Abstract
Research on the composition of the tumor micro-environment has demonstrated that membrane delimited microvesicles are shed from many types of malignant tumors, in the peripheral blood of cancer patients as well as in culture media of tumor cells propagated in vitro (Ginestra et al. Anticancer Res 18:3433–3437, 1998). Their documented effects involve the activation of signal transduction pathways by cellular cross-talk that are associated with epigenetic mechanisms that may be important in tumor progression, metastasis, and the activation of angiogenesis (Distler et al. Arthritis Rheum 52:3337–3348, 2005). Live cell imaging microscopic studies conducted in our laboratory of the formation of solid tumor spheroids in vitro show that the shedding of microvesicular structures from tumor cells occurs during this process. The observed properties of the tumor microvesicles suggest a role in solid tumor formation and intercellular communication. The tumor associated microvesicles were shown to be non-apoptotic based on the absence of fluorescent nuclear staining by acridine orange/ethidium bromide staining. Increased concentration of extracellular Ca++ [5–20 mM] resulted in an increase in the production of tumor-derived microvesicles and also to result in the formation of tumor spheroids whose size was considerably smaller than controls. Increased extracellular [Ca++] was also observed to induce the rapid dissociation of solid tumor spheroids to smaller cell aggregates in the absence of significant apoptosis.
Keywords: Extracellular Ca++, Live cell imaging, Multicellular tumor spheroid, Tumor associated microvesicle
Introduction
Membranous vesicles shed from cells in culture and also detected in peripheral blood, once thought to represent only “cell debris”, actually are representative of a diverse group of vesicles first observed more than 40 years ago as secreted entities formed by blood platelets [3]. Subsequent research has shown that these secreted vesicular structures, ranging in size from 100 nm–1 μm, are shed by many types of cells and may play an important role in intercellular communication [4].
Research on the composition of the tumor microenvironment has demonstrated that membrane delimited microvesicles are shed from many types of malignant tumors and have been detected in the peripheral blood of cancer patients as well as in culture media of tumor cells propagated in vitro [1, 5]. Research by Skog et al [6] has shown that glioblastoma cells release microvesicless that contain mRNA, miRNA and proteins that stimulate angiogenesis. Microvesicles containing these cellular components have also been identified in the serum of patients with glioblastoma. These researchers have further demonstrated that tumor derived microvesicless are taken up by normal live endothelial cells, which then express the delivered mRNAs as functional proteins.
Tumor-derived microvesicless were also shown to stimulate the proliferation of glioma cell lines in vitro [7]. Additional documented effects of tumor cell microvesicless include immunosuppression associated with myeloid derived suppressor cell (MDSC) induction [8], T cell apoptosis and suppression of natural killer cell (NK) activities [9].
Among the documented triggers of tumor-associated microvesicle release are cell proliferation, inflammation, hypoxia and shearing stresses, as well as elevated cytosolic calcium levels [10–12]. Research by diVizio et al [13] has shown that tumor-associated microvesicles or “oncosome” production from prostate cancer cell lines (DU145 and LNCaP)) is stimulated by EGFR activation and overexpression of AKT1. These “oncosomes” were shown to be capable of stimulating the proliferation and migration of tumor cells in association with AKT pathway signalling.
Oncosome production was also observed to increase in response to actin nucleating protein Diaphanous Related Formin 3 (DRF3/Dia2) inhibition suggesting that these MVs may resemble non-apoptotic membrane blebs. Moreover, deletion of this locus was found to be more common in metastatic tumors than in primary prostate tumors. Taken together, these observations have led to the suggestion that tumor-associated microvesicles may be important in epigenetic mechanisms of tumor progression, metastasis and angiogenesis [14–16].
Research studies in our laboratory suggest that the process of solid tumor spheroid formation in vitro involves microvesicle shedding from tumor cell membranes that play a role in the formation of multicellular aggregations. Research studies by Sutherland [17], Kerbel [18] and others have explored the use of multicellular tumor spheroids as a model for the understanding some aspects of solid tumor biology in vivo. Multicellular tumor spheroids that form spontaneously in vitro when tumor cells are cultured under conditions in which substrate attachment is blocked are similar in their overall dimension (1–3 mm) to microscopic malignant lesions.
Tumor spheroids characteristically display three distinct cell layers defined by their local microenvironment: an outer layer comprised of mitotic cells, a middle layer of quiescent non-dividing cells and a dense necrotic core [17]. These concentric spheroid regions are generated by the gradients of nutrients, oxygen and growth factors that produce distinctive microenvironments within the tumor mass that are associated with in the build-up of acidic metabolic waste and hypoxic condition in the tumor core. Researchers have suggested that similar biophysical parameters may define the microenvironment of solid tumors in vivo and may account for some aspects of therapeutic sensitivity to chemotherapy and radiation [19].
