Abstract
It is well known that antioxidants have protective effects against oxidative stress. Unfortunately, in the presence of transition metals, antioxidants including polyphenols with potent antioxidant activities may also exhibit pro-oxidant effects, which may irreversibly damage DNA. Therefore, antioxidants with strong free radical scavenging abilities and devoid of pro-oxidant effects would be of immense biological importance. We report two antioxidant dendrimers with a surface rich in multiple phenolic hydroxyl groups, benzylic hydrogens and electron donating ring substituents that contribute to their potent free radical quenching property. In order to minimize their pro-oxidant effects, the dendrimers were designed with a metal chelating tris(2-aminoethyl)amine (TREN) core. The dendritic antioxidants were prepared by attachment of six syringaldehyde or vanillin molecules to TREN by reductive amination. They exhibited potent radical scavenging properties: 5 times stronger than quercetin and 15 times more potent than Trolox according to the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. The antioxidant dendrimers also protected low-density lipoprotein, lysozyme and DNA against 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced free radical damage. More importantly, unlike quercetin and Trolox, the two TREN antioxidant dendrimers did not damage DNA via their pro-oxidant effects when incubated with physiological amounts of copper ions. The dendrimers also showed no cytotoxicity towards Chinese hamster ovary cells.
Keywords: Polyphenolic dendrimer, antioxidant, pro-oxidant, lipoprotein, lysozyme, DNA
Introduction
Oxygen- and nitrogen-based free radicals are produced as a result of metabolism and the immune response. At low levels, radicals have important physiological roles, such as cell signal transduction resulting in gene expression and modulation of cell growth characteristics. However, excessive amounts of radicals can be harmful to biomolecules including DNA, proteins, and lipid. Persistent overproduction may result in a condition of oxidative stress to cells, leading to the pathogenesis of many ailments, such as inflammation, cancer, asthma, cardiovascular and neurological diseases, and in the etiology or progression of age-related disorders. Among the myriad of antioxidants, polyphenols have been reported to possess numerous health benefits. Anti-cancer [1], anti-lipoperoxidation [2], anti-ischemic [3], anti-allergic and anti-inflammatory [4] properties have been reported for these compounds. Quercetin, a popular polyphenolic flavonoid has been clinically shown to be a chemo-preventive agent [5]. Unfortunately, antioxidants including polyphenols with potent antioxidant activity also exhibit carcinogenic and mutagenic effects [6]. Rats fed with excessive amounts of quercetin developed renal tubule adenomas, adenocarcinomas, intestinal and bladder cancer [7]. Although seldom seen clinically, there is substantial evidence associating high concentrations of flavonoids and other popular antioxidants, such as vitamin C and E and carotenoids, with mutagenic and DNA damaging ability [8]. It is well known that chelation of iron by ascorbate can lead to the formation of hydroxyl and superoxide radicals. Some of the damaging effects on DNA by antioxidants are associated with their pro-oxidant activity in the presence of transition metals like copper and iron [9]. Other potential mechanisms of the genotoxic effects of antioxidants have been proposed [8]. Identification of natural or synthetic potent antioxidants that have little or no pro-oxidant effects would clearly be beneficial for biological applications.
A vast number of antioxidants have been synthesized in attempts to develop more potent and bioavailable compounds. Strong antioxidants share certain common structural features. They often possess multiple phenolic hydroxyl groups (e.g. flavonoids) or extensively conjugated π system (e.g. carotenoids). In the case of phenolic antioxidants, stronger antioxidant capacity is observed with increasing number of phenolic hydroxyl groups [10]. The high antioxidant efficiency of polyphenols stems from their ability to form stable phenoxyl radicals that can resonate with the benzene rings after donation of H atoms to free radicals. If the ring has an extended conjugation ortho or para to the hydroxyl group, the resonance stabilization of the antioxidant radical increases, resulting in enhanced antioxidation capacity [11]. Substitution of the phenolic ring also plays an important role in antioxidant potential. For example, an electron-donating group ortho or para to hydroxyl increases the hydrogen donating capability by stabilizing phenoxyl radical via electron donation, increasing antioxidant efficiency [12]. Commonly found electron-donating groups in potent antioxidants include substituents that do not form intramolecular hydrogen bonds with the phenolic hydroxyl groups (e.g. methyl groups in α-tocopherol) or that can form nonlinear intramolecular hydrogen bonds with phenolic hydrogen at the ortho position (such as methoxy) [13]. Benzylic hydrogens have also been reported to be beneficial toward enhancing antioxidant potential [14]. These hydrogen atoms are chemically labile and analogous to phenolic hydrogens in terms of their ability to stabilize the resulting radical by resonance delocalization with the benzene ring.
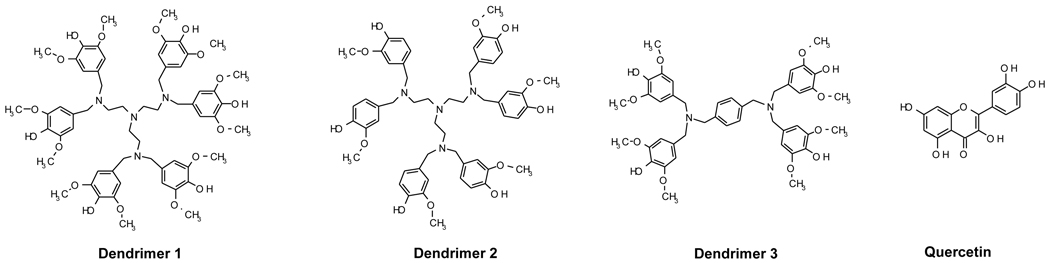
The dendritic architecture allows us to incorporate multi-functionality in a molecule. Dendrimers are “soft” nanomaterials whose size can be systematically increased to provide precise structures (generations). We recently reported the synthesis and antioxidant properties of three generation 1 (G1) dendritic polyphenols consisting of syringaldehyde, vanillin and 5-iodovanillin emanating from a 4-aminomethylbenzylamine core [15]. Among these three dendritic antioxidants, quercetin and a vitamin E analog (Trolox), the syringaldehyde-based antioxidant dendrimer showed the strongest antioxidant activity (measured by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical assay), and was the most effective in protecting LDL, linoleic acid and DNA from free radical attack. Interestingly, when the pro-oxidant effects of the G1 antioxidants on copper-induced DNA oxidation was compared with quercetin and Trolox, they were found to be less harmful than the latter two antioxidants. These promising results led us to prepare similar G1 antioxidants which possess interior amines that have potential metal chelation property surrounded by a peripheral layer of phenol rings which are efficient radical scavenging groups. An antioxidant dendrimer of this design should show beneficial antioxidant potential with reduced undesirable pro-oxidant activity towards DNA. Structures of the two newly synthesized syringaldehyde and vanillin-based dendrimers with a tris(2-aminoethyl)amine (TREN) core (1 and 2), a previously reported syringaldehyde-based dendrimer with a 4-aminomethylbenzylamine core (3), and a naturally occurring polyphenol (quercetin) are shown in Figure 1.
