Abstract
Effective response inhibition is a key component of recovery from addiction. Some research suggests that response inhibition can be enhanced through reward contingencies. We examined the effect of monetary incentive on response inhibition among adolescents with and without substance use disorder (SUD) using a fast event-related fMRI antisaccade reward task. The fMRI task permits investigation of how reward (monetary incentive) might modulate inhibitory control during three task phases: cue presentation (reward or neutral trial), response preparation, and response execution. Adolescents with lifetime SUD (n=12; 100% marijuana use disorder) were gender and age-matched to healthy controls (n=12). Monetary incentive facilitated inhibitory control for SUD adolescents; for healthy controls, the difference in error rate for neutral and reward trials was not significant. There were no significant differences in behavioral performance between groups across reward and neutral trials, however, group differences in regional brain activation were identified. During the response preparation phase of reward trials, SUD adolescents, compared to controls, showed increased activation of prefrontal and oculomotor control (e.g., frontal eye field) areas, brain regions that have been associated with effective response inhibition. Results indicate differences in brain activation between SUD and control youth when preparing to inhibit a prepotent response in the context of reward, and support a possible role for incentives in enhancing response inhibition among youth with SUD.
Keywords: adolescent, substance use disorder, fMRI, response inhibition, incentive, antisaccade
1. Introduction
During adolescence, developmental changes in reward-related processing and limitations in the cognitive control of behavior are thought to contribute to a propensity for risk-taking behavior, such as substance use (Spear, 2000; Ernst et al., 2009). A reward-sensitive temperament, in combination with behavioral disinhibition, has been associated with risk for progression to substance use disorder (SUD) in adolescence (review: Dawe et al., 2004). These findings suggest that adolescent substance users, compared to healthy controls, might demonstrate greater sensitivity to reward and reduced response inhibition. Increased understanding of the impact of incentives on response inhibition has potential implications for improving substance use outcomes among youth in recovery. This study examined the effect of reward, in the form of monetary incentive, on inhibitory control, comparing adolescents with and without SUD, on a reward-mediated antisaccade (AS) fMRI task (Geier et al., 2010).
Performance of a typical AS requires halting a prepotent eye movement toward a salient stimulus in favor of a voluntary movement to the opposite spatial location (Hallett, 1978). Saccadic eye movements in general, and particularly antisaccades, have proven to be a sensitive marker of psychopathology related to the cognitive control of behavior and frontal lobe integrity (review: Munoz & Everling, 2004). Other advantages of using AS to measure cognitive control over behavior include the ability to isolate activity related to response preparation, which is critical to effective response inhibition, and the extensive characterization of the neural circuitry underlying AS (Everling et al., 1998; 2000; Luna et al., 2008). Brain systems subserving AS performance include the frontal eye field (FEF), supplementary eye field (SEF), dorsolateral prefrontal cortex (DLPFC), posterior parietal cortex, anterior cingulate cortex, basal ganglia, thalamus, superior colliculus, brainstem reticular formation and cerebellum (reviews: Munoz & Everling, 2004; Luna et al., 2004). Developmentally, adult-like levels of response inhibition, based on AS performance, begin to stabilize around mid- to late adolescence (Luna et al., 2004).
Studies of youth at high risk for substance involvement suggest poorer AS behavioral performance and less activation of brain regions supporting AS, compared to youth at low risk. A behavioral AS study found that children of fathers with an alcohol use disorder had a higher rate of response inhibition errors compared to children of fathers with no alcohol use disorder (Habeych et al., 2006). Likewise, an fMRI study of high risk youth (ages 12-19) reported that neurobehavioral disinhibition score (e.g., greater impulsivity, conduct problems) was negatively correlated with total percentage of frontal lobe activation, suggesting that youth with greater neurobehavioral disinhibition may have deficits in the neural circuitry supporting AS, particularly in frontal brain regions (McNamee et al., 2008). Although these findings suggest deficits in AS performance that may be associated with lower activation of brain regions supporting AS among high risk youth, studies to date have not examined AS behavioral and neuroimaging data together, and have not contrasted adolescent substance users with healthy controls.
The use of incentives can improve performance on tasks that require strong cognitive control over behavior, such as AS (e.g., Jazbec et al., 2005, 2006; Hardin et al., 2007; Geier et al., 2010). In youth with depression or anxiety, use of monetary incentive improved AS performance by reducing inhibitory errors (Hardin et al., 2007). However, monetary incentive did not affect AS performance among youth with bipolar disorder, possibly due to impaired reward-related processing and limited room for performance improvement due to disorder-related impairment (Mueller et al., 2010). Taken together, extant research suggests that sensitivity to reward can modulate cognitive control processes, and that reward sensitivity may differ depending on type of psychopathology. Little is known regarding sensitivity to reward in adolescents with SUD (Hardin & Ernst, 2009). Given that adolescent substance users may have a reward-seeking temperament (Dawe et al., 2004), we predicted that monetary incentive would improve AS performance among adolescents with SUD, which would provide support for the modulation of effortful cognitive control by reward in this subgroup.
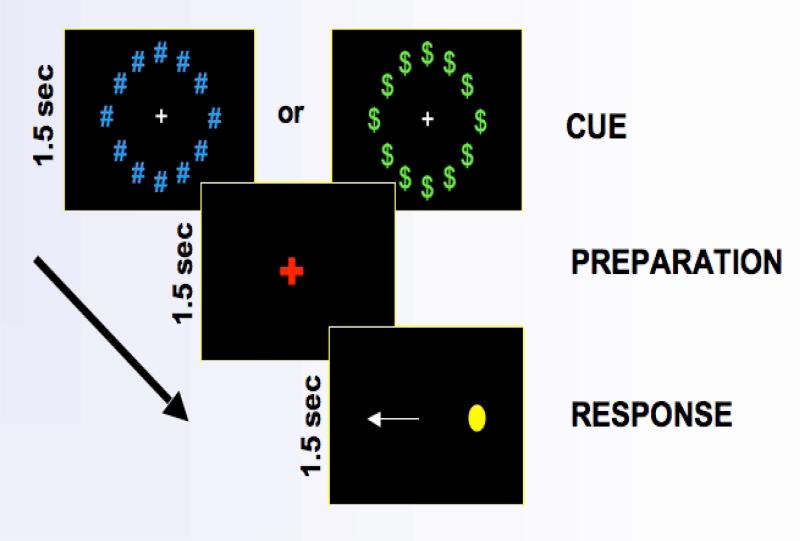
To examine the effect of reward on AS behavioral performance and its supporting neural circuitry, we used a fast event-related AS task that included three epochs (incentive cue presentation, response preparation, response execution), and reward and non-reward trials (Figure 1; Geier et al., 2010). The use of three epochs in each trial facilitated evaluation of temporally distinct phases of cue exposure (i.e., reward versus non-reward “cue”), anticipation of reward and response preparation, and AS response execution, as well as the distinct patterns of regional brain activation that may be associated with each epoch of the trial (review: Geier & Luna, 2009). We hypothesized that incentives would improve AS performance through activation of the ventral striatum (VS), which may enhance activity in premotor and parietal oculomotor control regions, as well as prefrontal cortex, brain regions that support correct AS performance (Geier et al., 2010). In support of this model, greater activity in the frontal cortex was observed during response preparation for reward, compared to non-reward trials, in healthy adolescents (Geier et al., 2010). These findings suggest modulation of prefrontal and oculomotor control regions supporting correct inhibitory response by monetary incentive. Thus, the fMRI AS reward task provides a method to compare adolescent substance users and healthy controls on processes relevant to understanding adolescent substance use: reward sensitivity, response inhibition, and the effect of incentive on cognitive control of behavior.
