Abstract
Prostate cancer (PC) is now the second most prevalent cause of death in men in the USA and Europe. At present, the major treatment options include surgical or medical castration. These strategies cause ablation of the production of testosterone (T), dihydrotestosterone (DHT) and related androgens by the testes. However, because these procedures do not affect adrenal, prostate and other tissues androgen production, they are often combined with androgen receptor antagonists to block their action. Indeed, recent studies have unequivocally established that in castration-resistant prostate cancer (CRPC) many androgen-regulated genes become re-expressed and tissue androgen levels increase despite low serum levels.
Clearly, inhibition of the key enzyme which catalyzes the biosynthesis of androgens from pregnane precursors, 17α-hydroxy/17,20-lyase (hereafter referred to as CYP17) could prevent androgen production from all sources. Thus, total ablation of androgen production by potent CYP17 inhibitors may provide effective treatment of prostate cancer patients. This review highlights the role of androgen biosynthesis in the progression of prostate cancer and the impact of CYP17 inhibitors, such as ketoconazole, abiraterone acetate, VN/124-1 (TOK-001) and TAK-700 in the clinic and in clinical development.
1. Introduction
The dependence of prostate cancer (PC) on androgen signaling has been recognized for approximately 70 years, yet PC remains a leading cause of male death in the Western world. In the USA alone 192,280 new cases and 27,360 PC related deaths were estimated for 2009 [1]. Improvements in the methodology and timing of PC screening have enabled the detection of PC tumors at an early stage, when the disease may be cured through surgical excision or radiotherapy. However, despite the positive treatment outcomes possible for localized PC, advanced stage disease presents a much poorer prognosis.
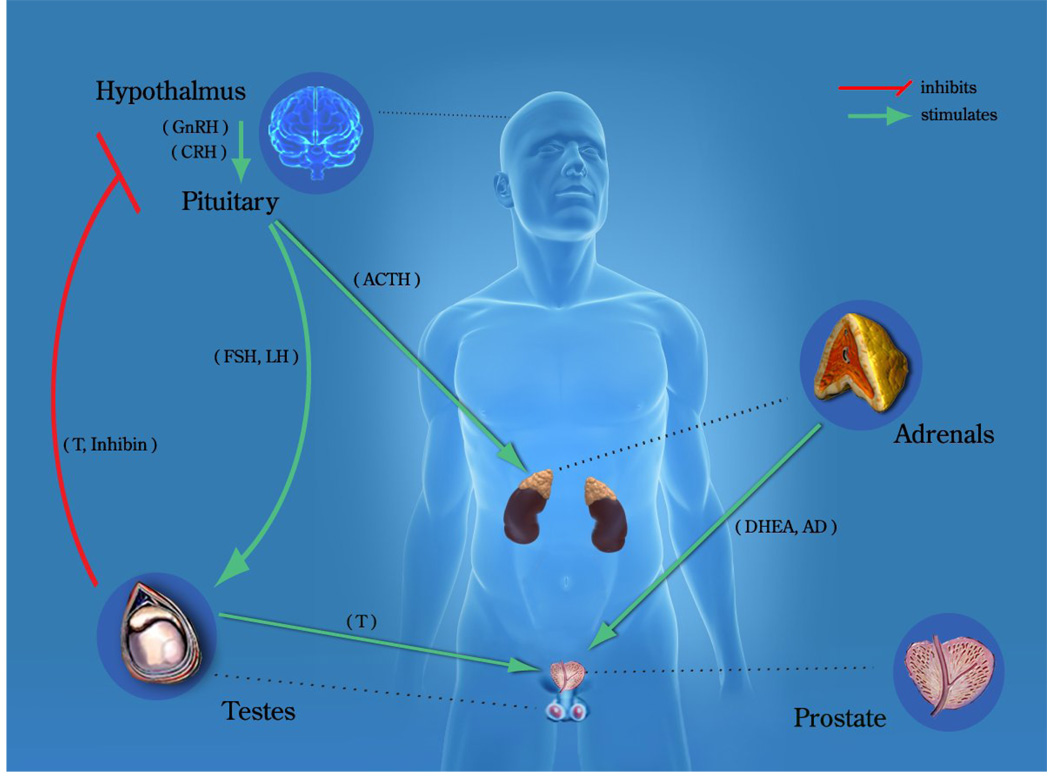
Prostate cancer growth and progression is stimulated by androgens acting through the androgen receptor (AR). Androgen levels are predominately regulated through the hypothalamic-pituitary-adrenal/gonadal axis (Figure 1). The majority of androgens are synthesized in the testis and adrenal glands from pregnane precursors. The testes are the primary source of testosterone in men, contributing approximately 90–95% of the circulating androgens [2, 3]. The adrenal glands are responsible for the remaining androgens, which are enzymatically converted to testosterone and DHT in the prostate and peripheral tissues. Of the two major androgens, testosterone (T) and dihydrotestosterone (DHT), T is the most abundant in systemic circulation and DHT has the highest affinity for the AR.
Figure 1. Endocrine control of prostatic growth.
The growth and development of the normal prostate requires a functioning androgen signaling pathway, which is regulated by the hypothalamic–pituitary–adrenal/gonadal axis. Androgens [testosterone (T), androstenedione (AD), dehydroepiandrosterone (DHEA)] and other steroids are synthesized in the testes or adrenal glands and released into the circulation in response to specific hormonal signals [follicle stimulating hormone (FSH), gonadotropin releasing hormone (GnRH), luteinizing hormone (LH), luteinizing hormone releasing hormone (LHRH)]. Testosterone is transported by steroid hormone binding globulin (SHBG) to the prostate, where it is predominantly converted by 5α-reductase to its more active metabolite, 5α-dihydrotestosterone (DHT). The adrenals are stimulated to produce AD and DHEA by adrenocorticotropic hormone, released by the pituitary.