Figure 1.
Structures of dendrimers 1–3 and quercetin.
Materials and Methods
Syringaldehyde, vanillin, quercetin, TREN (97%), sodium triacetoxyborohydride (Na(OAc)3BH), tetra-butylammonium fluoride (n-Bu4NF, 75 wt% solution in water), tert-butyldimethylsilyl chloride (TBDMS-Cl, 50% in toluene), DPPH, Fat Red 7B, phosphate-buffered saline (PBS), potassium persulfate, glacial acetic acid, sodium acetate and methanol were purchased from Sigma Aldrich and were used without further purification. Lysozyme (egg white) was purchased from Worthington Biochemical Corporation. 2,2'-Azobis(2-amidinopropane) dihydrochloride (AAPH) was obtained from Cayman Chemical (Ann Arbor, MI, USA). Human low-density lipoprotein (LDL) was obtained from Kalen Biomedical (Montgomery Village, MD, USA). The lipoprotein solution (protein = 5 mg/mL) contained 154 mM NaCl and 0.34 mM EDTA.
1H-NMR spectra were recorded with a Varian Mercury-300 spectrometer operating at 300 MHz with tetramethylsilane (Si(CH3)4) as an internal standard. 13C-NMR spectra were recorded using a Varian Mercury-300 spectrometer operating at 75 MHz with Si(CH3)4 as an internal standard. The mass spectra were obtained on Bruker Autoflex MALDI-TOF using 2,5-dihydroxybenzoic acid as a matrix. All spectrophotometric data were obtained using Perkin Elmer UV/Vis spectrometer (Lambda 20) and Molecular Devices Corp. Spectra Max (M2e).
General procedures for the protection of phenolic hydroxyl groups with TBDMS-Cl
Phenolic hydroxyl groups were protected with TBDMS as previously described [15].
TBDMS-protected syringaldehyde
Yield: 98%; Rfvalue: 0.75 (hexane-ethyl acetate = 1:1); 1H-NMR (300 MHz, CDCl3) δ 0.15 (s, 6H, Si(CH3)2C(CH3)3), 1.00 (s, 9H, Si(CH3)2C(CH3)3), 3.86 (s, 6H, 2 × OCH3), 7.09 (s, 2H, C2-H and C6-H), 9.82 (s, 1H, CHO); 13C-NMR (75 MHz, CDCl3) δ −4.3 (Si(CH3)2C(CH3)3), 19.0 (Si(CH3)2C(CH3)3), 25.9 (Si(CH3)2C(CH3)3), 56.0 (OCH3), 106.9 (C2 and C6), 129.5(C1), 140.8(C4), 152.2 (C3 and C5), 191.3 (CHO); MS: m/z 297 [M+H+].
General procedures for the formation of dendrimers 1 and 2
The dendrimers 1 and 2 were synthesized using the previously reported methods [15].
Dendrimer 1
Yield: 63%; Rf: 0.33 (acetone); 1H-NMR(300 MHz, CD3COCD3) δ 2.49 (s, 6H, 3 × core N-CH2-CH2-N-), 2.63 (s, 6H, 3 × core N-CH2-CH2-N-), 3.41 (s, 12H, 6 × Ph-CH2-N-), 3.77 (s, 36H, 12 × OCH3), 5.4 (s, 6H, 6 × OH), 6.65 (s, 12H, 6 × Ph C2-H and C6-H); 13C-NMR (75 MHz, CD3COCD3) 50.9 (3 × core N-CH2-CH2-N-), 52.7 (3 × core N-CH2-CH2-N-), 56.4 (12 × OCH3), 59.0 (6 × Ph-CH2-N-), 106.5 (6 × Ph C2 and C6-H), 130.5 (6 × Ph C1-CH2-), 135.4 (6 × Ph C4-OH), 148.4 (6 × Ph C3 and C5-OCH3); MS: m/z 1143.5 [M+H+].
Dendrimer 2
Yield: 51%; Rf = 0.21 (acetone); 1H-NMR (300 MHz, CD3COCD3) δ 2.46 (t, J = 5.7 Hz, 6H, 3 × core N-CH2-CH2-N-), 2.57 (t, J = 5.4 Hz, 6H, 3 × core N-CH2-CH2-N-), 3.42 (s, 12H, 6 × Ph-CH2-N-), 3.79 (s, 18H, 6 × OCH3), 6.75 (s, 12H, 6 × Ph C2-H and C6-H), 6.95 (s, 6H, 6 × Ph C5-H); 13C-NMR (75 MHz, CD3COCD3) δ 51.3 (3 × core N-CH2-CH2-N-), 53.1 (3 × core N-CH2-CH2-N-), 56.1 (6 × OCH3), 58.8 (6 × Ph-CH2-N-), 112.9 (6 × Ph C2-H), 115.3 (6 × Ph C5-H), 122.1 (6 × Ph C6-H), 131.6 (6 × Ph C1-CH2), 146.3 (6 × Ph C4-OH), 148.1 (6 × Ph C3-OCH3); MS: m/z 963.2 [M+H]+.
DPPH assay
The reduction of DPPH radical was determined for dendrimers, quercetin, syringaldehyde, and vanillin as previously reported [16]. The DPPH reagent and antioxidants were dissolved in methanol. Antioxidant sample (10 µL) was added to 1.0 mL of reagent and the absorbance was monitored at 515 nm until a plateau was reached. All samples were run in triplicates at room temperature. The within-run coefficient of variation of the % inhibition values was less than 6%.
LDL oxidation-electrophoresis
Low-density lipoprotein was incubated with 20 mM AAPH in PBS and increasing concentration of antioxidant (0–37 µM, made in methanol) at 37 °C for 21 h. The mixture was then subjected to electrophoresis on 1% agarose gels (Helena Labs, Beaumont, TX) using the Ciba Corning Clinical Electrophoresis System. The gels were stained with Fat Red 7B.