Research studies by Espina et al [19] have shown that ex vivo organoid cultures of breast ductal carcinoma in situ (DCIS) spontaneously produce hundreds of spheroids in the first several weeks in culture that are capable of anchorage independent growth, stromal invasion and tumorgenicity in SCID mice. Moreover, these researchers observed that most of the organoid cultures of DCIS displayed microcalcification, which has been linked to the presence of high grade DCIS by mammography [20].
Additional clinical research has established a physiological link between malignancy to cancer progression and systematic disease. It has been estimated that hypercalcemia is present in up to 40% of patients with advanced cancer and its presence is invariably associated with systemic disease and poor patient prognosis [21]. Most forms of hypercalcemia of malignancy are associated with increased bone resorption resulting in elevated levels of serum calcium [22]. Recent studies suggest that up to 80% of these effects may be due to tumor-derived, circulating humoral factors that affect bone metabolism and stimulate the release of calcium in the circulation [23]. These observations raise the possibility that elevated calcium levels may play a role in disease progression by contributing to a tumor microenvironment that promotes tumor spread and metastasis.
The goal of this research study was to explore the effects of increased calcium concentration on multicellular tumor spheroid formation and tumor-associated microvesicle production. The results of the research reported in this paper suggest that elevated concentration of extracellular calcium may enhance the production of TAMV and affect parameters of spheroid formation and morphology.
Materials and Methods
Cell Lines and Culture Conditions
DBTRG-.05MG (glioblastoma), SJSA-1 (juvenile osteosarcoma, and Hs578T (breast carcinoma) tumor cell lines were obtained from the American Type Culture Collection (ATCC) and cultured in standard culture medium RPMI 1640 or DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) (Whittaker) and incubated at 37C in 5–10% CO2. Multicellular tumor spheroid cultures were prepared by culturing cells in tissue culture dishes coated with 1% agarose (Sigma) in phosphate buffered saline (PBS) solution.
The effects of exogenous added calcium on cell cultures were assessed by adding CaCl2 in concentrations ranging from 5–25 mM to culture media (see also below).
Photomicroscopy Studies
Live cell imaging studies of tumor spheroid formation were performed using tumor cells from human cancer cell lines plated onto culture dishes coated with sterile 1% agarose solution to prevent substrate attachment. Under these conditions, tumor cells spontaneously aggregate to form micro-tumor masses called spheroids. This process was recorded by videography and photomicroscopy from the time of cell culture initiation continuously for 4–5 h post-plating that was subsequently followed by intermittent photomicroscopy over a period of several days to several weeks in culture. Aquis imaging software was used to record photomicroscopy imaging data.
Assessment of the Effects of CaCl2 Supplementation of Culture Media on Cultured Tumor Cells
CaCl2 at concentrations ranging from 5–25 mM was added to complete culture media (RPMI 1640 or DMEM) to assess the effects of increased extracellular Ca++ on spheroid formation and viability. Trypsinized tumor cells were plated onto culture dishes coated with 1% agarose in CaCl2 supplemented media and spheroid formation parameters were assessed by photomicroscopy.
Alternatively, CaCl2 was added to culture media to preformed spheroids that had been in culture for approximately 1 week to assess the effects of increased [Ca++] on spheroid morphology and viability.
Spheroid Viability Assay
Spheroid viability was assessed by determining the capacity of preformed spheroids to attach to uncoated culture dishes and to proliferate laterally from substrate attachment sites at the tumor periphery. Measurements of average diameter of the proliferating area of the attached tumor spheroid were made approximately 3–5 days after spheroid transfer to uncoated dishes. A comparison of the diameters of the proliferating zones of spheroids in increased CaCl2 concentration to control spheroids cultured under identical conditions with the exception of added CaCl2 was used as an estimate of spheroid viability.
A second measure of spheroid viability was the fluorescent microscopy imaging (Biorad Cellmap) of tumor spheroids stained with acridine orange (Sigma) and ethidium bromide (Sigma) to assess the fluorescent properties of the stained nuclei. Lasersharp2000 imaging software was used in the fluorescent microscopy assessment.
A third measure of spheroid viability involved the use of trypsin to dissociate spheroid aggregates. Cell viability assays were then performed using erythrosin B (Sigma) viability stain to distinguish viable cells (clear) from non-viable cells (dark pink) based on standard membrane exclusion parameters of dye uptake as a measure of cell viability. Measurements were made using Aquis Montage (Biorad) software.