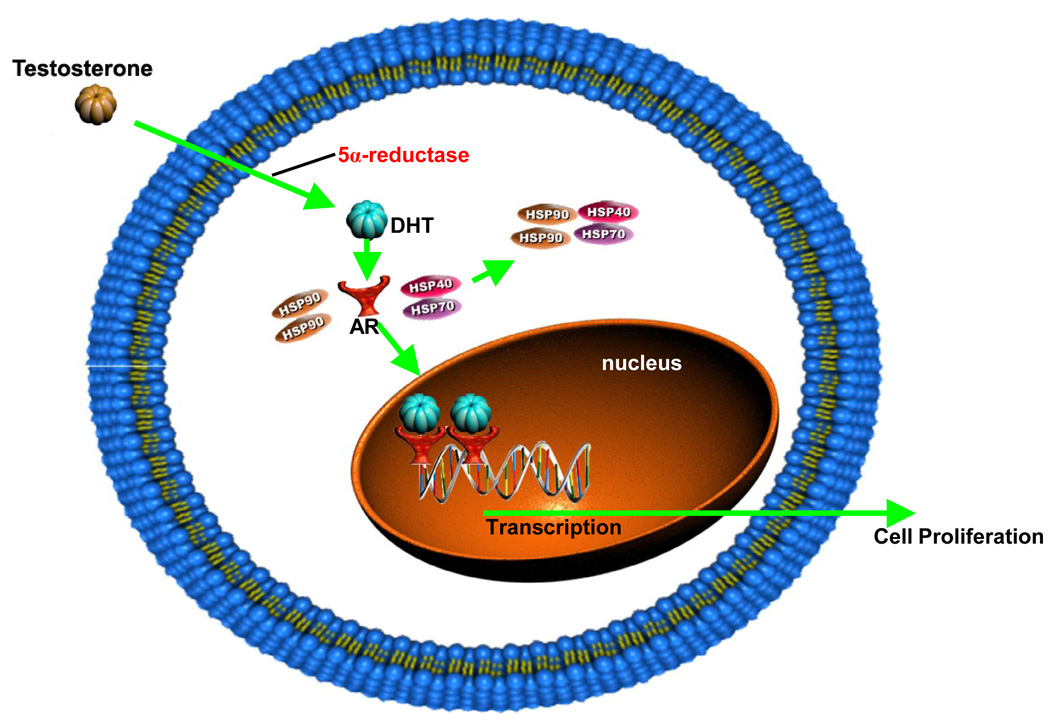
The inactive AR is primarily found in the cytoplasm bound to heat shock proteins [4]. Androgen binding to the AR induces conformational changes in the ligand-binding domain and heat-shock protein dissociation from the AR (Figure 2). The transformed AR undergoes dimerization, phosphorylation and translocation to the nucleus [5]. The translocated receptor dimer binds to androgen response elements located in the promoter or enhancer region of AR target genes [6], leading to the transactivation of AR-regulated gene expression and cell proliferation [7]. AR signaling mediates a range of physiological responses in addition to prostate growth and differentiation, including development and maintenance of the male phenotype. Conversely, androgen deprivation induces apoptosis in the prostate [8]. The dependence of prostate cells on AR signaling persists after neoplastic transformation, and thus forms the basis of metastatic PC therapy.
Figure 2. Mechanism of androgen (DHT) action.
Testosterone enters the cell and is converted to DHT by the enzyme 5α-reductase. DHT binding to the AR induces conformational changes in the ligand-binding domain and causes heat shock protein dissociation from the AR. The transformed AR undergoes dimerization, phosphorylation and translocation to the nucleus. The translocated receptor dimer binds to androgen response elements in the DNA, thereby activating transcription of AR target genes and ultimately leading to cell proliferation.
The mainstay treatment of advanced PC is androgen-deprivation therapy (ADT), alone or in combination with an anti-androgen, which results in temporary disease regression or stabilization in the majority of patients. Androgen deprivation therapy is frequently accomplished through chemical castration with leutinizing–hormone releasing–hormone (LHRH) analogs, or surgical castration (orchiectomy). ADT typically results in a 90% or greater reduction of serum testosterone (T) [9, 10], and clinical response is observed in 80–90% of patients [11]. However, ADT does not affect adrenal or intra-tumoral androgen production, which may be clinically relevant in disease recurrence. Unfortunately, treatment of advanced stage PC nearly always fails, with recurrent castration-resistant tumors typically developing after 18–24 months. Recurrence is marked by increases in serum prostate specific antigen (PSA) levels and the manifestation of disease symptoms.
Previously, the failure of ADT was suspected to evidence an escape from androgen dependence. Castrate serum testosterone levels were classically defined as 50 ng/dl, and surgical bilateral orchiectomy reduces serum T levels to below 20 ng/dl. Chemical castration via LHRH agonists similarly reduces serum T [12–14], although it has been reported that up to 13% of men on depot LHRH agonists will have testosterone values between 20 and 50 ng/dl [13]. In light of the low serum T, recurrent disease under castrate conditions was thought to be driven by adaptive mechanisms distinct from androgen receptor signaling; therefore it was deemed to be androgen-independent. However, despite the drastic reduction of circulating T, it has been shown that serum testosterone levels do not directly correlate with equivalent changes in intra-prostatic androgen levels [15]. In the mid 1980’s, Geller et al. reported that castration failed to completely eliminate intra-prostatic androgens in men with PC [16]. This important finding has been revisited, with accumulating evidence that total body and intra-prostatic androgen levels remain significantly high after castration. Through measurement of the serum levels of glucuronide metabolites of androgens, Labrie et al. demonstrated that 40% of the total body androgen pool remains after castration as compared to intact men [9]. Relevant studies by Mohler [3], Nishiyama [15], and Mohstaghel [17] have further supported the role and mechanisms of persistent intra-prostatic androgens in PC, and are summarized in an excellent review by Marks et al. [18]. This suggests that some PC recurrence is not resultant of true androgen independence but rather adaptations that allow continued response under low levels of circulating androgens. Furthermore, advances in the understanding of the mechanisms of castration resistance have confirmed continued activity of the androgen signaling pathway, as the AR has been found to be active and often overexpressed in CRPC [10, 19, 20]. Additional mechanisms that may mediate continued AR signaling include AR mutation, increased expression of transcriptional co-activator proteins, and activation of other signal transduction pathways [21, 22]. These observations clearly indicate that the goal of complete androgen abrogation has not been achieved with current PC therapeutic strategies, and highlights the potential of improved inhibitors of androgen signaling. This review covers the historical development, current state, and future outlook of inhibitors of 17α-hydroxylase/17,20-lyase (CYP17), a multifunctional and critical enzyme in the pathway of androgen biosynthesis, in regard to PC therapy.
2. CYP17, a key enzyme in steroid biosynthesis
The multifunctional 17α-hydroxylase/17,20-lyase is a cytochrome P450 enzyme localized to the endoplasmic reticulum in the adrenals, testes, placenta and ovaries, and lies at the crossraods of sex steroid and glucocorticoid synthesis. It is encoded for by a single gene found on chromosome 10 in humans [23]. The 1527 bp CYP17 cDNA encodes a protein consisting of 508 amino acids, containing a heme prosthetic group at the active site, with an iron-oxygen species required for catalytic activity. The enzyme possesses both 17α-hydroxylase activity and C17,20-lyase activity, and requires P450 reductase to transfer electrons in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) for both catalytic activities. The degree of C17,20-lyase activity, modulated by cytochrome b5 (b5), determines which metabolic pathway the substrate will follow in terms of sex steroid or glucocorticoid formation [24–26]. Cytochrome b5 is required for the C17,20 lyase activity, and high b5/CYP17 ratios are associated with the nearly exclusive production of androgens in the testes as opposed to the low ratios observed in the adrenals where glucocorticoids are produced [25–27]. It should be noted that the role of b5 in CYP17 actions is described in detail in this volume by Akhtar et al.