Enzyme inactivation
Lysozyme (1.8 mg/mL) was incubated with 20 mM AAPH in PBS and 80 µM antioxidant (made in methanol) at 37 °C for 24 h. Appropriate control with solvent (methanol) instead of antioxidant was also run. After incubation, the sample was diluted with PBS and assayed for lysozyme activity. Lysozyme assays were carried out at room temperature (25 °C) by monitoring the loss of apparent absorbance at 450 nm, which results from the addition of lysozyme to a suspension of lyophilized Micrococcus lysodeikticus in 67 mM phosphate buffer (pH 6.2). Coefficient of variation for within-run % enzyme activity was < 8%.
Antioxidant effects on DNA
DNA electrophoresis was performed on pBR 322 after 4 h incubation at 37 °C in the presence of 10 mM AAPH in PBS and various concentrations (0–45 µM) of antioxidants (made in methanol).
Pro-oxidant effects on DNA
DNA (pBR 322) was incubated with 10 µM CuCl2 in the presence of 0–45 µM antioxidants (made in methanol) at 37 °C for 1 h. DNA damage was monitored by agarose electrophoresis.
Cell culture
Cytotoxic effects of dendrimer 1 and 2 were assessed using 3-(4,5-di-methylthizol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Chinese hamster ovary (CHO-K1) cells were cultured in F-12K medium supplemented with 10% fetal bovine serum. The cells (3 × 105/mL) were seeded in 100 µL volumes in 96 well culture plates and incubated with the solvent control (DMSO, 0.3%), dendrimer 1 or 2 at 3.1, 6.2, 12.5, 25, or 50 µM for 1, 3, or 5 days. Two hours prior to experimental termination, 20 µL of a 5 mg/mL MTT solution in 0.01M PBS was added to each well. Plates were centrifuged at 450 × g for 10 min and supernatant was removed. The resultant formazan crystals were dissolved in 100 µL DMSO and mixed. Absorbance was measured using a Biolog microplate reader (Biotek Instruments) at dual wavelengths, 590 and 650 nm. Percent control response was calculated as follows: (absorbance of treatment/absorbance of control) × 100. All experiments were performed in triplicate and repeated. The data were analyzed using Systat 12 for windows. Multiple groups were compared using a one-way analysis of variance (ANOVA) and a Tukey test for mean separation. A p value <0.05 was considered statistically significant.
Results and Discussion
Since the dendrimer has interior and exterior layers, which can be appropriately manipulated to produce a nanomolecule with various desirable properties, the dendritic architecture is ideal for the design of a nanoantioxidant that is able to chelate metal ions as well as scavenge free radicals. Dendrimers such as poly(amidoamine) (PAMAM) and poly (propylene imine) (PPI) are known to encapsulate metal ions such as Cu2+ and Fe3+. If the exterior layer of the dendrimer does not bind metal ions, then most of the bound metal ions are found in the interior of the dendrimer attached to its tertiary amines [17]. In the case of our antioxidants 1–3, such a binding can occur with the tertiary amines located in the core since TREN is well known for its metal ligating abilities and compounds derived from TREN have been reported to bind cobalt, nickel, copper, lanthanides and actinides [18]. Unlike quercetin with a catechol group that is known to bind metal ions such as Cu2+ [19], the surface groups of 1 and 2 lacking the catechol moiety is not expected to bind metal ions favorably. In addition to the metal chelating capability of the TREN core in compounds 1–2, six phenol rings, either derived from syringaldehyde or vanillin, on the exterior of the antioxidant dendrimers form a potent radical scavenging layer. The presence of multiple phenolic hydroxyl groups, benzylic hydrogens, and electron donating substituents (on the benzene rings) should contribute favorably towards the radical quenching property of these antioxidants.
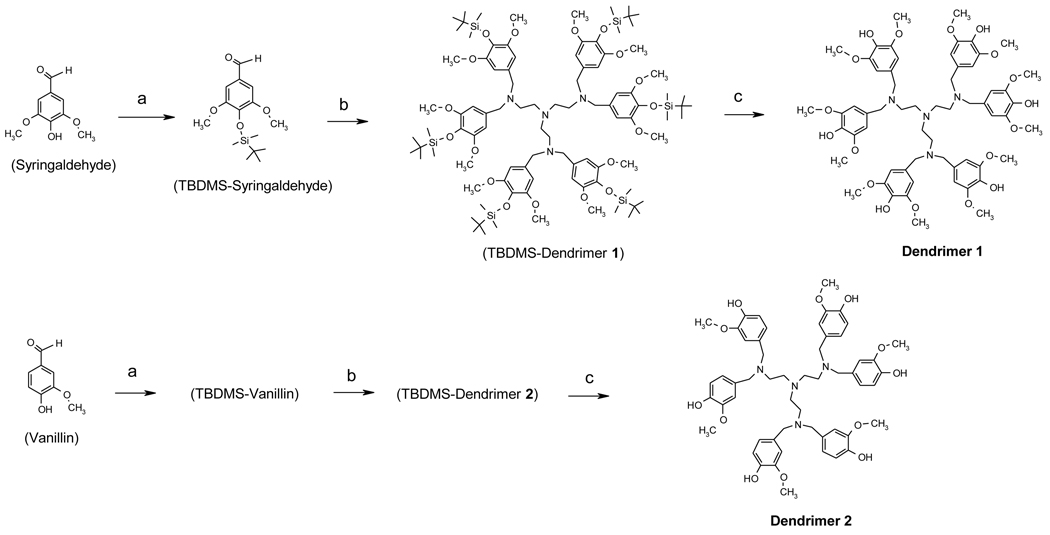
Syntheses of compounds 1 and 2 were initiated by protecting building blocks (syringaldehyde and vanillin) with TBDMS-Cl (Scheme 1). The protected building blocks were then attached to the amino groups of TREN via their aldehyde groups. The three resulting unstable imine bonds were subsequently reduced with Na(OAc)3BH to form secondary amines which further react with additional aldehyde groups, eventually forming tertiary amines after reduction by Na(OAc)3BH. Unlike other amine alkylation using alkyl halide (which usually produce a mixture of species ranging from primary amine to quaternary ammonium), the reaction between amine and aldehyde can only form a tertiary amine as an end product. Hence, it is an attractive method to form branches in a controlled manner, producing a well-defined dendritic structure. Although Na(OAc)3BH has been reported to be an efficient reductant for imine and iminium bonds [20], we found that its selective reduction of these groups over aldehyde was poor. The arylaldehydes reduced to their corresponding alcohols fairly rapidly when all reagents were mixed together in one pot. To avoid this problem, three equivalents of TBDMS-protected building block was first reacted with one equivalent of TREN core and the reaction was allowed sufficient time (> 5 h) before addition of Na(OAc)3BH. This was to ensure that the aldehyde reacted with three amino groups first with limited exposure to the reducing agent. After Na(OAc)3BH addition, the reaction was run for at least 24 h before another three equivalents of building block was added. Based on our experience, Na(OAc)3BH worked more efficiently in 1,2-dichloroethane than other solvents like CH2Cl2. Protection of syringaldehyde and vanillin with TBDMS-Cl enhanced percent yield by increasing solubility of the dendrimers 1 and 2 in 1,2-dichloroethane. Both TBDMS-dendrimer 1 and 2 have much better solubility than the corresponding dendrimers in the solvent. Although the water by-product cleaved some of the TBDMS protecting groups, the target compounds with varying number of TBDMS groups remained soluble during the reaction. Prior to column purification, the protecting groups were cleaved with n-tetrabutyl ammonium fluoride in ethanol, affording the antioxidant dendrimers 1 and 2.