Figure 1.
fMRI antisaccade in the context of reward task: “incentive cue”, “preparation” and “response” epochs
Given that reward enhances performance that leads to reward receipt, we predicted that adolescents with substance use disorder (SUD adolescents) and healthy controls would generate fewer behavioral AS errors during the reward, compared to neutral condition, and that during the reward condition, there would be increased activity in brain regions associated with reward processing (e.g., VS, orbito-frontal cortex (OFC)). We also predicted, based on the literature on risk for adolescent substance use, that SUD adolescents would show greater sensitivity to reward compared to controls, that is, greater effect of reward on behavioral AS performance (i.e., decreased errors during reward compared to neutral trials), and greater effect of reward on brain regions subserving correct AS performance (e.g., greater DLPFC activation in SUD adolescents compared to controls during response preparation).
2. Methods
2.1 Participants
Adolescent participants (ages 15-18) in this study were drawn from a longitudinal, naturalistic study of adolescent SUDs, which involved recruitment of youth from substance abuse treatment (SUD adolescents) and a representative sample of similar aged youth from the community (Chung & Maisto, 2009). Community control participants were identified using random digit dialing by trained interviewers at the University Center for Social and Urban Research. Table 1 summarizes the sample characteristics by study group. SUD adolescents (n=12, 50% female, mean age = 17.0 [SD=0.9], 83% Caucasian) had a lifetime DSM-IV SUD (75% alcohol use disorder, 100% cannabis use disorder; 75% other drug use disorder), and were sex and age matched to a community control participant (n=12, 50% female, mean age = 16.9 [SD=0.9], 83% Caucasian). Community controls had no lifetime DSM-IV SUD. Participants represented a broad range (1-5 scale, 5=high) of socio-economic status (Hollingshead, 1975; SUD sample mean = 2.7 [SD=1.3], control sample mean = 2.2 [SD=0.9]; p>.05). All were right-handed. Youth with a history of significant brain injury or other MRI contraindication (e.g., metal in the body) were excluded from the neuroimaging study. Full scale IQ, determined by the Wechsler Abbreviated Scale of Intelligence (Psychological Corporation, 1999), did not significantly differ for SUD youth (mean=103.7, SD=10.6) and controls (mean=110.1, SD=11.4).
Table 1.
Sample descriptive statistics for substance use disorder (SUD) and control groups
| SUD group (n=12) | Control group (n=12) | |
|---|---|---|
| Gender (% female) | 50.0 | 50.0 |
| Age: Mean (SD) | 17.0 (0.9) | 16.9 (0.9) |
| Ethnicity (% Caucasian) | 83.0 | 83.0 |
| Socio-economic status: Mean (SD) | 2.7 (1.3) | 2.2 (0.9) |
| Frequency of substance use (past 6 months): Mean (SD) | ||
| Alcohol use | 3.1 (1.9) | 1.4 (1.4) |
| Cannabis use | 5.5 (2.7) | 0.2 (0.6) |
| Tobacco use | 6.1 (3.2) | 1.2 (2.5) |
| Lifetime DSM-IV alcohol use disorder (%) | 75.0 | 0.0 |
| Alcohol Abuse | 66.7 | 0.0 |
| Alcohol Dependence | 8.3 | 0.0 |
| Mean (SD) age of diagnosis onset | 15.4 (1.2) | -- |
| Lifetime DSM-IV cannabis use disorder (%) | 100.0 | 0.0 |
| Cannabis Abuse | 66.7 | 0.0 |
| Cannabis Dependence | 33.3 | 0.0 |
| Mean (SD) age of diagnosis onset | 14.6 (1.6) | -- |
| Lifetime DSM-IV nicotine dependence diagnosis (%) | 41.7 | 0.0 |
| Mean (SD) age of diagnosis onset | 15.4 (0.9) | -- |
| Lifetime DSM-IV “other drug” diagnosis (%) | 75.0 | 0.0 |
| Other drug abuse | 50.0 | 0.0 |
| Other drug dependence | 25.0 | 0.0 |
| Mean (SD) age of diagnosis onset | 16.2 (0.8) | -- |
| Total DSM-IV symptom count, past 6 months: Mean (SD) | ||
| Alcohol symptoms | 1.5 (2.0) | 0.2 (0.4) |
| Cannabis symptoms | 3.9 (2.4) | 0.0 (0.0) |
| Nicotine symptoms | 1.5 (1.6) | 0.0 (0.0) |
| DSM-IV psychopathology in past 6 months | ||
| Major depression (%) | 17.0 | 0.0 |
| Conduct disorder (%) | 33.0 | 0.0 |
| Attention deficit hyperactivity disorder (%) | 17.0 | 0.0 |
| Oppositional defiant disorder (%) | 8.0 | 0.0 |
| Full scale intelligence quotient score: Mean (SD) | 103.7 (10.6) | 110.1 (11.4) |
Notes: SD= standard deviation. Frequency of substance use: 0=never tried, 1=no use in past 6 months, 2=less than once per month, 3=once per month, 4=2-3 times per month, 5=once per week, 6=2-3 times per week, 7=4-6 times per week, 8=daily. “Other drug” refers to substances other than alcohol, cannabis or nicotine. For other drug abuse (none had other drug dependence): n=3 opiate abuse, n=1 cocaine abuse, n=1 opiate and cocaine abuse, n=1 cocaine, stimulant, and hallucinogen abuse. For other drug dependence: n=2 opiate dependence (1 also met criteria for cocaine abuse), n=1 cocaine dependence (also had opiate, sedative, and stimulant abuse).