2.1. CYP17 in androgen biosynthesis
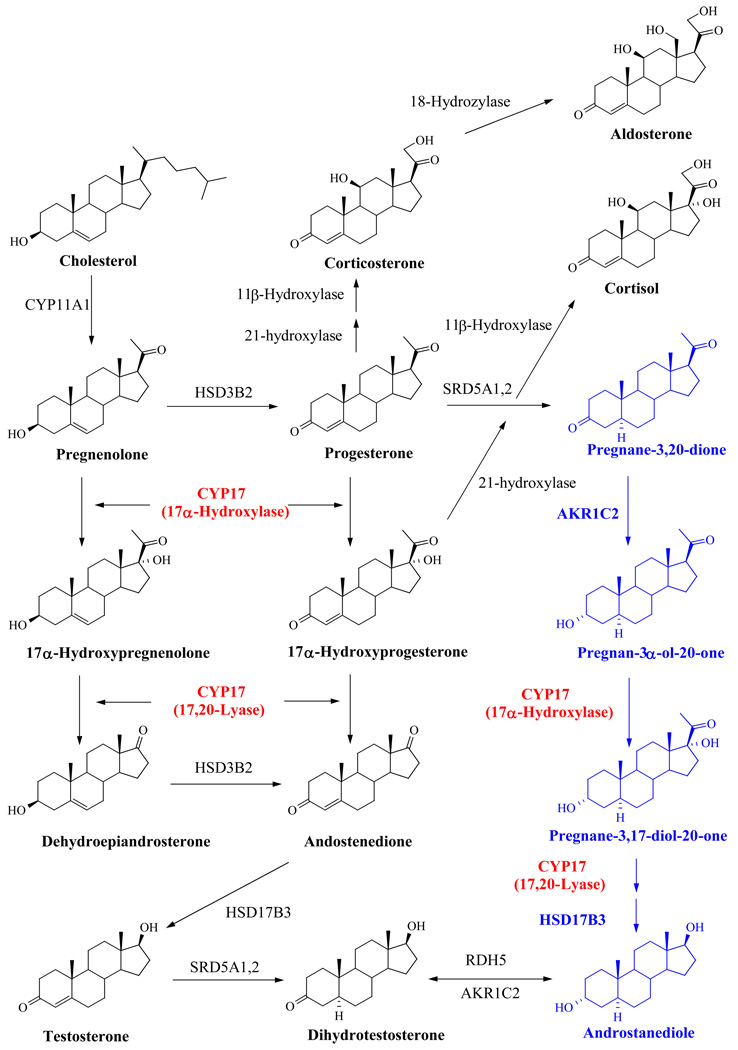
The formation of steroid hormones proceeds via the hypothalamic-pituitary-gonadal/adrenal axis, with the testes and adrenal cortex producing the majority of androgenic steroids in men. All steroid hormone synthesis follows the conversion of cholesterol to pregnenolone, which can subsequently progress down the androgen formation pathway, or be converted to progesterone by 3β-hydroxysteroid dehydrogenase (Figure 3). CYP17 catalyzes two key reactions involved in the production of sex steroids, which occur sequentially - the 17α-hydroxylase activity typically converts pregnenolone to 17α-hydroxypregnenolone and progesterone to 17α-hydroxyprogesterone, while the C17,20-lyase activity converts 17α-hydroxypregnenolone to DHEA (Δ5 pathway) and 17α–hydroxyprogesterone to androstenedione (Δ4 pathway) [28, 29]. CYP17 activity is species specific, and in comparison with the enzyme from pig [30], rat [30], and trout [31], the human form of CYP17 has a significantly lower affinity for 17α-hydroxyprogesterone than 17α-hydroxypregnenolone [30]. Therefore, the metabolic route of T formation in humans favors pregnenolone as the starting precursor rather than progesterone [28]. The precursor androgens DHEA and androstenedione may be subsequently transformed to T by other enzymes. T can then be converted to the more potent DHT by 5a-reductase in target tissue such as the prostate (Figure 3). Recently, an additional “backdoor pathway” has been described by Auchus et al. [32] in which DHT synthesis bypasses testosterone as an intermediate (Figure 3). It should be noted that CYP17 is still active in the backdoor pathway, and therefore emphasizes its importance as a therapeutic target.
Figure 3. Pathway of Steroid Biosynthesis.
2.2. CYP17 in glucocorticoid synthesis
In addition to catalyzing androgen biosynthesis, CYP17 is also important in glucocorticoid production, with 17α-hydroxypregnenolone and 17α-hydroxyprogesterone serving as precursors for cortisol synthesis. Therefore, treatment with inhibitors affecting the 17α-hydroxylase activity requires the additional management of the resultant change in glucocorticoid/mineralocorticoid levels. ACTH secretion, normally inhibited by cortisol through a negative feedback mechanism, drives the production of cortisol in the adrenals by promoting the conversion of cholesterol to pregnenolone. Consequently, suppression of cortisol and its precursors via inhibition of CYP17 results in a compensatory increase in ACTH [33]. Corticosterone, a weaker glucocorticoid upstream of CYP17, is not directly reduced by CYP17 inhibition, and may also inhibit ACTH secretion [34]. However, much higher corticosterone levels are necessary for this effect to occur. The increase in ACTH due to loss of cortisol modulated negative feedback may therefore result in a new homeostatic set point with increased levels of corticosterone and its precursors. This may lead to hypertension and other symptoms of mineralocorticoid excess such as hypokalemia and fluid retention [35]. This effect was predicted, as a similar condition has been observed in rare cases of congenital CYP17 deficiencies which result in congenital adrenal hyperplasia with impaired adrenal and gonadal steroidogenesis [36]. Management of these adverse effects is often achieved through concomitant administration of prednisone, hydrocortisone or dexamethasone. Ryan et al. noted that the addition of dexamethasone to abiraterone treatment resulted in orthostatic hypotension in two patients, which was not seen in patients on prednisone or hydrocortisone [37], and therefore recommended limiting dexamethasone usage in this context in favor of prednisone or hydrocortisone. Additionally, glucocorticoids have been shown to possess anti-PC activity, and therefore concomitant administration limits the ability to quantify the effectiveness of CYP17 inhibition alone.
2.3. Additional activities of CYP17
It is important to note that CYP17 has been shown to have additional properties, namely metabolizing xenobiotics and catalyzing the formation of a third class of active steroids, the 16-ene steroids. A growing body of research has focused on the role of 16-ene steroids in pigs, in which androstenol was identified as a pheromone [38]. In humans, androstenol was proposed to be involved in behavioral effects, including reducing tension, nervousness and other negative emotional states in women [39]. 5α-16-androsten-3α-ol has also been shown to modulate the activity of both the pregnane-X-receptor (PXR) and the constitutive androstane receptor (CAR) [40]. However, the overall functional effect of the 16-ene steroids in normal physiology is still poorly understood. In regards to xenobiotic metabolism, aminopyrine-N-demethylation activity has been observed with both human and porcine CYP17 [41, 42]. The ability to metabolize xenobiotics could result in inactivation or bioactivation of therapeutics and procarcinogens, as well as the possibility of CYP17 inactivation due to xenobiotic/substrate competition.
3. CYP17 inhibitors
As the critical catalytic step in all androgen biosynthesis, therapeutic CYP17 inhibition to treat PC and other androgen dependent diseases has been envisioned for over 50 years [43–45], with recent discoveries in PC pathology driving much of the current refocus on CYP17 as a therapeutic target. Additionally, some evidence suggests that CYP17 mRNA and protein expression correlate with disease stage and relapse [46, 47]. Despite the strong rational for targeting CYP17, this target has been largely unexploited with relatively few inhibitors progressing to clinical trials and only ketoconazole, an unspecific inhibitor of CYP17, in widespread clinical use.