Scheme 1.
Syntheses of dendrimers 1 and 2.
Reagents and conditions: (a) TBDMS-Cl, triethylamine, CH2Cl2, 0 °C (b) TREN, Na(OAc)3BH, 1,2-dichloroethane (c) n-Bu4NF, ethanol.
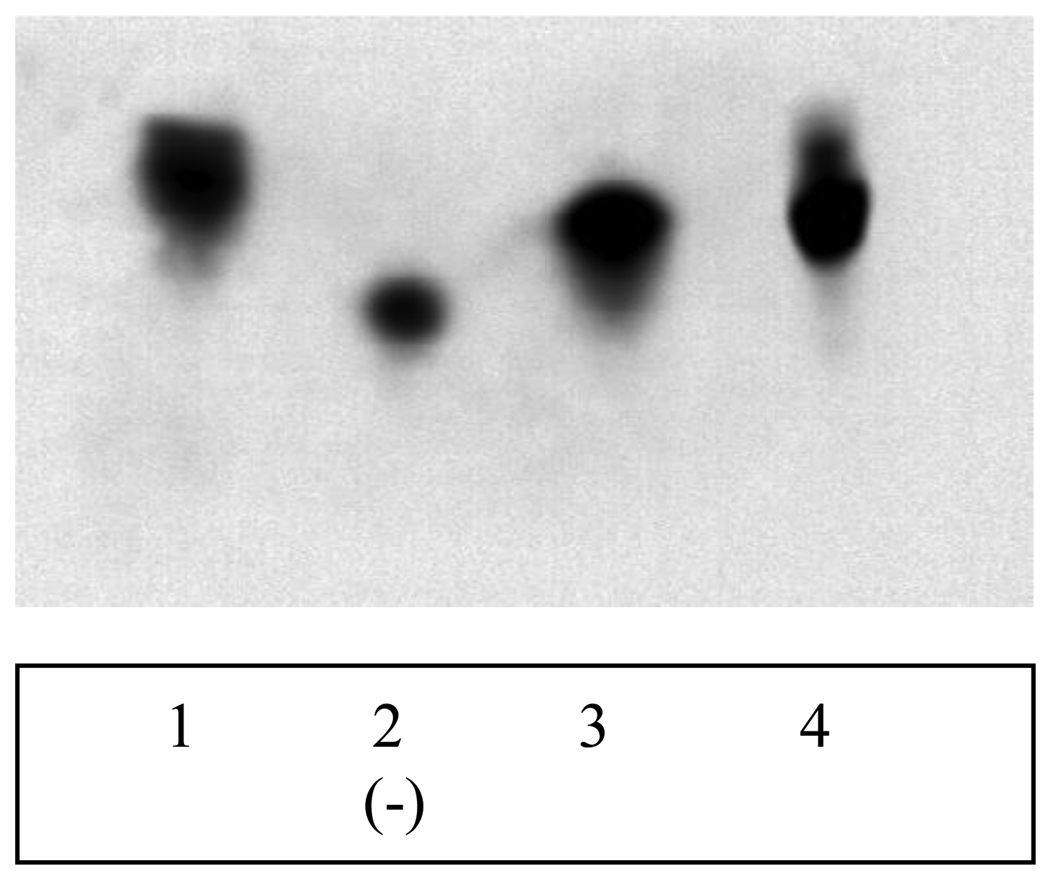
Polyacrylamide gel electrophoresis (PAGE) is a useful tool for characterization of dendrimers [21]. Like proteins, these low-polydispersity nanoparticles show bands in polyacrylamide gels. The antioxidant dendrimers 1–3 were run under acidic conditions on a 15% PAGE gel and stained with Coomassie Blue (Figure 2). Each lane contained 10 nmol of antioxidant. All antioxidants produced well-stained bands. For comparison, a generation 4 PAMAM dendrimer with amine surface is shown in lane 1. Dendrimer 2 (lane 2) showed the highest mobility towards the cathode followed by 3 (lane 3) and 1 (lane 4). In addition to the major band, dendrimer 1 also showed a broad minor band with lower mobility while 3 displayed a less intense band with higher mobility. These minor bands probably represent defective target compounds (e.g., species with missing arms).
Figure 2.
Electrophoresis of antioxidant dendrimers.
Lane 1 (G4 PAMAM); lane 2 (dendrimer 2); lane 3 (dendrimer 3); lane 4 (dendrimer 1). Each lane contains 10 nmol dendrimer.
The free-radical scavenging ability of the antioxidants was evaluated by the DPPH assay [16]. The two TREN-based dendrimers, 1 and 2, showed similar potency (IC50 mean of three experiments = 2 µM; SD = 1 µM at 120 min). Within experimental error, these values were also similar to the previously reported syringaldehyde-based 4-aminomethylbenzylamine core dendrimer 3 (IC50 mean = 4 µM; SD = 0.9 µM at 120 min). Under identical experimental conditions, average IC50 value for quercetin was 10 µM (SD = 0.2 µM) at 120 min, Trolox was 30 µM (SD = 0.2 µM) at 120 min while the starting materials used for syntheses of the antioxidants displayed negligible DPPH activity (IC50 values of syringaldehyde and vanillin were >100 µM).