In the 6 months prior to the scan, among SUD adolescents, average frequency of alcohol use was once per month, and average frequency of marijuana use was once per week. In the 30 days prior to the scan, 25% (n=3) of SUD adolescents reported alcohol use (range of 4-10 days prior to scan), and 42% (n=5) reported marijuana use (range of 1 day [>24 hrs] - 7 days prior to scan). In the control sample, average frequency of alcohol and marijuana use was less than once per month in the 6 months prior to the scan. In the 30 days prior to the scan, 33% (n=4) of controls reported alcohol use (range of 1 day [>24 hrs] - 19 days prior to scan), and none reported marijuana use. With regard to current (i.e., 6-months prior to scan) psychopathology, in the SUD sample, 17% (n=2) had major depression, 33% (n=4) had conduct disorder, 17% (n=2) met criteria for attention deficit hyperactivity disorder, and 8% (n=1) had oppositional defiant disorder. The control sample had no lifetime history of these disorders.
2.2 Procedures
Adolescents in the parent longitudinal project were invited to participate in a neuroimaging study of brain structure and functioning (Thatcher et al., 2010), and completed comprehensive substance use and psychiatric assessments at baseline and 1-year follow-up as part of the parent project. SUD adolescents were scanned at baseline, within one month of entry into intensive outpatient treatment for substance use (n=11), or at the 1-year follow-up assessment (n=1; this adolescent reported marijuana use in the 30 days prior to the scan, met criteria for a lifetime DSM-IV diagnosis of marijuana abuse, but did not have a current SUD at the time of the scan). Control adolescents were scanned within one month of completing the baseline assessment (n=3), or at 1-year follow-up (n=9). Youth were instructed to abstain from alcohol and illicit substance use for at least 24 hours prior to the scan; no adolescent included in the analyses reported substance use <24 hours prior to the scan. The study protocol was approved by the University's Institutional Review Board. Written informed consent (or assent from minor adolescents, and consent from the youth's parent) was obtained prior to conducting any study procedures. Youth were compensated for participation in the imaging study.
2.2.1 Measures of substance use and psychopathology
Measures of substance use and psychopathology were obtained at baseline and at 1-year follow-up by highly trained interviewers. For those who completed the scan at baseline, “lifetime” and “current” (past 6-months) status was based on data from the baseline assessment; for those who completed the scan at 1-year follow-up, data obtained at the 1-year follow-up assessment was used. Participants reported on frequency of alcohol, marijuana, tobacco, and other substance use in the past 6-months on a 9-point scale (0=never tried to 8=daily use). The Structured Clinical Interview for DSM-IV SUDs (SCID; First et al., 1997), adapted for adolescents (Martin et al., 1995), was used to assess the presence and age at onset of lifetime SUD diagnoses. The adapted SCID has good inter-rater reliability for ratings of symptoms, diagnosis, and age of onset, and has support for concurrent validity (e.g., Martin et al., 2000). Lifetime and current other DSM-IV Axis I psychopathology (e.g., conduct disorder, major depression) were assessed using the adolescent version of the Schedule for Affective Disorders and Schizophrenia (K-SADS: Kaufman et al., 1997). The K-SADS has good inter-rater reliability (Clark et al., 1997).
2.2.2 fMRI antisaccade (AS) task
A fast-event related design was used to assess reward contingency effects on activity unique to each epoch (i.e., cue, preparation, response execution) of the AS task (Geier et al., 2010). At the beginning of each AS trial, participants first viewed (1.5s) either (1) a ring of green dollar bill signs ($) around a central white fixation cross as a cue for reward trials, or (2) an equivalently sized, isoluminant ring of blue pound signs (#) around the fixation cross for neutral trials (Figure 1). The ring then disappeared, and a red fixation cross was displayed for 1.5s signifying that an AS is to be performed. Finally, a peripheral target (yellow dot) appeared (75 msec) at an unpredictable horizontal location (± 3, 6, and 98 degrees visual angle). Adolescents were instructed to look at the mirror location (i.e., perform an antisaccade) during this time (1475 msec). Because the AS task is a compound trial with an invariant sequence of components (i.e. motor response always follows response preparatory period), we included approximately 30% partial or “catch” trials, randomly inserted, in addition to jittered inter-trial intervals (Ollinger et al., 2001a, b) in order to deconvolve trial components. Two catch trial variants were presented and consisted of the trial terminating after either (1) the incentive cue (circles of “$” or “#”) (i.e. no response preparation or cue to perform an antisaccade) or (2) when the response preparation period ended (red fixation cross) (i.e. no peripheral target was presented). A jittered fixation period of 1.5, 3, or 4.5 seconds (randomly inserted), during which time subjects simply fixated on a central white cross presented on a black background, was included between all trials, compound and partial. Inclusion of partial trials and jittered inter-trial fixations allowed activity unique to each component of the task to be estimated independently (i.e., activity associated with response preparation can be estimated uniquely from reward image processing and motor processing).
The protocol included 14 complete reward trials, 6 partial reward trials (3 of each variant), 14 complete neutral trials, and 6 partial neutral trials (3 of each variant) in each run, presented in random order. Each run lasted 5 min 9 sec and was presented 4 times for a total of 56 complete reward trials and 56 complete neutral trials. Participants were told that they could win up to $10 for correct AS performance, with no monetary loss (i.e., no “punishment” for incorrect response), and were told to try to obtain the maximum amount. For the reward condition, the value of a correct response was intentionally ambiguous (to prevent participants from keeping a running total of earnings during the task), and no feedback was provided regarding whether a particular trial was successfully performed. At the end of the imaging session, all participants who made an effort to comply with the task received the full $10 “reward”. All participants in the sample complied with the task and received full compensation.
2.2.3 Eye tracking
Participants were first oriented to the AS task outside of the scanner to ensure that they were able to perform the task. In the scanner, eye movements were monitored using a long-range optics eye-tracking system (Model 504LRO, Applied Science Laboratories, Bedford, MA), which recorded eye position by pupil-corneal reflection obtained by a relay mirror mounted on the head coil with a resolution of 0.5° of visual angle. Simultaneous video monitoring provided data on task compliance. At the beginning of the session, a 9-point calibration procedure was performed. Stimuli were presented using E-prime (Psychology Software Tools, Inc., Pittsburgh, PA), projected onto a flat screen positioned behind the magnet. Subjects viewed the screen using a mirror mounted on a standard radiofrequency head coil. Eye movements were scored off-line using ILAB software (Gitelman, 2002) and in-house scoring programs written in MATLAB (Mathworks, Inc.) that calculated the direction, latency, and accuracy of responses.