Due to the unavailability of a 3D crystal structure of the enzyme, inhibitor design has been based on information gleaned utilizing various strategies including docking and molecular modeling techniques, site directed mutagenesis, comparative interspecies studies, and trial and error modifications of the enzyme’s natural substrates. Generally, CYP17 inhibitors have been structurally categorized as steroidal or non-steroidal. The steroidal inhibitors are similar in structure to the natural substrates of CYP17, pregnenolone or progesterone, and often involve modification of the substrates D-ring at the C17 position. Classification of steroidal CYP17 inhibitors can be further divided into: Type I competitive inhibitors, Type II competitive inhibitors, mechanism-based inhibitors, and affinity labeling agents.
The Type I and Type II classifications are designated based on the binding of the inhibitor to the active site of the enzyme. Type I inhibitors displace water as the sixth ligand of the Fe heme, allowing the Fe to exist in a pentacoordinate state. This induces a shift in the UV–absorption spectrum Soret band maximum from approximately 420 nm to approximately 390 nm [48–50]. Type II competitive inhibitors interact as the sixth ligand with the heme atom and in some instances with amino acid residues in close proximity to the heme site. These compounds produce a Type II difference spectrum with a Soret maximum at 421 to 430 nm corresponding to a hexacoordinate Fe [51, 52]. The most commonly studied Type II inhibitors contain a nitrogen heteroatom.
It is difficult to directly compare the potency of inhibitors from different studies due to variations in evaluation and assay procedures [53], and we continue to advocate for the inclusion of data of known potent CYP17 inhibitors for comparison. However, a few considerations must be noted. First, some compounds show variations in potency in a species dependent manner [54]. Additionally the concentration and Km of the substrate used should also be considered when comparing IC50 values and Ki values, respectively.
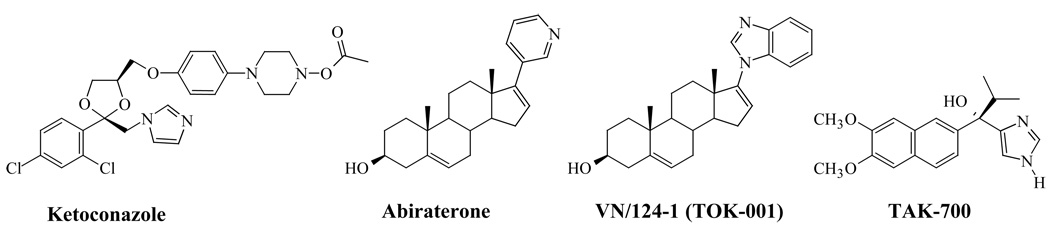
A large number of CYP17 inhibiting compounds have been developed as potential PC therapy over the years, and have been extensively reviewed elsewhere [53, 55–57]. However, in recent years, possibly resultant of the high expectations surrounding abiraterone, we have seen a reemergence in CYP17 inhibitor interest. The structures of CYP17 inhibitors that are discussed in this review, including ketoconazole, abiraterone, VN/124-1 (TOK-001) and TAK-700 are presented in Figure 4.
Figure 4. Chemical Structures of CYP17 Inhibitors in the Clinic and Clinical Trials.
4. CYP17 inhibitors in clinical use and clinical trials
4.1. Ketoconazole
Ketoconazole (Figure 4) is a broad spectrum anti-fungal agent that has been extensively used off-label as second-line hormonal therapy for PC. It was shown to induce gynecomastia in patients [58, 59] in the early 1980’s, which eventually lead to the discovery that ketoconazole is an inhibitor of testicular and adrenal androgen synthesis [60, 61]. Ketoconazole inhibits 11β-hydroxylation, cholesterol side chain cleavage to pregnenolone and CYP17 [62, 63]. Ketoconazole has typically been used in high dosage (800–1200 mg/d) for PC treatment. However, high dose ketoconazole (HDK) has been associated with significant potential side effects including hepatotoxicity, gastrointestinal toxicity, and adrenal insufficiency. Concomitant corticosteroid replacement is required with ketoconazole treatment. Furthermore, the inhibition of cytochrome P450 enzymes creates the possibility of drug-drug interactions.
Despite the aforementioned limitations ketoconazole remains widely used as secondary hormonal therapy for CRPC, and multiple trials have been undertaken to assess its efficacy in this context. Two single center trials in on the use of HDK in CRPC found PSA declines > 50% in 55% (11/20) [64] and 63% (30/48) of patients [65]. A larger phase III study of HDK therapy in 260 patients with post-ADT metastatic PC on anti-androgen withdrawal (AAWD) demonstrated a PSA decline > 50% in 27% of patients treated with HDK plus AAWD. Overall survival was not different between the treatment groups, however, those patients with a > 50% PSA decline had a median survival of 41 months compared to 13 months for those without a PSA decline. Time to PSA progression in PSA responders was 5.9 vs 8.6 months in AAWD alone and AAWD + HDK groups, respectively [66]. Androstenedione, DHEA, and DHEAS levels decreased with HDK therapy. However, there was no change in testosterone level from baseline in either treatment group. All three adrenal androgen levels rose at disease progression, indicating an escape from HDK induced androgen suppression and highlighting the potential for more effective agents. A follow-up study [62] reported that response to HDK was associated with baseline adrenal androgen levels, suggesting that higher patient androstenedione levels predict improved survival and response to ketoconazole treatment.
In an effort to reduce adverse events associated with HDK, ketoconazole has been tested at a lower dosage of 600 mg/d (LDK). PSA Response rates have been generally equivalent to HDK. Ngo et al. [67] observed a 32% (12/32). In a retrospective analysis of 138 patients treated with LDK [68], 23% of patients (39/138) experienced PSA declines ≥ 50%. Treatment was subsequently escalated to HDK in 55 patients, 12.7% (7/55) of which demonstrated a subsequent PSA decline ≥ 50%.
Multidrug therapy combining ketoconazole with chemotherapeutic agents has been of interest for some time. In addition to blocking CYP17 activity, ketoconazole also inhibits other important metabolizing enzymes such as CYP3A and CYP24A1, suggesting that concomitant ketoconazole administration may alter drug exposure or pharmacokinetic variability [69–71]. This necessitates careful monitoring for increased side effects due to inhibition based drug-drug interaction, but may reduce the amount of therapeutic agent required for optimal treatment. In a recent phase I study of 42 patients treated with docetaxel and ketoconazole, PSA decreases of 50% or greater were observed in 62% of patients. Docetaxel naïve patients had a median overall survival of 36.8 months on a combined regimen of docetaxel and 600 mg ketoconazole [72]. Docetaxel exposure was increased 1.3 to 1.5 fold due to concomitant ketoconazole administration. In addition to evaluating ketoconazole combined with traditional chemotherapeutics, CYP17 inhibitors in combination with other steroid biosynthesis enzyme inhibitors are being examined. A study by Taplin et al. [73] investigated the effect of ketoconazole combined with hydrocortisone and the 5α-reductase inhibitor dutasteride in asymptomatic castration-resistant PC. As compared to previous investigations of ketoconazole+hydrocortisone therapy alone, this combination resulted in an increased duration of response with an overall median time to PSA progression of 14.5 months as compared to the 8.6 months reported in the largest previous study that analyzed ketoconazole+hydrocortisone given concurrently or subsequent to antiandrogen withdrawl [66]. These results warrant the investigation of stronger CYP17 inhibitors as components of multidrug therapy.