Monolayer Culture Viability Assay
Tumor cells were plated onto culture dishes in medium containing increased concentrations of CaCl2 (5–25 mM) and incubated for approximately 24 h prior to assay. Cell viability assays were performed using erythrosin B (Sigma) viability stain to distinguish viable cells (clear) from non-viable cells (dark pink) based on standard membrane exclusion parameters of dye uptake as a measure of cell viability. Measurements were made using Aquis Montage (Biorad) software.
Microscopic Assessment of Tumor Derived Microvesicles
Tumor spheroids cultured in standard culture media or at elevated [Ca++] were fractionated by centrifugation for 5 min at 10,000 rpm to pellet spheroid cell aggregates. The culture supernatant was then removed and examined by standard microscopy imaging at 400× magnification to ascertain the presence of microvesicles. The microvesicles were stained with acridine orange/ethidium bromide (Sigma) and examined immediately by confocal laser fluorescent microscopy (Cellmap by Biorad) using Lasersharp 2000 imaging software.
Results
Live Cell Imaging Studies of Spheroid Formation
Live cell videomicroscopy studies were carried out on human tumor cell lines, including glioblastoma (DBTRG.05MG), osteosarcoma, (SJSA-1) and breast carcinoma (Hs578T) to assess early stages of spheroid formation (see “Materials and Methods” for detailed procedure). Figure 1 shows time-lapse images obtained for glioblastoma cells in the process of spheroid formation immediately after post-plating up to several hours. This sequence of events, culminating in the spontaneous aggregation of tumor cells to form multicellular spheroids, was observed to involve the budding and release of microvesicles. These highly motile structures attached to tumor cell membranes and appeared to promote cell-to-cell interactions associated with spheroid formation. Additional studies of these tumor associated microvesicles generated during the process of spheroid formation revealed that these structures initially appeared to bud from the cell surface or, alternatively, could form intracellularly. The tumor-derived microvesicles were observed to fuse with tumor cells or with other microvesicles to form tubular structures serving as conduits for microvesicle transport and as structural links between different spheroids (see Fig. 2). Video imaging studies carried out on additional tumor cell lines, including breast cancer (Hs578T) and osteosarcoma (SJSA-1) produced similar results.
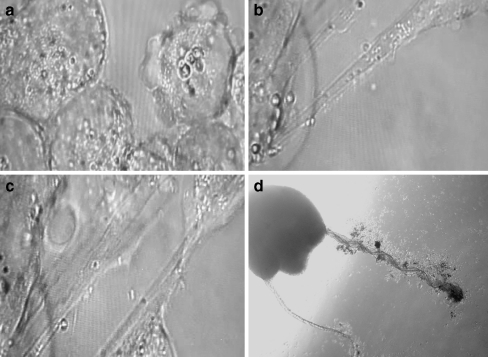
Fig. 1.
Time-lapse live cell imaging of glioblastoma cells cultured on 1% agarose to block cell-to-substrate attachment to promote spheroid formation in first 1.5 h post plating. Cell-to-cell interactions show microvesicle association. a Time 20 min; b time 40 min. c time 105 min (400× magnification)
Fig. 2.
Live cell imaging of tumor microvesicles. a incipient microvesicles can originate as membrane surface blebs; b and c tubules serve as conduits for microvesicle transport; d solid tumor with satellites showing tubular interconnections; (a,b,c-400×; d. -100× magnification)
Effects of Increased Extracellular Ca++ Concentration on Spheroid Formation and Microvesicle Production
To assess the effects of increased Ca++ concentration on spheroid formation, tumor cells were cultured in media containing added CaCl2 at concentrations ranging from 5–25 mM. Live cell imaging studies of spheroid formation under these conditions showed that increased production of microvesicles occurred within several hours of incubation at increased CaCl2 (10 mM) concentration in glioblastoma cultures (Fig. 3). Moreover, increased concentration of CaCl2 in glioblastoma cultures significantly affected the formation of multicellular spheroids, resulting in the formation of spheroids that were considerably smaller in size than control spheroids (see Fig. 3c and 3d). Glioblastoma spheroids cultured in varying concentrations of CaCl2 were assayed for viability by re-plating the spheroids onto uncoated culture dishes to allow cell reattachment to occur. The diameter of the monolayer growth zone emanating from the reattached spheroids was measured after several days post plating as an assay for tumor viability (see “Materials and Methods” for detailed procedure). These data indicated that the recovered tumor cells incubated in 10 mM Ca++ were capable of proliferating as attached cultures (see Fig. 3e, f). Higher concentrations of Ca++ were associated with a loss of culture viability.