Behavioral variables of interest included correct and incorrect AS latencies and errors in inhibitory response on rewarded and neutral trials. A correct response in the AS task was one in which the first eye movement during the response execution epoch with velocity greater than or equal to 30°/s (Gitelman, 2002) was made toward the mirror location of the peripheral cue and extended beyond a 2.5° visual angle central fixation zone. AS errors occurred when the first saccade during the response execution epoch was directed toward the peripheral target and exceeded the 2.5° visual angle central fixation zone. Prosaccade errors were consistently followed by movement to the appropriate location, indicating that instructions were understood, but the reflexive saccade was not effectively inhibited (cf. Velanova et al., 2008). Sensitivity to reward on this task was indicated primarily by reduced inhibitory errors, and secondarily by shorter latency to correct AS, during reward compared to non-reward (neutral) trials (Hardin et al., 2007; Geier et al., 2010).
2.2.4 Image Acquisition and Preprocessing
Imaging data were acquired using a Siemens 3T Allegra Scanner. A gradient-echo echo-planar imaging sequence sensitive to blood oxygenation level dependent (BOLD) contrast (T2*) was used. Acquisition parameters were TR=1.5s TE=25ms, flip angle=70°, 64 × 64 acquisition matrix with field of view 20 × 20 cm. Twenty-nine 4mm-thick axial slices with no gap were collected, aligned to the anterior and posterior commissure (AC-PC line), generating 3.125 × 3.125 × 4mm voxels, which covered the entire cortex and most of the cerebellum. A series of magnetization-prepared rapid gradient echo (MPRAGE) images were acquired. Tools from the Functional MRI of the Brain (FMRIB) software library (FSL) were used for data preprocessing (Smith et al., 2004). MPRAGE images were brain extracted (BET), then registered and transformed to standard space using FLIRT and FNIRT. Functional images were slice-time and head-motion corrected (cf. Geier et al., 2010). Allowable head motion was 1.5mm, given that this is the amplitude at which most artifacts occur. Functional images were transformed to standard space, then spatially smoothed with a 5-mm full-width at half maximum kernel and subjected to high-pass temporal filtering (sigma = 37.5 s) to remove low-frequency scanner drift. Signal intensity for each run was scaled to a mean of 100 and multiple runs were concatenated.
Analysis of Functional Neuroimages (AFNI; Cox, 1996) was used for individual case deconvolution and group-based analyses. The deconvolution (regression) model consisted of six orthogonal regressors (i.e., reward cue, neutral cue, reward preparation, neutral preparation, reward saccade response, and neutral saccade response), regressors for reward and neutral error trials (consisting of the entire trial), regressors modeling baseline, linear, and non-linear trends, as well as motion parameters that were included as ‚nuisance’ regressors. Analyses included only correct AS trials. Cubic spline basis functions were used to estimate a unique estimated impulse response function (i.e., hemodynamic response function [HRF]) for each regressor of interest (reward and neutral cue, preparation, and saccade response). We estimated the HRF duration for each epoch of the trial (18sec from stimulus onset; 13 TR), but did not make assumptions about its specific shape, as is the case when a canonical HRF shape is used. The overall baseline was calculated as the mean activation for each voxel across all fixation time points. Goodness of fit statistics were calculated from the deconvolution, including partial F-statistics for each task regressor (e.g., reward cue, response preparation, response execution) and t-scores comparing each of the 5 estimated beta weights with zero.
2.2.5 fMRI Group-level analyses
Whole-brain analyses were conducted using data only from correct AS trials. Voxel-wise linear mixed-effects modeling (3dLME program within AFNI) was run using subjects’ mean estimated impulse response (beta weights from deconvolution scaled to reflect percent signal change) maps with condition (reward and neutral), group (SUD versus control), and time as fixed factors and subjects as a random factor for each trial epoch: (1) cue/reward assessment, (2) response preparation, and (3) response execution. These whole brain analyses generated a series of statistical maps that were used to compare regional brain activation across incentive cue conditions and SUD/control groups, separately for each of the three trial epochs. The Incentive condition (reward versus neutral) by Group (SUD versus control) by Time (I × G × T) interaction provided information on how brain regions differed in terms of percent signal change across the 2 incentive conditions, 2 groups, and over time (13 TRs).
Correction for multiple comparisons was applied at the cluster level. Image-level cluster significance of p<.05 was obtained using a combination of individual voxel thresholding (p ≤.001) and a minimum cluster size (17 contiguous voxels). Estimation of whole brain overall significance levels was done using Monte Carlo simulations (AFNI AlphaSim). Regions of interest (ROIs) were defined as significant voxels that were included in non-overlapping spheres with a 9-mm radius centered on the maximum voxel in the corrected I × G × T image. We then used these functionally defined ROIs as masks to extract the estimated time courses for each subject, for both incentive conditions, and for each of the three trial epochs separately.
2.2.6 Time course analyses
The average (across subjects) time courses extracted from the I × G × T interaction maps for each epoch (i.e., cue, preparation, response execution) were analyzed using repeated measures analysis of variance (RM ANOVA). RM ANOVA tested for I × G × T interactions in the time course data, using group (SUD versus control) as the between subjects factor, and incentive condition (reward, neutral) and time (0-12 TR) as within subjects factors. Analyses of all time points within an epoch resulted in no significant I × G interactions for any of the ROIs. Given that a biphasic or temporally later peak (i.e., peak >6 seconds after onset of the epoch) was observed for some ROIs, we used a more conservative approach to test for I × G interactions, focusing on estimated responses at TRs 3-6. We restricted the I × G analyses to these early time points (i.e., 3-7.5 seconds after epoch onset) because this period includes the initial peak in a stereotyped hemodynamic response, which generally occurs between 4-6 seconds after stimulus presentation (cf. Geier et al., 2010). Greenhouse-Geisser sphericity corrected levels of significance are reported. Analyses of average time courses did not correct for multiple comparisons, given that this was a pilot study.