4.2. Abiraterone Acetate
Abiraterone (Figure 4), a highly selective irreversible CYP17 inhibitor, was developed as a mechanism-based steroidal inhibitor of CYP17 following observations that nonsteroidal 3-pyridyl esters had improved selectivity for inhibition [74]. The concentration of abiraterone required to reduce CYP17 activity by 50% in human microsomes is 1/10 that of ketoconazole [75, 76]. Furthermore, abiraterone has been shown to reduce serum testosterone levels to below a detection threshold of 1 ng/dL [75, 77]. As would be expected, abiraterone also decreases estradiol, dehydroepiandrosterone, and androstenedione. Due to abiraterone’s poor bioavailability, a 3β-acetate prodrug (abiraterone acetate) that is rapidly deacetylated to the active metabolite in vivo was synthesized [78, 79].
Promising results from clinical trials of AA in CRPC patients have recently been reported. In a phase I trial of abiraterone acetate treatment of both ketoconazole pre-treated and ketoconazole naïve CRPC patients [37], PSA declines of ≥ 50% were seen in 18 (55%) of 33 patients, including nine (47%) of 19 patients with prior ketoconazole therapy and nine (64%) of 14 patients without prior ketoconazole therapy. Significantly, the antitumor activity was nearly equivalent in both populations. The activity observed in castrate, ketoconazole naïve patients confirms that abiraterone acetate is an active agent, whereas the activity in ketoconazole pre-treated patients implies that a more selective and potent inhibitor of CYP17 may be an improvement beyond ketoconazole, or an additional sequential therapeutic option. The most common adverse events in patients treated with abiraterone acetate were fatigue, hypertension, headache, nausea, and diarrhea. As predicted, the levels of mineralocorticoids upstream of CYP17 were increased along with a decrease in cortisol. Management of excessive mineralocorticoids was achieved through administration of exogenous corticosteroids such as hydrocortisone or prednisone. The highest evaluated dosage, 1,000 mg/d, did not create dose limiting toxicities and was also used in additional trials [37, 75, 80, 81].
In addition to chemotherapy-naïve patients, a multicenter phase II study evaluated the efficacy of abiraterone in patients with docetaxel-treated CRPC [81]. All patients were treated with 1,000 mg/d. Forty seven patients were enrolled, and treatment resulted in observed PSA declines ≥ 50% in 51% (24/47) of patients at least once, whereas 68% (32/47) and 15% (7/47) had PSA declines ≥ 30% and ≥ 90%, respectively. Partial responses (by RECIST criteria) were reported in 27% (8/30) patients with measurable disease. Decreases in circulating tumor cell (CTC) counts were also observed [81].
Another phase II trial was conducted to evaluate the safety and efficacy of abiraterone acetate in combination with prednisone to reduce the symptoms of secondary hyperaldosteronism associated with abiraterone acetate monotherapy [80]. The subject population consisted of 58 CRPC patients who had progressed during chemotherapy. All subjects had undergone hormonal therapy including antiandrogens (91%), ketoconazole (47%) and estrogens (16%). Additionally, all subjects previously experienced failure on docetaxel-based chemotherapy, with 24% having undergone secondary chemotherapy. The addition of prednisone to the treatment regimen resulted in significant decreases in symptoms related to hyperaldosteronism including lower incidence of hypokalemia (5% vs 55%), hypertension (5% vs 17%), and fluid retention (10% vs 15%) relative to the aforementioned study of abiraterone acetate without prednisone [81]. Abiraterone showed activity similar to previous studies, with observed PSA declines ≥ 50% in 36% (22/58) of patients, and CTC conversion from ≥ 5 to < 5 in 34% (10/29) patients. Interestingly, patients pretreated with ketoconazole experienced PSA progressed in 99 days, as opposed to a 198 day time to progression observed in ketoconazole-naïve patients. The possibility of cross resistance is planned to be addressed in a future trial [80].
Two Phase III clinical trials of abiraterone acetate are now in progress. The first of these trials is designed to evaluate abiraterone + prednisone against a placebo + prednisone in patients with progressive CRPC after docetaxel chemotherapy. This trial has an estimated study completion date of June 2011 [82]. The second study will evaluate abiraterone + prednisone against a placebo + prednisone in CRPC patients prior to chemotherapy ([83]). The estimated study completion date is in 2014. Both trials list prior ketoconazole treatment in their exclusion criteria.
4.3. VN/124-1 (TOK-001)
VN/124-1 (3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene, now called TOK-001) (Figure 4) was rationally designed as an inhibitor of androgen biosynthesis via inhibition of CYP17. VN/124-1 is one of a series of novel Δ16-17-azolyl steroids which unlike previously known 17-heteroaryl steroids, the azole moiety is attached to the steroid nucleus at C-17 via a nitrogen of the azole. Utilizing intact CYP17 expressing E. coli, VN/124-1 was shown to be a potent inhibitor of the enzyme with an IC50 value of 300 nM, being 6-fold less potent that our previously reported VN/85-1 (IC50 = 50 nM). Under the same assay conditions, abiraterone had an IC50 value of 800 nM. However, VN/124-1 was subsequently found to disrupt androgen signaling through multiple targets [84, 85]. The synthesis of VN/124-1 has previously been reported [84] and a facile and large scale preparation (commercial process) of the compound has recently been developed but is yet to appear in the literature.
The increased efficacy of VN/124-1 in several prostate cancer models both in vitro and in vivo is believed to arise from its ability to downregulate the AR as well as competitively block androgen binding. In competitive binding studies against the synthetic androgen [3H]R1881, VN/124-1 was equipotent to bicalutamide in LNCaP cells, but had a slightly higher affinity for the wild-type receptor in PC3-AR cells. Transcriptional activation assays utilizing a luciferase reporter showed VN/124-1 to be a pure AR antagonist of the wild-type AR and the T877A mutation found in LNCaP cells [79, 80]. In prostate cancer cell lines, VN/124-1 inhibited the growth of CRPCs, which had increased AR and were no longer sensitive to bicalutamide. In addition, VN/124-1 demonstrated superior synergy for growth inhibition in combination with everolimus or gefitinib compared with bicalutamide [86].
VN/124-1 (0.13 mmol/kg twice daily) caused a 93.8% reduction (P = 0.00065) in the mean final LAPC-4 xenograft volume compared with controls, and this efficacy was significantly more effective than castration or VN/85-1 [79]. In another anti-tumor efficacy study, treatment of VN/124-1 (0.13 mmol twice daily) was very effective in preventing the formation of LAPC4 tumors (6.94 versus 2410.28 mm3 in the control group). VN/124-1 (0.13 mmol/kg twice daily) and VN/124-1 (0.13 mmol/kg twice daily) + castration induced regression of LAPC4 tumor xenografts by 26.55 and 60.67%, respectively [85]. On the basis of these impressive preclinical data, VN/124-1 is currently in further preclinical and clinical development by Tokai Pharmaceutical Cambridge, Mass. Indeed, Tokai Pharmaceuticals recently (November 5, 2009) initiated ARMOR1 (Androgen Receptor Modulation Optimized for Response 1) phase 1/2 trials in castrate resistant prostate cancer patients. We eagerly await the trial findings.