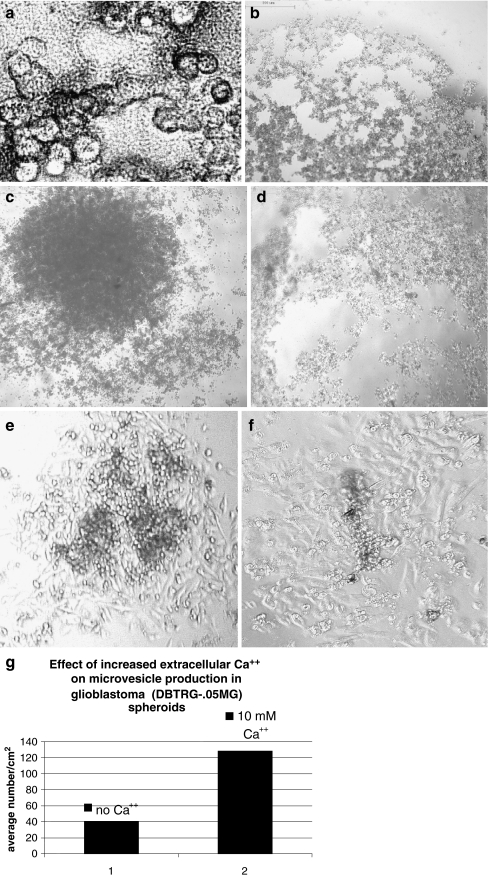
Fig. 3.
Live cell imaging of the effects of elevated CaCl2 on glioblastoma spheroid formation. a 400× magnification reveals vesicular network in intercellular spaces at 3 h in 10 mM Ca++; b spheroid after 3 h in culture 10 mM Ca++ (4× magnification); c control at 5 days; d spheroids cultured in 10 mM CaCl2 at 5 days; e, f spheroid viability post substrate attachment e control; f 10 mM Ca++. Note zone of proliferation. Part g is a graph showing effect of increased Ca++ on quantitative production of microvesicles in glioblastoma spheroids measured using Aquis Montage software
Microvesicles were harvested from the media of glioblastoma (DBTRG-.05MG) spheroids (see “Materials and Methods” for procedure) cultured in 5–10 mM CaCl2, analyzed for the presence of nucleic acid by staining with acridine orange/ethidium bromide and examined by fluorescence laser microscopy. The majority of microvesicles produced at increased concentrations of Ca++ were observed to be devoid of nucleic acid, indicating that they were not the cell products of apoptosis (Fig. 4).
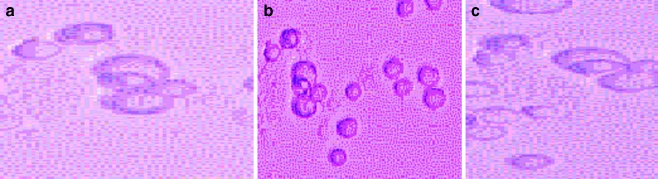
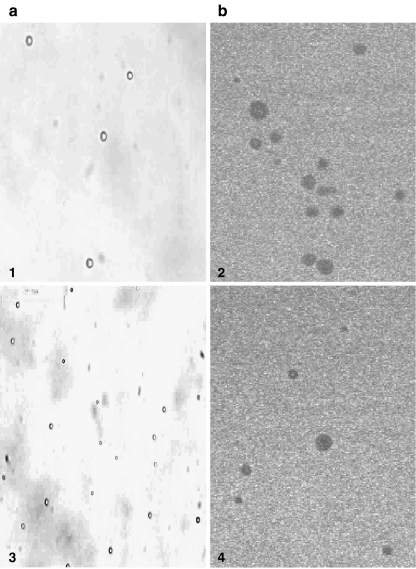
Fig. 4.