3. Results
3.1 Behavioral Results
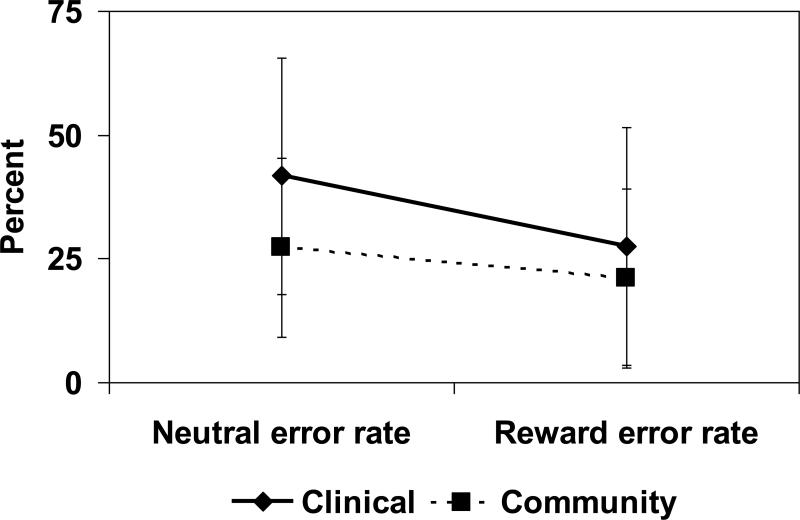
Descriptive statistics for behavioral AS performance across neutral and reward conditions are provided in Table 2. As predicted, RM ANOVA of errors in inhibitory response across incentive conditions indicated a significant main effect of incentive type ([F(1,22) = 15.81, p<.01), with decreased errors in the reward, compared to neutral condition. In the SUD group, reward trials resulted in fewer errors compared to neutral trials (F(1,11) = 12.25, p<.01). Controls, however, did not show a difference in error rate by incentive condition p=.07; see Figure 2). There were no between-group differences in error rates during reward or neutral trials (ps>.11). Contrary to prediction, the I × G interaction was not significant (F(1,22) = 2.44, p=.13).
Table 2.
Behavioral results for reward and neutral antisaccade trials
| SUD Group (n=12) Mean (SD) | Control Group (n=12) Mean (SD) | |
|---|---|---|
| Neutral: Error rate (%) | 41.69 (24.21) | 27.28 (18.32) |
| Reward: Error rate (%) | 27.40 (22.93) | 21.06 (19.94) |
| Neutral: Latency of AS errors (ms) | 379.18 (55.70) | 351.09 (70.71)1 |
| Reward: Latency of AS errors (ms) | 359.06 (67.71) | 344.94 (71.60)1 |
| Neutral: Latency of correct ASs (ms) | 439.58 (47.73) | 423.53 (42.00) |
| Reward: Latency of correct ASs (ms) | 432.39 (59.70) | 418.11 (57.23) |
| Overall correct response rate (%) | 66.52 (23.77) | 76.10 (17.60) |
Note:
n=11 for control group (1 subject had division by 0)
SUD=Substance Use Disorder, AS=antisaccade, ms=millisecond
Figure 2.
Antisaccade behavioral results
Consistent with the literature (e.g., Ford et al., 2005), latency to correct AS was longer compared to AS errors (F(1,21)=19.63, p<.001), with no significant group × latency to AS (i.e., correct vs. error trials) interaction (p=.87). In addition, there was no significant main effect of incentive type on latency to initiate a correct AS (F(1,22)=.94, p=.34), and the I × G interaction for latency to correct AS was not significant (F(1,22)=.02, p=.89). There were no between-group differences in latency to correct AS during reward or neutral trials (ps>.39). RM ANOVA of latencies for erroneous saccades (i.e., prosaccade errors) also indicated no significant main effect of incentive condition (F(1,21)=.49, p=.49), and no significant difference for the I × G interaction (F(1,21)=.14, p=.71). There were no between-group differences in latency for erroneous saccades during reward or neutral trials (ps>.30).
3.2 fMRI Results
When considering the whole trial, a network of brain regions, particularly in frontal and parietal areas, was activated to support correct AS performance. In addition, reward trials were associated with greater activation of regions associated with reward-related processing (e.g., anterior cingulate, OFC; cf. Goldstein et al., 2007), compared to neutral trials, in the total sample. However, no significant differences in activation of reward-related brain regions (e.g., ventral striatum, anterior cingulate, OFC) between SUD and control adolescents across reward and neutral trials were identified.
3.2.1 Incentive cue/reward assessment
A significant I × G × T interaction was identified only in the right cuneus (Table 3). Across early time points (i.e., TRs 3-6), the I × G interaction in the right cuneus was significant (Table 3). SUD youth showed greater activation in the right cuneus than controls during reward, and lower activation than controls during neutral trials. SUD youth also showed greater activation in this area during reward, compared to neutral trials. Controls showed the reverse, that is, greater activity in this region during neutral versus reward trials.
Table 3.
