. Statins--a cure-all for the brain? Nat Rev Neurosci. 2005;6:325–331. doi: 10.1038/nrn1652. [DOI] [PubMed] [Google Scholar]
- 148.Florent-Bechard S, Malaplate-Armand C, Koziel V, Kriem B, Olivier JL, Pillot T, Oster T. Towards a nutritional approach for prevention of Alzheimer’s disease: biochemical and cellular aspects. J Neurol Sci. 2007;262:27–36. doi: 10.1016/j.jns.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 149.Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs. 2008;9:735–743. [PubMed] [Google Scholar]
- 151.Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008;373:341–344. doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 152.Anekonda TS. Resveratrol--a boon for treating Alzheimer’s disease? Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 153.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 154.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 155.Ponto LL, Schultz SK. Ginkgo biloba extract: review of CNS effects. Ann Clin Psychiatry. 2003;15:109–119. doi: 10.1023/a:1024688326023. [DOI] [PubMed] [Google Scholar]
- 156.Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1:469–480. doi: 10.1089/ars.1999.1.4-469. [DOI] [PubMed] [Google Scholar]
- 157.Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–678. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 158.Luo Y, Smith JV, Paramasivam V, Burdick A, Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H, Butko P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci U S A. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, Huettel SA. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- 160.Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- 161.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 162.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 163.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 164.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM. Selective increase of cortical thickness in high-performing elderly-structural indices of optimal cognitive aging. NeuroImage. 2005;29:984–994. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 166.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 167.Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiol Aging. 2006;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 168.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 169.Ghisletta P, de Ribaupierre A. A dynamic investigation of cognitive dedifferentiation with control for retest: evidence from the Swiss Interdisciplinary Longitudinal Study on the Oldest Old. Psychol Aging. 2005;20:671–682. doi: 10.1037/0882-7974.20.4.671. [DOI] [PubMed] [Google Scholar]
- 170.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 171.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 172.Rosen AC, Prull MW, O’Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- 173.Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 174.Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- 175.Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- 176.Polich J, Lardon MT. P300 and long-term physical exercise. Electroencephalogr Clin Neurophysiol. 1997;103:493–498. doi: 10.1016/s0013-4694(97)96033-8. [DOI] [PubMed] [Google Scholar]
- 177.McDowell K, Kerick SE, Santa Maria DL, Hatfield BD. Aging, physical activity, and cognitive processing: an examination of P300. Neurobiol Aging. 2003;24:597–606. doi: 10.1016/s0197-4580(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 178.Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Age-related differences in attention to novelty among cognitively high performing adults. Biol Psychol. 2006;72:67–77. doi: 10.1016/j.biopsycho.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 179.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 180.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 181.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 185.Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM. Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J Am Geriatr Soc. 2009;57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Poon IO. Effects of antihypertensive drug treatment on the risk of dementia and cognitive impairment. Pharmacotherapy. 2008;28:366–375. doi: 10.1592/phco.28.3.366. [DOI] [PubMed] [Google Scholar]
- 188.McGuinness B, Todd S, Passmore P, Bullock R. The effects of blood pressure lowering on development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease. Cochrane Database Syst Rev. 2006:CD004034. doi: 10.1002/14651858.CD004034.pub2. [DOI] [PubMed] [Google Scholar]
- 189.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 190.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 191.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 192.Probstfield JL, Applegate WB, Borhani NO, Curb JD, Cutler JA, Davis BR, Furberg CD, Hawkins CM, Lakatos E, Page LB, et al. The Systolic Hypertension in the Elderly Program (SHEP): an intervention trial on isolated systolic hypertension. SHEP Cooperative Research Group. Clin Exp Hypertens A. 1989;11:973–989. doi: 10.3109/10641968909035386. [DOI] [PubMed] [Google Scholar]
- 193.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 194.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 195.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 196.Heart Protection Study Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 197.Parale GP, Baheti NN, Kulkarni PM, Panchal NV. Effects of atorvastatin on higher functions. Eur J Clin Pharmacol. 2006;62:259–265. doi: 10.1007/s00228-005-0073-z. [DOI] [PubMed] [Google Scholar]
- 198.Yaffe K. Antioxidants and prevention of cognitive decline: does duration of use matter? Arch Intern Med. 2007;167:2167–2168. doi: 10.1001/archinte.167.20.2167. [DOI] [PubMed] [Google Scholar]
- 199.Lim WS, Gammack JK, Van Niekerk J, Dangour AD. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev. 2006:CD005379. doi: 10.1002/14651858.CD005379.pub2. [DOI] [PubMed] [Google Scholar]
- 200.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71:430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 201.Global Nutrition Sciences. [Accessed on August 13, 2009];Red wine’s amazing secret. 2009 revealed! http://www.gns-online.com/resv/rx10im/, Last updated 2009.
- 202.Karr V. The fountain of youth has been discovered through Resveratrol Anti-aging! [Accessed on August 13, 2009];2009 http://oolongteaforweightloss.com/index.php?option=com_content&view=article&id=107:the-fountain-of-youth-has-been-discovered-through-resveratrol-anti-aging&catid=47:resveratrol-study&Itemid=152, Last updated 2009.
- 203.Live Healthy Live Longer. Introducing a remarkable product that is so powerful, it could add 25 years to your life and help you feel 25 years younger. [Accessed on August 13, 2009];2009 http://www.livehealthylivelonger.com/VIVIX_Home.hmtl Last updated 2009.
- 204.DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD. Ginkgo biloba for Prevention of Dementia: A Randomized Controlled Trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Canter PH, Ernst E. Ginkgo biloba is not a smart drug: an updated systematic review of randomised clinical trials testing the nootropic effects of G. biloba extracts in healthy people. Hum Psychopharmacol. 2007;22:265–278. doi: 10.1002/hup.843. [DOI] [PubMed] [Google Scholar]
- 206.Solomon PR, Adams F, Silver A, Zimmer J, DeVeaux R. Ginkgo for memory enhancement: a randomized controlled trial. JAMA. 2002;288:835–840. doi: 10.1001/jama.288.7.835. [DOI] [PubMed] [Google Scholar]
- 207.Carlson MC, Saczynski JS, Rebok GW, Seeman T, Glass TA, McGill S, Tielsch J, Frick KD, Hill J, Fried LP. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist. 2008;48:793–801. doi: 10.1093/geront/48.6.793. [DOI] [PubMed] [Google Scholar]
- 208.Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 209.BCC Research. [Accessed on August 13, 2009];Nutraceuticals: Global Markets and Processing. 2008 http:/www.bccresearch.com/report/FOD013C.html Last updated 2008.
- 210.McDaniel MA, Maier SF, Einstein GO. “Brain-specific” nutrients: a memory cure? Nutrition. 2003;19:957–975. doi: 10.1016/s0899-9007(03)00024-8. [DOI] [PubMed] [Google Scholar]