Microvesicles prepared from cultures of glioblastoma (DBTRG-.05MG) in 5–10 mM CaCl2 (see “Materials and Methods”). Panel A shows live imaging and panel B shows microvesicles stained with acridine orange/ethidium bromide visualized by fluorescence laser microscopy (Biorad Cellmap) using Lasersharp 2000 software. Note absence of fluorescence; vesicles appear as dark spots due to the absence of nucleic acid (400× magnification (+2× zoom for images 1, 2 and 4)
Effects of Increased Extracellular Ca++ Concentration on Osteosarcoma (SJSA-1) and Breast Carcinoma (Hs578T) Spheroids
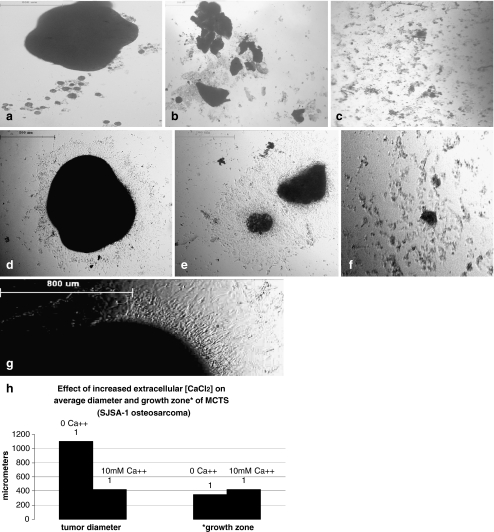
The addition of increased concentrations of CaCl2 also affected the formation of osteosarcoma (SJSA-1) spheroids. Figure 5 shows that spheroid size was markedly reduced compared to the control for tumors formed in 5–20 mM CaCl2. Tumor viability was assessed by measuring the proliferation of the tumor periphery following re-plating onto uncoated dishes to allow for substrate attachment of tumor spheroids. The zone of proliferation for spheroids at 10 mM Ca++ was observed to be comparable to the control spheroids (see Fig. 5d–f). Higher concentrations of Ca++ produced significant effects on spheroid reattachment to uncoated culture dishes and cell viability.
Fig. 5.
Osteosarcoma spheroids 5 days post plating in culture medium with a no added CaCl2 or b in 10 mM CaCl2; c 20 mM CaCl2. Viability of osteosarcoma spheroids 2 days post substrate reattachment at d 0; e 10 and f 20 mM CaCl2; g breast carcinoma Hs578T spheroid reattachment in no added Ca++; h 10 mM Ca++; i 20 mM Ca++; k graph shows SJSA1 spheroid size and diameter of proliferation areas. Enlarged photo (j) shows zone of proliferation for SJSA-1 spheroid
Effect of Increased Extracellular Ca++ on Breast Carcinoma Hs578T Spheroid Formation
Breast carcinoma (Hs578T) spheroid formation was similarly affected by increased Ca++, such that their overall size at 48 h post plating was significantly smaller than spheroids formed in the absence of added extracellular CaCl2 (Fig. 6). Even relatively small increases in extracellular Ca++ resulted in the production of smaller spheroids that were less dense, based on the increased uptake of acridine orange/ethidium bromide stain by the spheroid interior. In contrast, dye uptake in control spheroids was largely restricted to the periphery. At Ca++ concentrations greater than 5 mM spheroid reattachment to culture dishes was dramatically reduced; however, acridine orange/ethidium bromide staining indicated that the cell aggregates were, nevertheless, viable based on the presence of bright green fluorescent nuclei that displayed similar fluorescence to viable spheroid controls. The viability of the spheroid cultures was observed to decrease significantly at Ca++ concentrations greater than 15 mM.
Fig. 6.
Breast carcinoma Hs578T spheroid formation 48 h post plating in increasing concentrations of extracellular CaCl2 (0–15 mM). Panel A shows live images. Panel B shows spheroids stained with acridine orange/ethidium bromide and imaged by fluorescent laser microscopy (Biorad Cellmap) using Lasersharp 2000 software
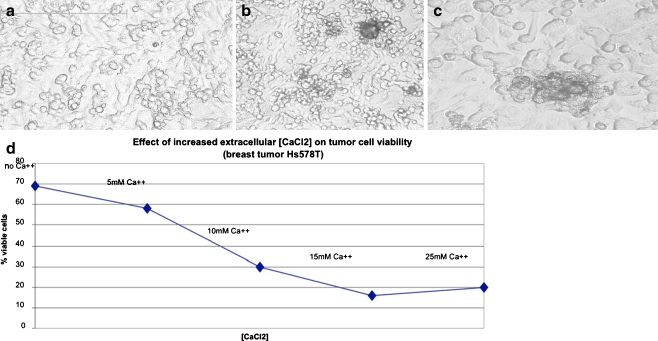
Effect of Ca++ on monolayer cultures of tumor cells
The effect of added CaCl2 on monolayer cultures of tumor cell lines was also evaluated in breast carcinoma (Hs578T) and glioblastoma (DBTRG-.05MG). The primary effect observed with the addition of increasing Ca++ was the clumping of cells in monolayer cultures (glioblastoma monolayer is shown) that was associated with increased microvesicle production (see Fig. 7a–c). Viable cell counts of erythrosin B stained Hs578T breast tumor cells indicated a linear decline in cell viability with increasing CaCl2 concentration, such that 30% culture viability was observed at 10 mM CaCl2 and 25% viability was observed at 25 mM ++(Fig. 7d). The results suggest that the effect of increased Ca++ viability was greater in monolayer cultures of breast carcinoma Hs578T than on either the proliferating zone of spheroid cultures (SJSA-1 osteosarcoma) or on spheroids of Hs578T based on fluorescence microscopy assessment of acridine orange/ethidium bromide stained spheroids. (see Figs. 5 and 6).