Regions showing significant BOLD response contrast on Incentive Condition × Group × Time interactions
| Talairach Coordinates | Incentive Condition × Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Anatomic region | Brod. Area | x | y | z | Peak F1 | volume (mL) | All time points | Early time points F(1, 22) |
| CUE | ||||||||
| R cuneus | 18 | 17 | -74 | 16 | 3.81 | 324 | F(2, 53)=5.56** | 4.91* |
| PREPARATION | ||||||||
| L superior parietal lobule | 7 | -10 | -62 | 55 | 5.78 | 1134 | F(2, 38)=6.16** | 6.94* |
| L superior parietal lobule | 7 | -31 | -59 | 49 | 4.92 | 918 | F(2, 45)=7.43** | 7.81* |
| R superior parietal lobule | 7 | 17 | -65 | 55 | 4.87 | 837 | F(2, 54)=5.56** | 6.05* |
| L inferior parietal lobule | 40 | -46 | -35 | 37 | 5.71 | 459 | F(2, 44)=6.48** | 7.14* |
| L middle occipital gyrus | 19 | -40 | -83 | 4 | 3.93 | 243 | F(2, 56)=4.89** | 1.52 |
| L precuneus, L middle occipital gyrus | 19 | -22 | -77 | 34 | 4.02 | 702 | F(2, 49)=6.29** | 3.52 |
| R cuneus | 18 | -1 | -77 | 25 | 5.10 | 243 | F(2, 41)=5.01** | 7.24* |
| R posterior cingulate | 30 | -1 | -62 | 10 | 4.91 | 270 | F(3, 60)=5.60** | 6.45* |
| R FEF (iPCS, R middle frontal gyrus) | 6 | 32 | -8 | 49 | 4.73 | 432 | F(2, 52)=5.10** | 6.63* |
| L FEF (iPCS, L middle frontal gyrus) | 6 | -25 | -8 | 46 | 4.07 | 378 | F(2, 51)=5.62** | 4.07 |
| L SEF (L PCS, middle frontal gyrus) | 6 | -16 | -14 | 61 | 3.82 | 324 | F(3, 67)=7.44** | 18.73** |
| L middle frontal gyrus | 9 | -40 | 28 | 31 | 3.61 | 243 | F(2, 42)=6.76** | 4.43* |
| DLPFC, R middle frontal gyrus | 10 | 44 | 52 | 7 | 4.34 | 378 | F(2, 46)=4.84* | 5.54* |
| L inferior frontal gyrus | 9 | -43 | 4 | 28 | 4.09 | 378 | F(3, 62)=4.98** | 3.53 |
| R superior frontal gyrus | 8 | 14 | 46 | 40 | 3.96 | 189 | F(3, 62)=6.35** | 5.91* |
| RESPONSE EXECUTION | ||||||||
| L Precentral gyrus | 3 | -28 | -29 | 49 | 4.32 | 216 | F(3, 68)=5.76** | 0.46 |
NOTES:
corrected p<.001
p<.05
p<.01; early time points (i.e., analysis of restricted time points) = TRs 3-6 (3 - 7.5 seconds)
Brod.= Brodmann, R=Right, L=Left, FEF=frontal eye field, iPCS=inferior precentral sulcus, PCS=precentral sulcus, DLPFC=dorsolateral prefrontal cortex
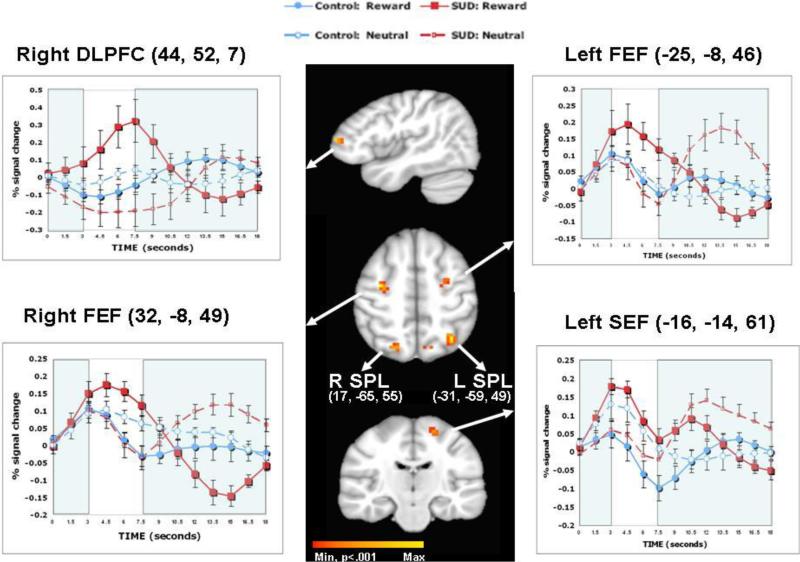
3.2.2 Response preparation
Significant I × G × T interactions were found in the right FEF, left FEF, left SEF, and DLPFC when analyzing all time points (Table 3). In the parietal cortex, across all time points, I × G × T interactions were found for regions in the left superior, right superior, and left inferior parietal lobe. Other areas that showed significant I × G × T interactions over all time points included: right cuneus, right posterior cingulate, areas of the frontal gyrus (i.e., left inferior, left middle, right superior), left middle occipital gyrus, and left precuneus.
Restricted time point analyses resulted in significant I × G interactions in the right FEF, left SEF, and right DLPFC (Table 3). Other areas with significant I × G interactions in restricted time point analyses included left superior, right superior, and left inferior parietal lobe; right cuneus; right posterior cingulate; and left middle and right superior frontal gyrus. I × G interactions were marginally significant for the left FEF (p=.056), left inferior frontal gyrus (p=.07), and left precuneus (p=.07).
During response preparation, when I × G interactions were significant, they were largely driven by activation during early time points. The pattern of activation was consistent for all significant I × G interactions, such that SUD, compared to control, youth showed greater activation during reward, and less activation during neutral trials (Figure 3; e.g., right FEF, left SEF, right DLPFC). Among SUD youth, there was greater activation in regions for which the I × G interaction was significant (e.g., right FEF, left SEF, right DLPFC) during reward, compared to neutral trials. In contrast, Figure 3 indicates that among controls, in regions for which the I × G interaction was significant (e.g., right FEF, left SEF, right DLPFC), there either appeared to be no difference in activation between reward and neutral conditions (i.e., in right FEF and left FEF), or greater activation in the neutral versus reward trials (i.e., in left SEF and right DLPFC).
Figure 3.
Comparison of Substance Use Disorder (SUD) and control adolescents during preparation epoch: Selected regions of interest and associated time courses
Notes: Whole brain activation maps during the preparation epoch for the I × G × T interaction are presented. Talairach coordinates are provided in parentheses. R = right, L = Left, DLPFC = dorsolateral prefrontal cortex, FEF = Frontal Eye Field, SEF = Supplementary Eye Field, SPL = Superior parietal lobule. Min=Minimum, p<.001. Max=Maximum. For the time course graphs, unshaded areas represent early time points of interest that were included in testing for Group × Incentive interactions.
3.2.3 Response Execution
A region in the left precentral gyrus resulted in a significant I × G × T interaction, but no significance for the I × G interaction for restricted time points in this region.
4. Discussion
This study examined the effect of incentive on response inhibition by comparing behavioral performance and regional brain activation on a rewarded AS reward task in adolescents with lifetime SUD and healthy controls. Among SUD youth, there was a decrease in errors during reward compared to neutral trials, an effect that was not significant in the control group. Imaging results indicated that SUD and control youth differed in BOLD response throughout the task in several brain regions, particularly when preparing to inhibit a prepotent response. During response preparation for neutral trials, SUD youth showed less activation, compared to controls, in brain regions known to support correct AS performance (e.g., FEF, SEF, DLPFC). However, during response preparation for reward trials, SUD youth had greater activation, compared to controls, in these brain regions (e.g., FEF, SEF, DLPFC). Further, within the SUD group, there was greater activation of these areas (e.g., FEF, SEF, DLPFC) during reward, compared to neutral trials. Increased activity in prefrontal and oculomotor control regions might have contributed to improvements among SUD youth in correct AS performance during reward trials. Study findings suggest that incentive can enhance response inhibition among SUD youth in a laboratory setting.