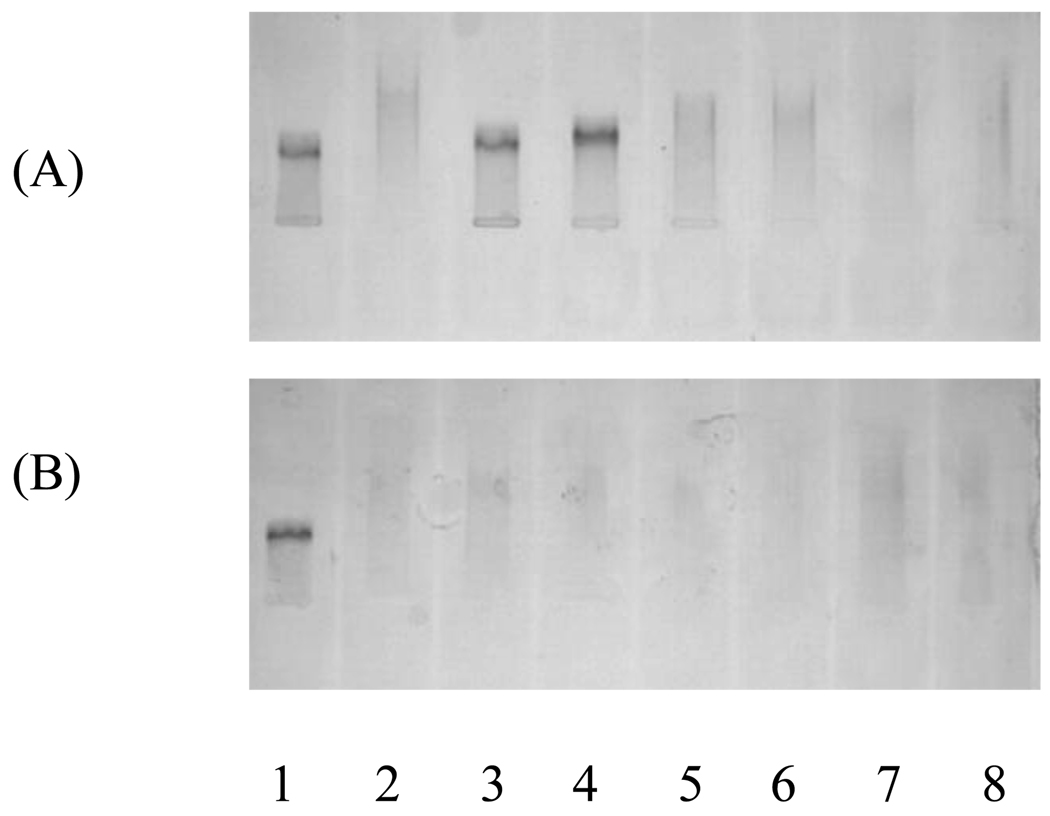
The ability of the antioxidant dendrimers to protect lipids, protein, and DNA from free radical damage was evaluated. For LDL, AAPH was used instead of Cu2+ to induce free radical damage since the latter may cause pro-oxidant interference [22,23]. Oxidation of human LDL was used as a model for evaluating the ability of the G1 dendrimers as chain-breaking antioxidants for lipid. LDL oxidation leads to changes in both the lipid and the protein moieties of the lipoprotein particle. Lipid peroxidation in LDL has been evaluated by a variety of methods. Due to the complexity of events that occur in lipoprotein oxidation, the electrophoretic migration pattern of oxidized LDL is one of the best methods to evaluate the extent of lipoprotein oxidation [24]. LDL was incubated with 20 mM AAPH and increasing concentrations of the G1 dendrimers at 37 °C for 21 h. The agarose gel obtained with dendrimer 1 is shown in Figure 3.
Figure 3.
Effect of antioxidants on LDL oxidation.
(A) Lane 1 (native LDL); lanes 2–8 (AAPH-oxidized LDL with 0, 37, 18.5, 9, 4.5, 2 and 1 µM dendrimer 1, respectively). (B) Lane 1 (native LDL); lanes 2–8 (AAPH-oxidized LDL with 0, 37, 18.5, 9, 4.5, 2 and 1 µM quercetin, respectively).
Under these conditions, oxidized LDL (lane 2, Figure 3A), unlike native lipoprotein (lane 1), showed a smear, characteristic of heterogeneous species formed as a result of lipoprotein oxidation. The lipoprotein was fully protected against free radical damage at 18.5 µM and 37 µM of dendrimer 1 (lanes 3 and 4, Figure 3A). Lower concentrations of dendrimer 1 (lanes 5–8, Figure 3A) were ineffective. Under similar conditions, dendrimers 2 and 3 were slightly less effective. They showed a sharp LDL band at 37 µM but not at 18.5 µM (data not shown). In comparison, quercetin, Trolox and the starting materials were ineffective at all antioxidant concentrations (1–37 µM) (lanes 3–8, Figure 3B).
Protective effect of the dendritic antioxidants on protein was determined using hen egg white lysozyme as a model protein. The enzyme was incubated with 20 mM AAPH and 80 µM antioxidant at 37 °C for 24 h. Both dendrimers 1 and 2 completely protected the enzyme (100% recovery), while dendrimer 3, quercetin and Trolox gave 54%, 34% and 12% recovery, respectively. All starting materials gave <10% recovery. Enzyme recovery decreased with lower antioxidant concentrations. At 40 µM antioxidant concentration, both dendrimers 1 and 2 gave 70% recovery, while dendrimer 3, quercetin and Trolox gave 50%, 19% and 10% recovery, respectively. Lower antioxidant amounts gave even lower recoveries. For example, dendrimer 1 gave recoveries of 40%, 16%, 13% at antioxidant concentrations of 20 µM, 10 µM, and 5 µM, respectively (control without antioxidant gave 7% activity).
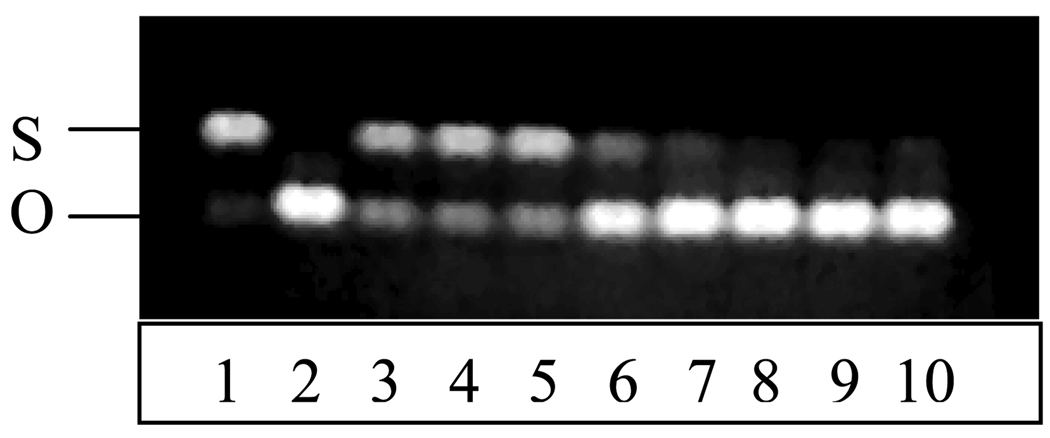
Protection of DNA from free radical damage by the antioxidant dendrimers was also evaluated. Plasmid DNA (pBR 322) samples were incubated with AAPH (final concentration, 10 mM) at 37 °C for 4 h with antioxidants (final concentrations, 0.35–45 µM) and subjected to agarose electrophoresis [25]. A gel obtained with dendrimer 1 is shown in Figure 4. The native DNA was mostly in its supercoiled (S) form (small amount of open circular, O, form was also visible, lane 1, Figure 4). In the presence of AAPH, the DNA was transformed almost entirely into its O form (lane 2, Figure 4). In the presence of 11–45 µM of dendrimer 1, DNA was well protected; the S band intensity was similar to control containing no AAPH (lanes 3–5, Figure 4). Quercetin as well as dendrimers 2 and 3 also displayed similar gel patterns to dendrimer 1 (data not shown). Some protection was also obtained for the three dendrimers at 5 µM as shown by traces of supercoiled DNA (lane 6, Figure 4) but not with quercetin (data not shown). Negligible DNA protection was afforded by all dendrimers at < 3 µM (lanes 7–10, Figure 4). Under similar conditions, Trolox as well as the starting materials for dendrimer synthesis were ineffective at all concentrations.