4.4. TAK-700
TAK-700 [(1S)-1-(6,7-dimethoxy-2-naphthyl)-1-(1H-imidazol-4-yl)-2-methylpropan-1-ol] (Figure 4) is a non-steroidal imidazole CYP17 inhibitor (IC50 = 28 nM) currently in clinical trials in prostate cancer patients [87–89]. The compound was found to be selective for CYP17 over 11β-hydroxylase by an impressive 260-fold, and exhibited suppressive effects on testosterone biosynthesis in rats and reduction in the weight of prostate and seminal vesicles in the rat. TAK-700 was also capable of reducing serum testosterone levels down to castration level after 8 hours following a single oral administration of 1 mg/kg to monkeys [90]. A practical asymmetric synthesis of TAK-700 has been established in eight steps from 2,3-dihydroxynaphthalene [91].
5. Structure Activity Relationship
A close look at the structures of CYP17 inhibitors with promising outlook, including ketoconazole, abiraterone, VN/124-1 and TAK-700 suggest that compounds that are capable of forming coordination to the heme-iron of CYP17 (type-II competitive inhibitors) may be superior to other types of inhibitors. This also seems to be true for other CYP inhibiting drugs such as antifungal agents and aromatase inhibitors.
6. Current usage and future development
The quest for more potent inhibitors of CYP17 continues with many new compounds in preclinical development. As of date, however, ketoconazole remains the only approved CYP17 inhibitor in clinical use, with abiraterone acetate, TAK-700, and the multi-mechanistic VN/124-1 in clinical trials. Although usage of ketoconazole was limited by toxicities, it remains one of the most active second line hormonal therapies for CRPC, and recent studies have investigated its usage in lower dosage and/or in combination with other PC treatment regimens. Overall, the CYP17 enzyme remains a largely untapped target with a growing body of data supporting the development of further novel inhibitors with potential benefit as single, sequential or concomitant PC therapy.
Acknowledgment
Part of this work was supported by NIH grants R21 CA11799-01 and start-up funds from Thomas Jefferson University (Njar, VCO). Vasaitis, TS was supported in part by US National Institutes of Health training grant T32 AG000219.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society Facts and Figures 2009. 2009. [Google Scholar]
- 2.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Seminars in surgical oncology. 2000;18(1):52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 4.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 5.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 6.Velasco AM, Gillis KA, Li Y, Brown EL, Sadler TM, Achilleos M, Greenberger LM, Frost P, Bai W, Zhang Y. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology. 2004;145(8):3913–3924. doi: 10.1210/en.2004-0311. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs JT. Role of androgens in prostatic cancer. Vitam Horm. 1994;49:433–502. doi: 10.1016/s0083-6729(08)61152-8. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee B. The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer. Mol Cell Biochem. 2003;253(1–2):89–101. doi: 10.1023/a:1026057402945. [DOI] [PubMed] [Google Scholar]
- 9.Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Belanger A, Vandenput L, Mellstrom D, Ohlsson C. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol. 2009;113(1–2):52–56. doi: 10.1016/j.jsbmb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Okihara K, Miyake H, Fujisawa M, Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, Ozono S, Kumon H, Ichikawa T, Miki T. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol. 2008;180(3):921–927. doi: 10.1016/j.juro.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 12.Morote J, Orsola A, Planas J, Trilla E, Raventos CX, Cecchini L, Catalan R. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178(4 Pt 1):1290–1295. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 13.Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56(6):1021–1024. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 14.Tammela T. Endocrine treatment of prostate cancer. J Steroid Biochem Mol Biol. 2004;92(4):287–295. doi: 10.1016/j.jsbmb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10(21):7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 16.Geller J, Albert JD, Nachtsheim DA, Loza D. Comparison of prostatic cancer tissue dihydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132(4):693–696. doi: 10.1016/s0022-5347(17)49829-6. [DOI] [PubMed] [Google Scholar]
- 17.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, Bremner WJ, Gleave ME, Nelson PS. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 18.Marks LS, Mostaghel EA, Nelson PS. Prostate tissue androgens: history and current clinical relevance. Urology. 2008;72(2):247–254. doi: 10.1016/j.urology.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22(14):2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 21.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 22.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351(15):1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 23.Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA (Mary Ann Liebert, Inc. 1987;6(5):439–448. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar MK, Kelly SL, Kaderbhai MA. Cytochrome b(5) modulation of 17{alpha} hydroxylase and 17–20 lyase (CYP17) activities in steroidogenesis. J Endocrinol. 2005;187(2):267–274. doi: 10.1677/joe.1.06375. [DOI] [PubMed] [Google Scholar]
- 25.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Archives of biochemistry and biophysics. 1995;317(2):343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 26.Lee-Robichaud P, Shyadehi AZ, Wright JN, Akhtar ME, Akhtar M. Mechanistic kinship between hydroxylation and desaturation reactions: acyl-carbon bond cleavage promoted by pig and human CYP17 (P-450(17)alpha; 17 alpha-hydroxylase-17,20-lyase) Biochemistry. 1995;34(43):14104–14113. doi: 10.1021/bi00043a015. [DOI] [PubMed] [Google Scholar]
- 27.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biology of reproduction. 2004;71(1):83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 28.Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38(5):1598–1606. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- 29.Fevold HR, Lorence MC, McCarthy JL, Trant JM, Kagimoto M, Waterman MR, Mason JI. Rat P450(17 alpha) from testis: characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both delta 4- and delta 5-steroid-17,20-lyase reactions. Mol Endocrinol. 1989;3(6):968–975. doi: 10.1210/mend-3-6-968. [DOI] [PubMed] [Google Scholar]
- 30.Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17 alpha-hydroxylase/C17,20-lyase cytochrome P-450. J Biol Chem. 1984;259(6):3971–3976. [PubMed] [Google Scholar]