Consistent with other behavioral AS studies (e.g., Geier et al., 2010; Jazbec et al., 2005, 2006; Hardin et al., 2007), we found that use of a monetary incentive to support correct AS resulted in decreased error rates, although this effect was limited to SUD adolescents. The absence of a significant reduction in error rate among control youth may have occurred because control youth were already performing at effortful levels during neutral trials, and the magnitude of the incentive was not large enough to significantly enhance performance in controls. In contrast, among SUD youth, incentive appeared to motivate enhanced inhibitory control, compared to neutral trials. In addition, although shorter latency to correct AS has been used to indicate increased reward sensitivity (Hardin et al., 2007; Geier et al., 2010), monetary incentive did not significantly modulate latency to AS in this study. The amount of monetary incentive used in this study, compared to others, was relatively small (maximum “winnings” of $10 across all trials). The relatively modest incentive magnitude across all trials of the task may have reduced the ability to detect differences in modulation of AS performance by incentive.
The fMRI AS reward task used in this study reliably elicits greater activation in brain regions associated with reward-related processing (e.g., VS, OFC) (Geier et al. 2010). However, in this study, no group differences in activation of reward-related brain areas were identified, possibly due to small sample size and differences across studies in the total amount of “reward” offered. Nevertheless, fMRI results revealed differences between adolescent substance users and healthy controls in the modulation of activity by reward incentive in certain brain regions supporting correct AS during all trial epochs (i.e., incentive cue presentation, response preparation, response execution), but particularly during response preparation. Thus, group differences were observed not in the activation of reward-related brain regions, but in how reward affected activation of specific brain regions that support cognitive control of behavior.
During presentation of the incentive cue, SUD youth showed greater activation in the right cuneus compared to control youth during reward trials, and lower activation of this area during neutral trials. Activation of the cuneus has been associated with AS and saccade generation more generally (Grosbas et al., 2005), although the functional meaning of activation in this area with regard to saccade generation remains unclear (Reuter et al., 2010). SUD adolescents’ greater activation of this area, compared to controls, during reward trials suggests their greater sensitivity to basic aspects of bottom-up reward cue assessment.
Most of the between-group interactions in regional activation over time for reward versus neutral trials occurred during the preparation epoch. Greater activity during response preparation in oculomotor control regions predicts successful inhibitory response (Everling & Munoz, 2000; Ford et al., 2005). Reward also has been found to increase activity in brain regions that support correct AS (Geier et al., 2010). Incentive condition by group analyses for early time points of response preparation indicated differences in activation involving oculomotor control regions (FEF, SEF), DLPFC, regions in the parietal lobe, and areas in the frontal gyrus. In each of these identified regions, SUD adolescents, compared to controls, showed lower activation during early time points of neutral trials, and greater activation during reward trials. SUD adolescents’ lower activation, compared to controls, of frontal regions on neutral trials is in accord with findings of less frontal activation during AS among high risk youth (McNamee et al., 2008), and raises the possibility that reduced frontal activation associated with response inhibition in adolescent substance users may pre-date and/or be exacerbated by substance use. Further, among SUD adolescents, incentive (compared to neutral trials) increased activation in brain regions known to support successful inhibitory response during response preparation.
Similar to other studies of response inhibition in marijuana users (e.g., Gruber & Yurgelun-Todd, 2005; Tapert et al., 2007), this AS study did not find significant SUD versus control group differences in behavioral performance on a response inhibition task. However, research has consistently identified differences in pattern and intensity of neural response, particularly involving the DLPFC, between substance users and healthy control groups (e.g., Schweinsburg et al., 2005; 2008). This study is the first to report modulation by reward of neural response supporting cognitive control among adolescent substance users, such that monetary incentive enhanced the activation of brain regions supporting cognitive control, among SUD youth, when preparing to inhibit a prepotent response and anticipating a reward.
The effect of incentive on response inhibition among SUD adolescents stands in contrast to some research on reward processing in SUD adults, which describes SUD adults’ blunted response to non-drug reward cues (e.g., Beck et al., 2009; Wrase et al., 2007), in line with predictions made by the Impaired Response Inhibition and Salience Attribution model of addiction (Goldstein & Volkow, 2002). The difference in results for SUD adolescents in this study and the IRISA model's prediction of blunted response to non-drug reward in SUD adults suggests that adolescents and adults with SUD differ in important ways. Compared to their adult counterparts, SUD adolescents tend to have shorter histories of use, lower dependence severity, and tend to report social (rather than compulsion-based) reasons for relapse (e.g., Deas et al., 2000; Brown et al., 1989). The generally early and milder form of substance involvement in adolescent SUD, compared to adults, suggests that the narrowing of reward to drug-related cues observed in SUD adults may still be developing in SUD youth, such that natural reinforcers (e.g., monetary incentive) may still be relevant in youth with SUD. Alternatively, a study of SUD adults (e.g., Bjork et al., 2008) did not find a blunting of response during anticipation of non-drug reward on a monetary incentive delay task, in accord with the incentive salience hypothesis (Robinson & Berridge, 2001). The incentive salience hypothesis proposes that SUD individuals’ response to non-drug cues essentially remains intact, in the context of increased salience of drug-related cues. Mixed findings across studies of response to non-drug reward in SUD adults may be due to differences in task parameters (e.g., differences in cognitive demand) and patient characteristics (e.g., differences in mean age) (Bjork et al., 2008). Another point to consider, which was not examined in the current study, involves potential differences in response between SUD and control groups during reward notification, in addition to reward anticipation or cue. Some research suggests greater limbic system sensitivity during reward notification among SUD adults compared to controls, a result that appears to be explained by individual differences in trait impulsivity and neuroticism in the SUD group (Bjork et al., 2008). The extent to which trait impulsivity and negative affectivity help to explain the effect of incentive cue on cerebral activation in SUD adolescents remains to be examined.