Figure 4.
Protection against AAPH-induced DNA oxidation by dendrimer 1.
Lane 1 (native DNA); lanes 2–10 (AAPH-oxidized DNA with 0, 45, 23, 11, 5, 3, 1.5, 0.7, and 0.35 µM antioxidant, respectively).
All of the above antioxidant activity tests clearly indicate that the antioxidant dendrimers are effective radical scavenging agents. This may be attributed to the presence of multiple phenolic hydroxyl groups, benzylic hydrogens, and electron donating substituents in these compounds.
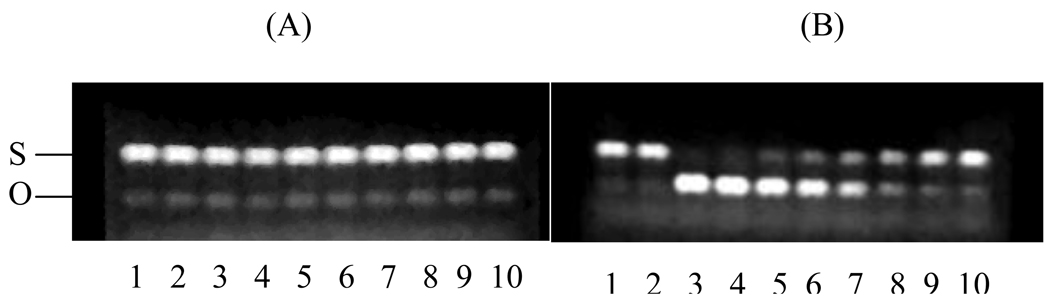
The pro-oxidant property of antioxidants, such as quercetin, in the presence of transition metals ions (e.g., Cu2+ and Fe3+) has been associated with their mutagenic and carcinogenic health hazard [7,19]. This is a serious drawback for their potential clinical applications. Since potent antioxidants often possess strong pro-oxidant activity, it was important for us to examine the pro-oxidant behaviors of our dendritic antioxidants. This was performed by co-incubation of DNA (pBR 322), copper (II) ion and antioxidant. It is believed that an antioxidant such as quercetin which displays strong pro-oxidant activity binds to Cu (II)-DNA complex through its catechol and forms a DNA-Cu(II)-quercetin complex. This ultimately forms DNA-Cu(I)OOH, initiating a free radical cascade by which DNA damage occurs [26]. pBR 322 was incubated with 10 µM Cu2+ in the presence of antioxidants at 37 °C for 1 h. Figure 5 shows an agarose gel obtained with dendrimer 1 (A) and quercetin (B). No DNA damage was obtained at all concentrations of dendrimer 1 between 0.35–45 µM. Dendrimer 2 also gave similar gel patterns (data not shown). As previously reported, DNA damage was clearly observed with dendrimer 3, quercetin (Fig. 5B) and Trolox at concentrations between 11–45 µM [15]. The starting materials did not show any pro-oxidant effect on DNA under these conditions. These results suggest that the lack of pro-oxidant effects displayed by the two TREN-based dendrimers may be due to chelation of copper ions by the tertiary amines in the core. At high copper concentrations (e.g. 60 µM), the O-form of DNA was also observed with the TREN dendrimers (data not shown). This suggests that the metal chelating property of the dendrimers was likely overwhelmed at higher copper concentrations resulting in pro-oxidative effects and DNA damage. However, it should be noted that physiological concentrations of copper are less than 10–20 µM in plasma and approximately 10−18 M for the cation in cells [27]. Therefore, chelation of even low amounts of cation can markedly affect free radical formation, especially in the chromatin region where one copper ion is present per kilobase [28].
Figure 5.
Pro-oxidant effect of dendrimer 1 (A) and quercetin (B) on DNA (pBR 322). Lane 1 (native DNA); lanes 2–10 (Cu2+ oxidized DNA with 0, 45, 23, 11, 5, 3, 1.5, 0.7, and 0.35 µM antioxidant, respectively).
Pro-oxidant effect of our novel antioxidant dendrimers was also evaluated with Fe3+. DNA was incubated at 37 °C for 1 h in the presence of physiological concentration of Fe3+ (30 µM) and antioxidant 1 (45 µM) or quercetin (45 µM). Neither the dendritic antioxidant 1 nor quercetin showed any pro-oxidant behavior (similar to lane 3, Figure 5A, data not shown). We also wanted to see if there was any deleterious interaction of our dendrimers with non-transition metal ions, such as Zn2+ that is present in significant amount in plasma (~12 µM), under similar conditions. Both dendrimer 1 and quercetin at 45 µM had no effect on DNA in the presence of 12 µM Zn2+ (similar to lane 3, Figure 5A, data not shown).