- 31.Sakai N, Tanaka M, Adachi S, Miller WL, Nagahama Y. Rainbow trout cytochrome P-450c17 (17 alpha-hydroxylase/17,20-lyase). cDNA cloning, enzymatic properties and temporal pattern of ovarian P-450c17 mRNA expression during oogenesis. FEBS Lett. 1992;301(1):60–64. doi: 10.1016/0014-5793(92)80210-8. [DOI] [PubMed] [Google Scholar]
- 32.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Lamberts SW, Uitterlinden P, de Jong FH. Rat prostatic weight regression in reaction to ketoconazole, cyproterone acetate, and RU 23908 as adjuncts to a depot formulation of gonadotropin-releasing hormone analogue. Cancer Res. 1988;48(21):6063–6068. [PubMed] [Google Scholar]
- 34.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 35.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69(12):4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 36.Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Seminars in reproductive medicine. 2004;22(4):281–288. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- 37.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, Molina A, Small EJ. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28(9):1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gower DB. 16-Unsaturated C 19 steroids. A review of their chemistry, biochemistry and possible physiological role. J Steroid Biochem. 1972;3(1):45–103. doi: 10.1016/0022-4731(72)90011-8. [DOI] [PubMed] [Google Scholar]
- 39.Grosser BI, Monti-Bloch L, Jennings-White C, Berliner DL. Behavioral and electrophysiological effects of androstadienone, a human pheromone. Psychoneuroendocrinology. 2000;25(3):289–299. doi: 10.1016/s0306-4530(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 40.Soucy P, Lacoste L, Luu-The V. Assessment of porcine and human 16-ene-synthase, a third activity of P450c17, in the formation of an androstenol precursor. Role of recombinant cytochrome b5 and P450 reductase. European journal of biochemistry / FEBS. 2003;270(6):1349–1355. doi: 10.1046/j.1432-1033.2003.03508.x. [DOI] [PubMed] [Google Scholar]
- 41.Niwa T, Fujimoto M, Kishimoto K, Yabusaki Y, Ishibashi F, Katagiri M. Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol Pharm Bull. 2001;24(9):1064–1067. doi: 10.1248/bpb.24.1064. [DOI] [PubMed] [Google Scholar]
- 42.Suhara K, Fujimura Y, Shiroo M, Katagiri M. Multiple catalytic properties of the purified and reconstituted cytochrome P-450 (P-450sccII) system of pig testis microsomes. J Biol Chem. 1984;259(14):8729–8736. [PubMed] [Google Scholar]
- 43.Arth GE, Patchett AA, Jefopoulus T, Bugianesi RL, Peterson LH, Ham EA, Kuehl FA, Jr, Brink NG. Steroidal androgen biosynthesis inhibitors. J Med Chem. 1971;14(8):675–679. doi: 10.1021/jm00290a003. [DOI] [PubMed] [Google Scholar]
- 44.Chart JJ, Sheppard H. Pharmacology and biochemistry of some amphenone analogues and other adrenal cortical inhibitors. Journal of medicinal and pharmaceutical chemistry. 1959;1:407–441. doi: 10.1021/jm50006a002. [DOI] [PubMed] [Google Scholar]
- 45.Gaunt R, Steinetz BG, Chart JJ. Pharmacologic alteration of steroid hormone functions. Clinical pharmacology and therapeutics. 1968;9(5):657–681. doi: 10.1002/cpt196895657. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stigliano A, Gandini O, Cerquetti L, Gazzaniga P, Misiti S, Monti S, Gradilone A, Falasca P, Poggi M, Brunetti E, Agliano AM, Toscano V. Increased metastatic lymph node 64 and CYP17 expression are associated with high stage prostate cancer. J Endocrinol. 2007;194(1):55–61. doi: 10.1677/JOE-07-0131. [DOI] [PubMed] [Google Scholar]
- 48.Schenkman JB, Cinti DL, Orrenius S, Moldeus P, Kraschnitz R. The nature of the reverse type I (modified type II) spectral change in liver microsomes. Biochemistry. 1972;11(23):4243–4251. doi: 10.1021/bi00773a008. [DOI] [PubMed] [Google Scholar]
- 49.Remmer H, Schenkman J, Estabrook RW, Sasame H, Gillette J, Narasimhulu S, Cooper DY, Rosenthal O. Drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1966;2(2):187–190. [PubMed] [Google Scholar]
- 50.Schenkman JB, Remmer H, Estabrook RW. Spectral studies of drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1967;3(2):113–123. [PubMed] [Google Scholar]
- 51.Dawson JH, Andersson LA, Sono M. Spectroscopic investigations of ferric cytochrome P-450-CAM ligand complexes. Identification of the ligand trans to cysteinate in the native enzyme. J Biol Chem. 1982;257(7):3606–3617. [PubMed] [Google Scholar]
- 52.Jefcoate CR. Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods in enzymology. 1978;52:258–279. doi: 10.1016/s0076-6879(78)52029-6. [DOI] [PubMed] [Google Scholar]
- 53.Njar VC, Brodie AM. Inhibitors of 17alpha-hydroxylase/17,20-lyase (CYP17): potential agents for the treatment of prostate cancer. Curr Pharm Des. 1999;5(3):163–180. [PubMed] [Google Scholar]
- 54.Jarman M, Smith HJ, Nicholls PJ, Simons C. Inhibitors of enzymes of androgen biosynthesis: cytochrome P450(17) alpha and 5 alpha-steroid reductase. Natural product reports. 1998;15(5):495–512. doi: 10.1039/a815495y. [DOI] [PubMed] [Google Scholar]
- 55.Hartmann RW, Ehmer PB, Haidar S, Hector M, Jose J, Klein CD, Seidel SB, Sergejew TF, Wachall BG, Wachter GA, Zhuang Y. Inhibition of CYP 17, a new strategy for the treatment of prostate cancer. Arch Pharm (Weinheim) 2002;335(4):119–128. doi: 10.1002/1521-4184(200204)335:4<119::AID-ARDP119>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Moreira VM, Salvador JA, Vasaitis TS, Njar VC. CYP17 inhibitors for prostate cancer treatment--an update. Curr Med Chem. 2008;15(9):868–899. doi: 10.2174/092986708783955428. [DOI] [PubMed] [Google Scholar]
- 57.Owen CP. 17alpha-hydroxylase/17,20-lyase (p450(17alpha)) inhibitors in the treatment of prostate cancer: a review. Anticancer Agents Med Chem. 2009;9(6):613–626. doi: 10.2174/187152009788680046. [DOI] [PubMed] [Google Scholar]
- 58.DeFelice R, Johnson DG, Galgiani JN. Gynecomastia with ketoconazole. Antimicrobial agents and chemotherapy. 1981;19(6):1073–1074. doi: 10.1128/aac.19.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz Y, Leung PC, Armstrong DT. Testosterone restores ovarian aromatase activity in rats treated with a 17,20-lyase inhibitor. Mol Cell Endocrinol. 1979;14(1):37–44. doi: 10.1016/0303-7207(79)90056-x. [DOI] [PubMed] [Google Scholar]
- 60.Pont A, Williams PL, Azhar S, Reitz RE, Bochra C, Smith ER, Stevens DA. Ketoconazole blocks testosterone synthesis. Archives of internal medicine. 1982;142(12):2137–2140. [PubMed] [Google Scholar]
- 61.Pont A, Williams PL, Loose DS, Feldman D, Reitz RE, Bochra C, Stevens DA. Ketoconazole blocks adrenal steroid synthesis. Annals of internal medicine. 1982;97(3):370–372. doi: 10.7326/0003-4819-97-3-370. [DOI] [PubMed] [Google Scholar]
- 62.Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13(7):2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 63.Santen RJ, Van den Bossche H, Symoens J, Brugmans J, DeCoster R. Site of action of low dose ketoconazole on androgen biosynthesis in men. J Clin Endocrinol Metab. 1983;57(4):732–736. doi: 10.1210/jcem-57-4-732. [DOI] [PubMed] [Google Scholar]
