Abstract
Characterization of the novel human protein MDGA1 (MAM Domain containing Glycosylphosphatidylinositol Anchor-1) has been reported in our laboratory in the past few years. hMDGA1 is a glycoprotein containing 955 aminoacids (137 kDa) attached to the eukaryotic cell membrane by a GPI (Glycosylphosphatidylinositol) anchor and localized specifically into membrane microdomains known as lipid rafts. Moreover, MDGA1 protein contains structural features found in different types of cell adhesion molecules (CAMs) such as the presence of immunoglobulin domains and a MAM domain (Meprin, A5 protein, receptor protein-tyrosine phosphatase μ), suggesting a role of MDGA1 in cell migration and/or adhesion. In order to investigate this aim, stable MDCK cell lines expressing MDGA1 or the truncated proteins IgGPI (lacking the MAM domain) and MAMGPI (lacking Ig domains) were generated. Our results reveal that MDGA1 increases the ability of MDCK cells to migrate, as it contains both Ig and MAM domains which have been implicated in cell motility. In addition, cell adhesion to extracellular matrix proteins, mainly to collagen IV, is reduced by MDGA1 and the IgGPI and MAMGPI truncated proteins. Accordingly, silencing MDGA1 by siRNA revealed a significant increase in adhesion to collagen IV. Furthermore, MDGA1 expression, through the intrinsic properties of the MAM domain, increases cell-cell adhesion independently of the cell monolayer used, suggesting that MDGA1 mediates cell-cell adhesiveness in a heterophilic manner.
Keywords: Adhesion, CAM (Cell Adhesion Molecule), Glycosylphosphatidylinositol (GPI), Immunoglobulin family, MAM, MDCK cells, MDGA1, Migration
Introduction
We have reported the characterization of the novel human protein MDGA1 (MAM Domain containing Glycosylphosphatidylinositol Anchor-1) [1]. MDGA1 is a 137 kDa protein anchored to the membrane of eukaryotic cells by a GPI motif, which is susceptible to be cleaved by phosphatidylinositol-specific phospholipase C (PI-PLC) [1, 2]. Similar to other proteins sorted via the secretory pathway, human MDGA1 (hMDGA1) undergoes post-translational modification consisting of N-glycosylation. Interestingly, hMDGA1 is localized in specialized membrane domains known as lipid rafts [1], providing a hypothetical platform to transduce signals within the cell.
Identification and characterization of the MDGA1 gene (originally termed GPIM) was previously performed in our laboratory [3]. MDGA1 is expressed in multiple human tissues such as brain, heart, skeleton muscle and placenta. Analysis of the 955-aa sequence of hMDGA1 indicated the presence of a signal peptide at the N-terminal, followed by six immunoglobulin-like (Ig) domains, one single fibronectin type III (FnIII) domain, a MAM (Meprin, A5 protein, receptor protein-tyrosine phosphatase μ) domain and a cleavage site for GPI located in the C-terminal anchoring the protein to the cell membrane [3]. Interestingly, identification and characterization of MDGA2, a homologue of MDGA1, containing the same structural organization was also reported in rat [2]. Since these structural motifs are present in multiple Cell Adhesion Molecules (CAMs) a functional role related to cellular adhesion may be speculated for MDGA1.
MDGA1 has been postulated as a member of the Ig superfamily (IgSF), the largest class of CAMs, and contains both a MAM domain and a GPI anchor [1–6]. The presence of these structural features makes it a unique protein, as it is the first GPI-linked IgSF molecule containing a MAM domain described to our knowledge. Some of the GPI-linked IgSF proteins are involved in a variety of specific cell-cell interactions and/or in migration, such as LAMP, BIG-1, neurotrimin, CEPU-1, GP55 [7–9], CEA, CEACAM-6, NCAM p120 [10–12], F3/F11/contactin and TAG1/axonin-1 [13, 14]. In addition, the presence of the MAM and/or Ig domains confers to these proteins the capacity to interact with other cells through homophilic and/or heterophilic interactions [2, 4, 15].
The MAM domain is thought to have an adhesive function, as it is widespread among various adhesive proteins implicated in cell to cell interactions. This adhesive domain was first recognized as a common sequence in the extracellular regions of meprin, A5 antigen and protein tyrosine phosphatases μ, κ [16–18]. Several MAM domain-containing proteins later identified, including zonadhesin [19], nephronectin [20], POEM [21], and neuropilins [22], have been shown to be involved in different aspects of cell adhesion and migration. It has been reported that the MAM domain mediates lateral (cis) homophilic interactions in PTP μ and κ [15, 23] and neuropilin-1 [24, 25]. In the developing chicken nervous system MDGA1 heterophilically interacts with axon-rich regions mainly through its MAM domain, and with differentiating muscle through its Ig-repeat-containing N-terminal region [4].