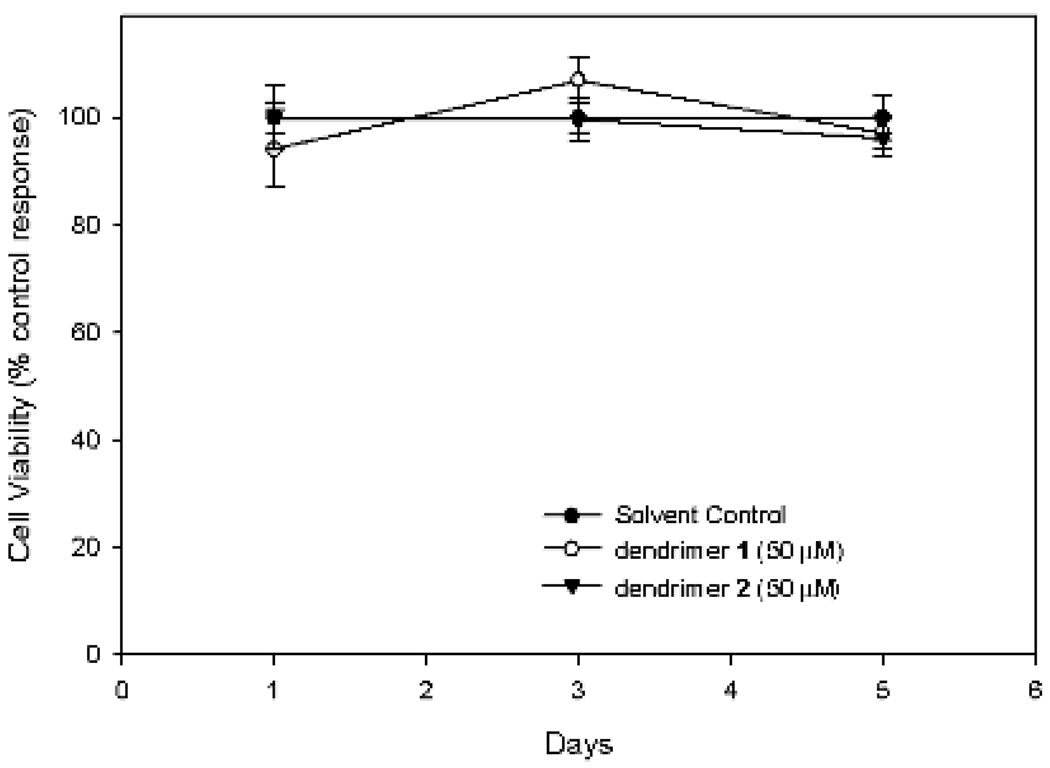
Preliminary data on cell toxicity of dendrimers 1 and 2 were obtained with Chinese hamster ovary (CHO) cells using 3-(4,5-di-methylthizol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) [29]. Incubation of the CHO cells (3 × 105/mL) in the presence of 3.1 – 50 µM of antioxidants did not show any reduction in cell viability over 5 days (Figure 7).
Conclusion
We have successfully demonstrated the concept of designing dendrimers with free radical scavenging as well as metal chelating properties. The antioxidant dendrimers we synthesized show high radical scavenging activity and strong protective effects on biomolecules (lipid, protein and DNA). In general, potent antioxidants also possess strong pro-oxidant activity. The potent TREN-based dendritic antioxidants were however devoid of pro-oxidant activity thus providing a significant biological benefit. Their strong radical scavenging potential and lack of pro-oxidant effects as well as cell toxicity make them promising candidates as therapeutic agents for pathological conditions strongly associated with oxidative stress including asthma, atherosclerosis and neurodegenerative diseases.
Figure 6.
Comparative effects of dendrimers 1 and 2 on CHO cell viability. CHO cells were incubated with DMSO control, dendrimer 1 or dendrimer 2 at 50 µM for 1, 3, and 5 days.
Acknowledgements
We are grateful to the Central Michigan University Office of Research and Sponsored Programs for funding (President’s Research Investment Funds). This work was supported in part by Award Number R15GM087697-01 and R15GM087697-01S1 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
List of abbreviations
- AAPH
2,2'-azobis(2-amidinopropane) dihydrochloride
- CDCl3
Deuterated Chloroform
- CD3COCD3
Deuterated acetone
- CHO-K1
Chinese hamster ovary cells
- DMSO
Dimethylsulfoxide
- DNA
Deoxyribonucleic acid
- DPPH
1-Diphenyl-2-picrylhydrazyl
- LDL
Low-density lipoprotein
- MALDI-TOF
Matrix Assisted Laser Desorption/Ionization-Time of Flight
- MTT
3-(4,5-Di-methylthizol-2-yl)-2,5-diphenyltetrazolium bromide
- Na(OAc)3BH
Sodium triacetoxyborohydride
- n-Bu4NF
Tetra-butylammonium fluoride
- PAGE
Polyacrylamide gel electrophoresis
- PAMAM
poly(amidoamine) dendrimer
- pBR 322
Plasmid DNA
- PBS
Phosphate-buffered saline
- PPI
poly (propylene imine) dendrimer
- Si(CH3)4
Tetramethylsilane
- TBDMS-Cl
Tert-butyldimethylsilyl chloride
- TREN
Tris(2-aminoethyl)amine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–1196. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]; (b) Elangovan V, Sekar N, Govindasamy S. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Lett. 1994;87:107–113. doi: 10.1016/0304-3835(94)90416-2. [DOI] [PubMed] [Google Scholar]; (b) Brown JP. A review of genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat. Res. 1980;75:243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 2.Terao J, Piskula M, Yao Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994;308:278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- 3.Rump AF, Schussler M, Acar D, Cordes A, Ratke R, Theisohn M, Rosen R, Klaus W, Fricke U. Effects of different inotropes with antioxidant properties on acute regional myocardial ischemia in isolated rabbit hearts. Gen. Pharmacol. 1995;26:603–611. doi: 10.1016/0306-3623(94)00209-6. [DOI] [PubMed] [Google Scholar]
- 4.(a) Gil B, Sanz MJ, Terencio MC, Ferrándiz ML, Bustos G, Payá M, Gunasegaran R, Alcaraz MJ. Effects of flavonoids on Naja naja and human recombinant synovial phospholipases A2 and inflammatory responses in mice. Life Sci. 1994;54:333–338. doi: 10.1016/0024-3205(94)90021-3. [DOI] [PubMed] [Google Scholar]; (b) Middleton E, Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 5.Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996;2:659–668. [PubMed] [Google Scholar]
- 6.(a) Rahman A, Fazal F, Greensill J, Ainley K, Parish JH, Hadi SM. Strand scission in DNA induced by dietary flavonoids: role of Cu(I) and oxygen free radicals and biological consequences of scission. Mol. Cell Biochem. 1992;111:3–9. doi: 10.1007/BF00229567. [DOI] [PubMed] [Google Scholar]; (b) Sahu SC, Washington MC. Effects of antioxidants on quercetin-induced nuclear DNA damage and lipid peroxidation. Cancer Lett. 1991;60:259–264. doi: 10.1016/0304-3835(91)90122-x. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita N, Tanemura H, Kawanishi S. Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II) Mutation Res. 1999;425:107–115. doi: 10.1016/s0027-5107(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 8.Rietjens IMCM, Boersma MG, Haan Ld, Spenkelink B, Awad HM, Cnubben NHP, van Zanden JJ, Woude Hvd, Alink GM, Koeman JH. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002;11:321–333. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 9.(a) Rahman A, Shahabuddin HSM, Parish JH, Ainley K. Strand scission in DNA induced by quercetin and. Cu(II): role of Cu(I) and oxygen free radicals. Carcinogenesis. 1989;10:1833–1839. doi: 10.1093/carcin/10.10.1833. [DOI] [PubMed] [Google Scholar]; (b) Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behaviour of flavonoids:structure-activity relationships. Free Radic. Biol. Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]; (c) Lebeau J, Furman C, Bernier JL, Duriez P, Teissier E, Cotelle N. Antioxidant properties of di-tert-butylhydroxylated flavonoids. Free Radic. Biol. Med. 2000;29:900–912. doi: 10.1016/s0891-5849(00)00390-7. [DOI] [PubMed] [Google Scholar]