Fig. 7.
Effects of Ca++ on monolayer cultures of glioblastoma (DBTRG-.05MG) at a 0; b 10 mM and c 15 mMCaCl2. Note cell clumping at elevated Ca++. Effects of Ca++ on viability of breast carcinoma (Hs578T) are shown on graph (d) based on erythrosin B assay data calculated using Acquis Montage software
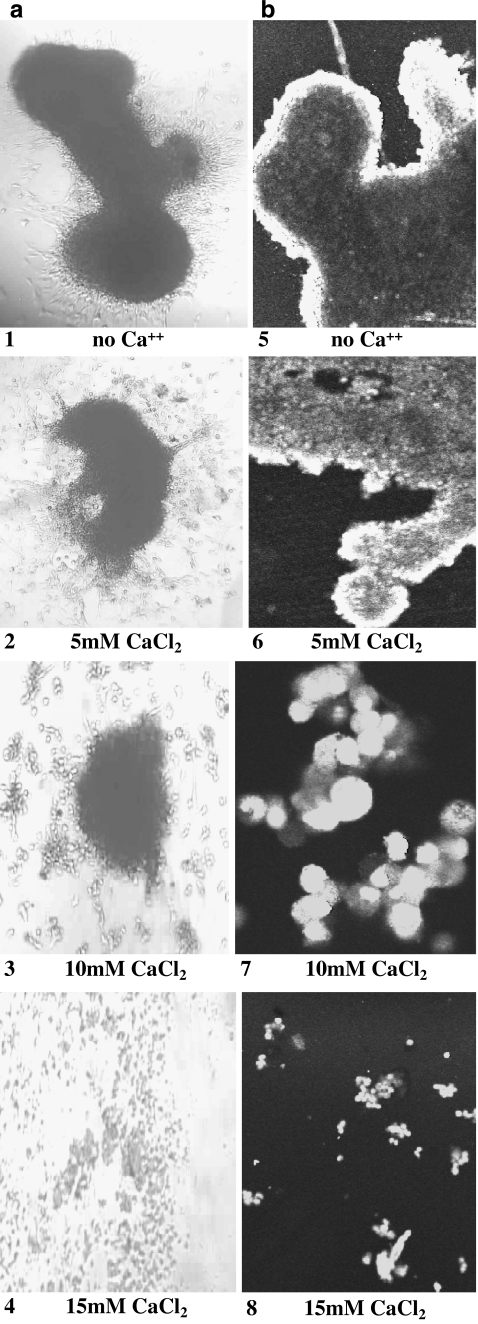
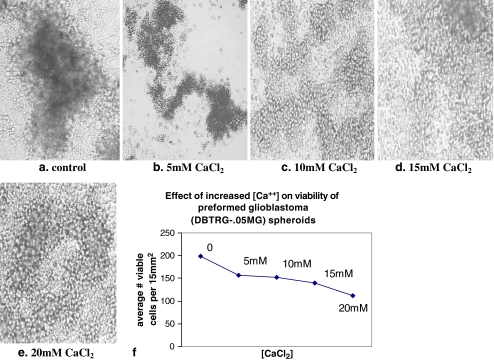
Effect of Increased Extracellular Ca++ on Preformed Spheroids from Glioblastoma (DBTRG-.05MG)
The addition of extracellular Ca++ (5–20 mM) to preformed spheroids from glioblastoma (DBTRG-.05MG) induced the rapid dissociation of solid cell masses to smaller and less dense cell aggregates within 4 h (Fig. 8). Subsequent plating of these smaller spheroids showed that they were capable of reattachment and the cells comprising these smaller masses were viable based on cell counts of erythrosin B stained cells, such that approximately 75% of the cells were viable at 5–15 mM Ca++; at 20 mM approximately 50% viability was observed. These data suggest that the effect of increased extracellular [Ca++] on spheroid morphology occurred in the absence of significant loss of culture viability. At concentrations greater than 5 mM Ca++, however, spheroid viability decreased dramatically after 24 h in culture.
Fig. 8.
Preformed spheroids of glioblastoma (DBTRG-.05MG) were cultured in media containing increased Ca++ concentration (0–20 mM) for 4 h and then transferred to untreated culture dishes to permit spheroid reattachment to substrate
Discussion
Results presented in this paper suggest that increased extracellular [Ca++] may affect biological properties of tumor spheroids from breast carcinoma (Hs578T), osteosarcoma (SJSA-1) and glioblastoma (DBTRG-.05MG) in vitro as they relate to the properties of tumor size (mass), which was observed to decrease, and microvesicle production, which increased in response to high (5–20 mM) concentrations of this divalent cation.