Study findings suggest that adolescent substance users do not necessarily lack the ability to consistently inhibit a prepotent response, but when inihibitory control is associated with anticipation of reward, processes supporting response inhibition can be enhanced. This finding could have potential implications for treatment in terms of maintaining long-term abstinence from substance use, for example in increasing understanding of brain mechanisms that may underlie the effectiveness of contingency management interventions used to support abstinence among adolescents in substance abuse treatment (e.g., Kamon et al., 2005; Godley et al., 2008; Stanger et al., 2009). However, despite the use of salient incentives, comorbid conditions (e.g., bipolar disorder, Mueller et al., 2010), brain injury, and other factors (e.g., competing rewards) may limit the extent to which an incentive facilitates cognitive control of behavior.
Certain study limitations warrant comment. Self-report of recent substance use prior to the scan was not biochemically verified. The small sample size limited statistical power to detect significant differences for some comparisons, and precluded inclusion of covariates (e.g., cooccurring psychopathology). Brain activation-behavior associations and performance errors were not studied here, and represent important directions for future research. In addition, the amount of reward provided for correct AS was relatively small, which may explain why some reward effects (e.g., effect of reward on reducing latency to correct AS) were not observed. Other aspects of the incentive condition, such as parametric effects of monetary incentive and monetary losses (i.e., punishment), and trial-by-trial incentives and performance feedback, were not examined, and warrant further study (cf. Hardin et al., 2007). Furthermore, although one SUD adolescent had lifetime, but not current (i.e., past 6 months), substance use disorder at the time of the scan, there is some evidence for long-term effects of heavy marijuana use on neurocognitive functioning (review: Schweinsburg et al., 2008), which supports the inclusion of this adolescent in the SUD group. Although all adolescents had a lifetime DSM-IV cannabis use disorder, due to the use of multiple substances by SUD youth in the sample, effects specific to a particular substance cannot be determined in this study. Laboratory-based performance on tasks involving inhibitory control may have limited generalizability to real-world contexts in which other factors, such as social context, also influence response inhibition (Steinberg, 2010). The effect of treatment on improving SUD youth's inhibitory control remains to be studied.
In summary, this study of the effects of reward on oculomotor inhibitory control found that monetary incentive facilitated cognitive control of behavior for adolescent substance users. Although SUD and control adolescents showed similar behavioral AS performance regardless of incentive condition, group differences in regional brain activation were identified. Results suggest that, particularly for SUD youth, incentive may enhance motivation and preparatory brain activity supporting effective response inhibition.
Acknowledgments
This research and the preparation of this manuscript were supported by National Institute on Alcohol Abuse and Alcoholism K02 AA018195, R01 AA014357, R21 AA017128, R21 AA016272, K02AA00291, and National Institute on Drug Abuse K01 DA018698. Portions of this research were presented at the 2009 Research Society on Alcoholism meeting, San Diego, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addict. Behav. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Chung T, Maisto SA. “What I got from treatment”: predictors of treatment content received and association of treatment content with 6-month outcomes in adolescents. J. Subst. Abuse Treat. 2009;37:171–81. doi: 10.1016/j.jsat.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Pollock NA, Bromberger JT, Bukstein OG, Mezzich AC, Donovan JE. Gender and comorbid psychopathology in adolescents with alcohol use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1195–1203. doi: 10.1097/00004583-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsivenss as dimensions of impulsivity: Implications for substance misuse. Addict. Behav. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, Brown S. Adolescents are not adults: Developmental considerations in alcohol users. Alcohol Clin. Exp. Res. 2000;24:232–237. [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacol. Biochem. Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J. Neurophysiol. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with prosaccades and anti-saccades in the primate frontal eye field. J. Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders, Research Version, Non-patient edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Ford KA, Goltz HC, Brown MRG, Everling S. Neural processes associated with antisaccade task performance investigated with event-related fMRI. J. Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol. Biochem. Behav. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for postesperimental eye movement analysis. Behav. Res. Meth. Instrum. Comput. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Godley SH, Godley M, Wright K, Funk R, Petry N. Contingent reinforcement of personal goal activities for adolescents with substance use disorders during post-residential continuing care. Am. J. Addict. 2008;17:278–286. doi: 10.1080/10550490802138798. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow N. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone L, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow N. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbas M-H, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum. Brain Mapp. 2005;25:140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Brain Res. Cogn. Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Folan MM, Luna B, Tarter RE. Impaired oculomotor response inhibition in children of alcoholics: The role of attention deficit hyperactivity disorder. Drug Alcohol Depend. 2006;82:11–17. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hardin M, Ernst M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents. J. Addict. Med. 2009;3:47–54. doi: 10.1097/ADM.0b013e31819ca788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin M, Schroth E, Pine D, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: Development and psychopathology related differences. J. Child Psychol. Psychiatry. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Four-factor index of social status. Author; New Haven: 1975. [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biol. Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin M, Schroth E, McClure E, Pine D, Ernst M. Age-related influence of contingencies on a saccade task. Exp. Brain Res. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamon J, Budney A, Stanger C. A contingency management intervention for adolescent marijuana abuse and conduct problems. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children – present and lifetime version (KSADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, Geier C. Development of eye-movement control. Brain Cogn. 2008;68:293–308. doi: 10.1016/j.bandc.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Kaczynski NA, Maisto SA, Bukstein OG, Moss HB. Patterns of DSMIV alcohol abuse and dependence symptoms in adolescent drinkers. J. Stud. Alcohol. 1995;56:672–680. doi: 10.15288/jsa.1995.56.672. [DOI] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Depend. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- McNamee RL, Dunfee KL, Luna B, Clark DB, Eddy EF, Tarter RE. Brain activation, response inhibition, and increased risk for substance use disorder. Alcohol Clin. Exp. Res. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Ng P, Temple V, Hardin MG, Pine DS, Leibenluft E, Ernst M. Perturbed reward processing in pediatric bipolar disorder: An antisaccade study. J. Psychopharmacol. 2010 doi: 10.1177/0269881109353462. published online 15 January 2010. DOI: 10.1177/0269881109353462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nature. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI: Part I. Neuroimage. 2001a;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shuldman GL. Separating processes within a trial in event-related functional MRI: Part II. Neuroimage. 2001b;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation . Wechsler Abbreviated Scale of Intelligence (WASI) manual. Author; San Antonio, TX: 1999. [Google Scholar]
- Reuter B, Kaufmann C, Bender J, Pinkpank T, Kathmann N. Distinct neural correlates for volitional generation and inhibition of saccades. J. Cogn. Neurosci. 2010;22:728–738. doi: 10.1162/jocn.2009.21235. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr. Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk taking. Dev. Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Thatcher DL, Pajtek S, Chung T, Terwilliger RA, Clark D. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug Alcohol Depend. 2010;110:55–61. doi: 10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]