- 10.(a) Pirvu L, Nichita C, Giurginca M, Meghea A. Correlation structure-antioxidant activity of some polyphenols of vegetal origin. Revista de Chime. 2006;57:699–705. [Google Scholar]; (b) Tripoli E, Guardia ML, Giammanco S, Majo DD, Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007;104:466–479. [Google Scholar]
- 11.(a) Natella F, Nardini M, Felice MD, Scaccini C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure–Activity Relation. J. Agric. Food Chem. 1999;47:1453–1459. doi: 10.1021/jf980737w. [DOI] [PubMed] [Google Scholar]; (b) Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]; (c) Cai YZ, Sun M, Xing J, Luo Q, Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]; (d) Cuvelier ME, Richard H, Berset C. Biosci. Biotech. Biochem. 1992;56:324–325. [Google Scholar]
- 12.(a) Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols:structure-activity relationship. Biosci. Biotech. Biochem. 1992;56:324–325. [Google Scholar]; (b) Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]; (c) Shahidi F, Janitha PK, Wanasundara PD. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 13.De Heer MI, Mulder P, Korth HG, Ingold KU, Lusztyk J. Hydrogen atom abstraction kinetics from intramolecularly hydrogen bonded ubiquinol-0 and other (poly)methoxy phenols. J. Am. Chem. Soc. 2000;122:2355–2360. [Google Scholar]
- 14.(a) Okada Y, Tanaka K, Sato E, Okajima H. Antioxidant activity of the new thiosulfinate derivative, S-benzylphenylmethanethiosulfinate, from Petiveria alliacea L. Org. Biomol. Chem. 2008;6:1097–1102. doi: 10.1039/b715727d. [DOI] [PubMed] [Google Scholar]; (b) Hu C, Yuan YV, Kitts DD. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007;5:2219–2227. doi: 10.1016/j.fct.2007.05.017. [DOI] [PubMed] [Google Scholar]; (c) Mendiara SN, Coronel MEJ. Evidence for the development of persistent carbon-centered radicals from a benzyl phenolic antioxidant: an electron paramagnetic resonance study. Appl. Magn. Reson. 2006;30:103–120. [Google Scholar]; (d) Nishiyama T, Sakita K, Fuchigami T, Fukui T. Antioxidant activities of fused heterocyclic compounds, xanthene-2,7-diols with BHT or catechol skeleton. Polym. Degrad. Stab. 1998;62:529–534. [Google Scholar]
- 15.Lee CY, Sharma A, Cheong JE, Nelson J. Synthesis and antioxidant properties of dendritic polyphenols. Bioorg. Med. Chem. Lett. 2009;19:6326–6330. doi: 10.1016/j.bmcl.2009.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. -Wiss. U.-Technol. 1995;28:25–30. [Google Scholar]
- 17.(a) Balogh L, Tomalia DA. Poly(amidoamine) dendrimer-templated nanocomposites. 1. Synthesis of zerovalent copper nanoclusters. J. Am. Chem. Soc. 1998;120:7355–7356. [Google Scholar]; (b) Niu Y, Crooks RM. Dendrimer-encapsulated metal nanoparticles and their applications to catalysis. C.R. Chimie. 2003;6:1049–1059. [Google Scholar]
- 18.Cregg PJ, editor. A practical guide to supramolecular chemistry. John Wiley and Sons; 2005. pp. 19–21. [Google Scholar]
- 19.Lebeau J, Furman C, Bernier JL, Duriez P, Teissier E, Cotelle N. Antioxidant properties of di-tert-butylhydroxylated flavonoids. Free Radic. Biol. Med. 2000;29:900–912. doi: 10.1016/s0891-5849(00)00390-7. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. J. Org. Chem. 1996;61:3849–3862. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 21.Sharma A, Desai A, Ali R, Tomalia D. Polyacrylamide gel electrophoresis separation and detection of polyamidoamine dendrimers possessing various cores and terminal groups. J. Chromatography A. 2005;1081:238–244. doi: 10.1016/j.chroma.2005.05.074. [DOI] [PubMed] [Google Scholar]
- 22.Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans CA. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 23.All solutions were made in ultra-pure water.
- 24.Foxx KK, Roberts RL, Waxdal MJ. Kalen Biomedical Technical Note 2: Effect of copper ion concentration on the oxidation of human LDL. [Accessed 2009 July 10]; Available: http://www.kalenbiomed.com/50tech_info.php via the INTERNET. [Google Scholar]
- 25.Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicol. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita N, Tanemura H, Kawanishi S. Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II) Mutat. Res. 1999;425:107–115. doi: 10.1016/s0027-5107(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 27.Babaeva EE, Vorobyova UA, Zharkova MS, Cheknyov SB. Human serum γ-globulin binds copper cations. Bull. Exp. Biol. Med. 2006;141:59–62. doi: 10.1007/s10517-006-0092-5. [DOI] [PubMed] [Google Scholar]
- 28.Drouin R, Rodriquez H, Gao SW, Gebreyes Z, O'Connor TR, Holmquist GP. Cupric ion/ascorbate/hydrogen peroxide-induced DNA damage: DNA-bound copper ion primarily induces base modifications. Free Radic. Biol. Med. 1996;21:261–273. doi: 10.1016/0891-5849(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 29.Pestka JJ, Uzarski RL, Islam Z. Induction of apoptosis and cytokine production in the Jurkat human T cells by deoxynivalenol: Role of mitogen-activated protein kinases and comparison to other 8-ketotrichothecenes. Toxicol. 2005;206:207–219. doi: 10.1016/j.tox.2004.08.020. [DOI] [PubMed] [Google Scholar]