Increased extracellular [Ca++] was associated with the formation of smaller, less dense spheroids of these tumor cell lines that displayed significant viability at [Ca++] less than 10 mM based on the relative sizes of the proliferating outer layer of the tumor spheroids following substrate reattachment and nuclear integrity based on fluorescence microscopy using acridine orange/ethidium bromide stain. Maximum spheroid viability was observed at 5 mM Ca++; higher levels (10 mM and greater) produced significant viability loss both in monolayer cultures and in tumor spheroids. The data also showed that increased extracellular Ca++ induced the rapid breakdown (within 4 h) of solid tumor spheroids that were, nevertheless, viable after the short-term exposure to Ca++ from 5–20 mM and remained viable for an extended period of time at lower Ca++ (5 mM) based on cell counts following staining with erythrosin B.
If microvesicle production plays a role in spheroid formation, then it would seem paradoxical that micro-environmental conditions that promote microvesicle production (elevated Ca++) also produce spheroids of smaller size. It will be necessary to conduct further studies to address the proposed relationship between microvesicle production and spheroid formation; the results of the present study suggest that Ca++ may be an important micro-environmental determinant of tumor spheroid structural parameters.
To explore the potential significance of these research findings, it is necessary to consider the relationship between tumor size (mass) and physiology. This complex relationship represents an important parameter of the tumor microenvironment. Cell-to-cell associations that characterize the spontaneous formation of tumor spheroids in vitro prevent anoikis and promote the survival of genetically transformed cells. Increases in tumor mass generate changes in the tumor environment that both sustain survival and promote cell death. The activation of pro-inflammatory pathways in response to hypoxia, for example, represents an important survival mechanism. The micro-environmental effects at the solid tumor core, in contrast, produce necrosis; moreover, the external cell layers of the tumor spheroid appear to comprise the mitotically active portion of the solid tumor. In this microcosm of natural selection, the genesis of small tumor masses of low density may represent a pathophysiological adaptation that may promote tumor survival. In this context, the effects of elevated extracellular Ca++ on spheroid size and density may affect tumor survival by increasing cell surface to volume ratio, thereby increasing the surface area to facilitate increased uptake of oxygen, growth factors and nutrients by tumor cells comprising the spheroid mass. That this biophysical effect is possible is suggested by the observed increased uptake of acridine orange/ethidium bromide by spheroids cultured in 5 mM Ca++ as compared to controls that display fluorescence only at the outer periphery of the spheroid.
Live cell imaging studies conducted in our laboratory suggest that the process of tumor spheroid formation in vitro is associated with microvesicle production that appears to play a role in mediating cell-to-cell associations that characterize spheroid aggregation. The tumor associated microvesicles produced at 5–10 mM Ca++ did not display a fluorescent interior following acridine orange/ethidium bromide staining, suggesting that the vesicles secreted by tumor spheroids at elevated Ca++ were not apoptotic bodies. Research on the molecular composition of tumor associated microvesicles and their biological functions have led to the suggestion that tumor-associated microvesicles may be important epigenetic determinants of tumor progression, metastasis and angiogenesis. The research presented in this paper suggests that tumor associated microvesicles may also play an important role in the cell-to-cell associations that mediate spheroid formation, an activity that is consistently observed by tumor cells in vitro and also organoid cultures of patient tumors. Taken together, the combined effects of increased extracellular [Ca++] on spheroid formation and microvesicle production suggests that Ca++ levels may represent an important micro-environmental component of tumor spheroid biology.
Oncologists generally consider hypercalcemia the most common life-threatening complication of malignancy as it is associated with systemic disease in up to 40% of patients with advanced cancer and generally signals a poor prognosis. [21–23]. Hypercalcemia involves the occurrence of elevated levels of serum Ca++ and is most commonly associated with breast and lung cancer, although it may also be observed in many types of solid tumor malignancies as well as in leukemia. Cancer associated hypercalcemia may occur by two mechanisms: the osteolytic destruction of bone tissue or by the secretion of humoral factors, most commonly parathyroid hormone-related peptide (PTHrP), which causes increased calcium resorption by the renal tubules and also promotes the release of calcium from bone [24]. PTHrP is related to parathyroid hormone and also to a protein involved in Ca++ transport and developmental biology. The gene encoding PTHrP is activated in patients with many types of solid tumors [24]. The physiological relationship between hypercalcemia and tumor biology has not yet been determined. A recent study by Anderson et al [25] provided evidence that PTHrP activity may affect adhesion via integrin-mediated attachment to substrate. Alterations in substrate adhesion associated with hypercalcemia could be associated with increased microvesicle formation. Thus, it is possible that tumor-induced hypercalcemia associated with bone resorption may generate micro-environmental conditions in the body that promote systemic disease, particularly in cancers that metastasize to the bone.