- 64.Small EJ, Baron A, Bok R. Simultaneous antiandrogen withdrawal and treatment with ketoconazole and hydrocortisone in patients with advanced prostate carcinoma. Cancer. 1997;80(9):1755–1759. doi: 10.1002/(sici)1097-0142(19971101)80:9<1755::aid-cncr9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 65.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157(4):1204–1207. [PubMed] [Google Scholar]
- 66.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, Torti FM, Kaplan E, Vogelzang NJ. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22(6):1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 67.Ngo LS, Yeo A, Wong AS, Tay MH. Efficacy of low-dose ketoconazole in hormone refractory prostate cancer patients at the National Cancer Centre and The Cancer Institute, Singapore. Annals of the Academy of Medicine, Singapore. 2007;36(10):811–814. [PubMed] [Google Scholar]
- 68.Nakabayashi M, Xie W, Regan MM, Jackman DM, Kantoff PW, Oh WK. Response to low-dose ketoconazole and subsequent dose escalation to high-dose ketoconazole in patients with androgen-independent prostate cancer. Cancer. 2006;107(5):975–981. doi: 10.1002/cncr.22085. [DOI] [PubMed] [Google Scholar]
- 69.Venkatakrishnan K, Rader M, Ramanathan RK, Ramalingam S, Chen E, Riordan W, Trepicchio W, Cooper M, Karol M, von Moltke L, Neuwirth R, Egorin M, Chatta G. Effect of the CYP3A inhibitor ketoconazole on the pharmacokinetics and pharmacodynamics of bortezomib in patients with advanced solid tumors: a prospective, multicenter, open-label, randomized, two-way crossover drug-drug interaction study. Clinical therapeutics. 2009;31(Pt 2):2444–2458. doi: 10.1016/j.clinthera.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Yong WP, Wang LZ, Tham LS, Wong CI, Lee SC, Soo R, Sukri N, Lee HS, Goh BC. A phase I study of docetaxel with ketoconazole modulation in patients with advanced cancers. Cancer Chemother Pharmacol. 2008;62(2):243–251. doi: 10.1007/s00280-007-0598-1. [DOI] [PubMed] [Google Scholar]
- 71.Muindi JR, Yu WD, Ma Y, Engler KL, Kong RX, Trump DL, Johnson CS. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology. 151(9):4301–4312. doi: 10.1210/en.2009-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Figg WD, Woo S, Zhu W, Chen X, Ajiboye AS, Steinberg SM, Price DK, Wright JJ, Parnes HL, Arlen PM, Gulley JL, Dahut WL. A phase I clinical study of high dose ketoconazole plus weekly docetaxel for metastatic castration resistant prostate cancer. J Urol. 183(6):2219–2226. doi: 10.1016/j.juro.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taplin ME, Regan MM, Ko YJ, Bubley GJ, Duggan SE, Werner L, Beer TM, Ryan CW, Mathew P, Tu SM, Denmeade SR, Oh WK, Sartor O, Mantzoros CS, Rittmaster R, Kantoff PW, Balk SP. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15(22):7099–7105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rowlands MG, Barrie SE, Chan F, Houghton J, Jarman M, McCague R, Potter GA. Esters of 3-pyridylacetic acid that combine potent inhibition of 17 alpha-hydroxylase/C17,20-lyase (cytochrome P45017 alpha) with resistance to esterase hydrolysis. J Med Chem. 1995;38(21):4191–4197. doi: 10.1021/jm00021a008. [DOI] [PubMed] [Google Scholar]
- 75.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 76.Haidar S, Ehmer PB, Barassin S, Batzl-Hartmann C, Hartmann RW. Effects of novel 17alpha-hydroxylase/C17, 20-lyase (P450 17, CYP 17) inhibitors on androgen biosynthesis in vitro and in vivo. J Steroid Biochem Mol Biol. 2003;84(5):555–562. doi: 10.1016/s0960-0760(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 77.Yap TA, Carden CP, Attard A, de Bono JS. Targeting CYP17: established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8(4):449–457. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50(5–6):267–273. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 79.Chan FC, Potter GA, Barrie SE, Haynes BP, Rowlands MG, Houghton J, Jarman M. 3- and 4-pyridylalkyl adamantanecarboxylates: inhibitors of human cytochrome P450(17 alpha) (17 alpha-hydroxylase/C17,20-lyase). Potential nonsteroidal agents for the treatment of prostatic cancer. J Med Chem. 1996;39(17):3319–3323. doi: 10.1021/jm950749y. [DOI] [PubMed] [Google Scholar]
- 80.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, Molina A, Anand A, Koscuiszka M, Larson SM, Schwartz LH, Fleisher M, Scher HI. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, Dearnaley D, Swennenhuis JF, Terstappen LW, Lee G, Kheoh T, Molina A, Ryan CJ, Small E, Scher HI, de Bono JS. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28(9):1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abiraterone Acetate in Castration-Resistant Prostate Cancer Previously Treated With Docetaxel-Based Chemotherapy. [Accessed 6/1/2010]; http://clinicaltrials.gov/ct2/show/NCT00638690.
- 83.Abiraterone Acetate in Asymptomatic or Mildly Symptomatic Patients With Metastatic Castration-Resistant Prostate Cancer. [Accessed 6/1/2010]; http://clinicaltrials.gov/ct2/show/NCT00887198.
- 84.Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, Newman D, Jr, Farquhar R, Guo Z, Qiu Y, Brodie AM. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48(8):2972–2984. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 85.Vasaitis T, Belosay A, Schayowitz A, Khandelwal A, Chopra P, Gediya LK, Guo Z, Fang HB, Njar VC, Brodie AM. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7(8):2348–2357. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schayowitz A, Sabnis G, Njar VC, Brodie AM. Synergistic effect of a novel antiandrogen, VN/124-1, and signal transduction inhibitors in prostate cancer progression to hormone independence in vitro. Mol Cancer Ther. 2008;7(1):121–132. doi: 10.1158/1535-7163.MCT-07-0581. [DOI] [PubMed] [Google Scholar]
- 87.Safety Study of TAK-700 in Subjects With Prostate Cancer. [Accessed 7/15/2010]; http://clinicaltrials.gov/ct2/show/NCT00569153.
- 88.Safety and Efficacy Study of TAK-700 in Patients With Nonmetastatic Castration-resistant Prostate Cancer and a Rising Prostate-specific Antigen. [Accessed 7/15/2010]; doi: 10.1158/1078-0432.CCR-14-0356. http://clinicaltrials.gov/ct2/show/NCT01046916. [DOI] [PubMed]
- 89.Study of TAK-700 in Combination With Docetaxel and Prednisone in Men With Metastatic Castration-Resistant Prostate Cancer. [Accessed 9/2/2010]; http://clinicaltrials.gov/ct2/show/NCT01084655.
- 90.Matsunaga N, Kaku T, Ojida A, Tanaka T, Hara T, Yamaoka M, Kusaka M, Tasaka A. C(17,20)-lyase inhibitors. Part 2: design, synthesis and structure-activity relationships of (2-naphthylmethyl)-1H-imidazoles as novel C(17,20)-lyase inhibitors. Bioorg Med Chem. 2004;12(16):4313–4336. doi: 10.1016/j.bmc.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 91.Matsunaga N, Kaku T, Ojida A, Tasaka A. Synthetic studies on (1S)-1-(6,7-dimethoxy-2-naphthyl)-1-(1H-imidazol-4-yl)-2-methylpropan-1-ol as a selective C17,20-lyase inhibitor. Tetrahedron: Asymmetry. 2004;15(13):8. [Google Scholar]