Normal physiological serum [Ca++] is measured as 2.2–2.6 mM. Hypercalcemia of malignancy is clinically diagnosed at [Ca++] = 3 mM and is considered severe at 4 mM [Ca++] [26]. Our research data show that elevated [Ca++] in this approximate range affects tumor spheroid biology in regard to spheroid size, density and the production of microvesicles without significantly impacting tumor spheroid viability. While one cannot extrapolate the findings from simple pre-clinical assessments of solid tumor behavior presented in this paper to define the complex dynamics of systemic malignancy, these observations may be useful in suggesting potentially important disease parameters associated with the tumor microenvironment that may enhance the design of further experiments to address solid tumor behavior in the context of systems biology.
References
- 1.Ginestra A, LaPlaca MD, Saladino F, et al. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18:3433–3437. [PubMed] [Google Scholar]
- 2.Distler JH, Pisetsky DS, Huber LC, et al. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005;52:3337–3348. doi: 10.1002/art.21350. [DOI] [PubMed] [Google Scholar]
- 3.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 4.Beaudoin AR, Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochim Biophys Acta. 1991;10:171–203. doi: 10.1016/0304-4157(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 5.Horstman LL, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med. 2004;53:210–230. doi: 10.2302/kjm.53.210. [DOI] [PubMed] [Google Scholar]
- 6.Skog J, Wurdinger T, Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muralidharan-Chari V, Clancy J, Sedgwick A, et al. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenti R, Huber V, Manuela I, et al. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2006;67:2912. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 9.Tl W. Tumor-derived exosomes or microvesicles: another mechanism of tumor escape from the host immune system? Br J Cancer. 2005;92:209–211. doi: 10.1038/sj.bjc.6602360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George JN, Thoi LL, McManus LM, et al. Isolation of human membrane microparticles from plasma and serum. Blood. 1982;60:834. [PubMed] [Google Scholar]
- 11.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 12.Ginestra A, Miceli D, Dolo V, et al. Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res. 1999;19:3439–3446. [PubMed] [Google Scholar]
- 13.DiVizio D, Kim J, Hager M, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69:5601–5609. doi: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidulescu C, Clejan S, O’Connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8:388–396. doi: 10.1111/j.1582-4934.2004.tb00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janoska-Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:3143–3149. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 16.Barry OP, Fitzgerald GA. Mechanisms of cellular activation by platelet microparticles. Thromb Haemost. 1999;82:794–800. [PubMed] [Google Scholar]
- 17.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;8:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 18.Francia G, Green S, Kerbel R (2005) Epigenetic aspects of environmentally mediated “multicellular” drug resistance. Am Assoc Cancer Res Educ Book, pp 114–118
- 19.Espina V, Mariani B, Gallgher R, et al. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS ONE. 2010;5:e10240. doi: 10.1371/journal.pone.0010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeRoos M, Vegt B, Vries J, et al. Pathological and biological differences between screen-detected and interval ductal carcinoma in situ of the breast. Ann Surg Oncol. 2007;14:2097–2104. doi: 10.1245/s10434-007-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 22.Hotte S, Hirte H, Rabbani S, Carling T, Hendy G, Major P. Hypercalcemia of malignancy: pathophysiology, diagnosis and treatment. Am J Cancer. 2002;1:179–187. doi: 10.2165/00024669-200201030-00003. [DOI] [Google Scholar]
- 23.Davidson TG. Conventional treatment of hypercalcemia of malignancy. Am J Health-Syst Pharm. 2001;15:S8–S15. doi: 10.1093/ajhp/58.suppl_3.S8. [DOI] [PubMed] [Google Scholar]
- 24.Sidler B, Alpert L, Henderson JE, Deckelbaum R, Amizuka N, Silva JE, Goltzman D, Karaplis AC. Amplification of the parathyroid hormone-related peptide gene in a colonic carcinoma. J Clin Endocrinol Metab. 1996;81:2841–2847. doi: 10.1210/jc.81.8.2841. [DOI] [PubMed] [Google Scholar]
- 25.Anderson J, Grabowska A, Watson S. PTHrP increases transcriptional activity of the integrin subunit α5. Br J Cancer. 2007;96:1394–1403. doi: 10.1038/sj.bjc.6603720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suva LJ, Winslow GA, Wettenhall RE, et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]