Over the last few years, the expression profile of rat and mouse MDGA1 and MDGA2 has been reported, suggesting a role in controlling neuronal adhesion, migration and axon outgrowth in the developing rat brain [2]. These authors report that MDGA1 is highly expressed by two distinct populations of migrating neurons, each of which undergo non-radial circumferential migration from their germinal zone, suggesting that this protein controls aspects of their migration [2]. Moreover, MDGA1 is expressed in cortical neurons that migrate to the superficial layer in late embryos and neonates in mice [5, 6]. Knock-down of MDGA1 expression by in vivo RNAi disrupts radial migration of these neurons [5], suggesting that MDGA1 is essential for neuronal cell migration. Recently, the expression of MDGA1 and MDGA2 has been described in the migration and differentiation of neurons at the periphery of the brain/neural tube in medaka embryos [26], suggesting that MDGAs are also implicated in neurons migration in the teleost fish.
The main goal of the present work was to investigate the role of human MDGA1 in cell migration and cellular adhesion in vitro. For this purpose, stable cell lines expressing MDGA1 or the truncated forms IgGPI and MAMGPI, were generated to be used for in vitro migration and adhesion assays, in order to characterize the functional domain(s) of this protein. Our results have shown that: (i) MDGA1 increases cell motility in MDCK cell lines; (ii) MDGA1 decreases cell adhesion to ECM proteins, principally to collagen IV and (iii) MDGA1 is involved in cell-cell adhesion, which is in part heterophilic, mainly through its MAM domain.
Material and Methods
Cloning
pFLAG-MDGA1, pFLAG-IgGPI and pFLAG-MAMGPI constructs were generated by PCR amplification of the MDGA1 full sequence cDNA clone 2782838 (IMAGE) using the primers: 5′-GGGAATTCGATGGAGGTGACCTGCCTTCT-3′ and 5′-GGTCTAGATCATCTCTGCAACGCCAAGAGG-3′ for pFLAG-MDGA1 (product length 2891 bp); 5′-GGGAATTCGATGGAGGTGACCTGCCTTCT-3′, 5′-GGCTCGAGGGTGTTGTCTGAAAGG-3′, 5′-GGCTCGAGAAGCAGACGGATCCC-3′ and 5′-GGTCTAGATCATCTCTGCAACGCCAAGAGG-3′ for pFLAG-IgGPI (product length 2415 bp); 5′-GGGAATTCGTGCCACTTTGAGGATGAG-3′ and 5′-GGTCTAGATCATCTCTGCAACGCCAAGAGG-3′ for the pFLAG-MAMGPI (product length 629 bp). PCR products were cloned into pSTBLUE-1 acceptor vector (Novagen), confirmed by sequencing, and then subcloned into pFLAG-CMV-3 (Sigma). In all cases, in-frame fusion proteins generated were confirmed by sequencing, and their expression was verified by Western blotting. pFLAG expression vector without coding regions served as empty vector control (EV).
Cell Culture Conditions and cDNA Transfections
The MDCK cell line, kindly provided by Dr. Miguel Quintanilla (Instituto de Investigaciones Biomédicas, CSIC), was cultured in DMEM medium (Gibco BRL) supplemented with 10% fetal calf serum (FCS) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), in a humidified chamber with 5% CO2 at 37°C. For stable transfections, MDCK cells were transfected with pFLAG-MDGA1, pFLAG IgGPI or pFLAG-MAMGPI partial constructs, or the empty pFLAG vector, using FuGene 6 transfection reagent (Roche Molecular Biochemicals, France) according to the manufacturer’s instructions. Transfected cells were selected by adding 500 μg/ml of neomycin (G418) during 3 weeks. All resistant clones were collected in order to obtain polyclonal stable cell lines (MDGA1, IgGPI or MAMGPI MDCK cells). Expression of all fusion proteins generated was confirmed by Western blotting.
Northern Blot
Twenty microgram of total RNA of subclonfluent cells was isolated by TRIZOL reagent (Invitrogen), separated on 1% formaldehyde agarose gels, blotted onto GeneScreen membranes (Perkin Elmer Life Sciences) by the capillary method, and UV cross-linked. cDNA labeling was carried out with [α-32P]dCTP by random priming reaction (Amersham). MDGA1 cDNA probe covering the nucleotides 1-1201 was obtained from digestion with XhoI and SacI restriction enzymes of the cDNA clone 2782838 (IMAGE) containing the MDGA1 full sequence.
Primary Antibodies
Anti-FLAG M2 and anti-FLAG M2 FITC-conjugated monoclonal antibodies were purchased from Sigma. In addition, a polyclonal antibody against MDGA1, termed as GP854, was obtained by immunization of two rabbits with a protein sequence belonging to the MAM domain (comprising aminoacids 753-918 of MDGA1 protein) coupled to KLH (Eurogentec).
Immunofluorescence Analysis
For Immunofluorescence analysis, cells were grown on glass coverslips, fixed with 5% formaldehyde, incubated with anti-FLAG M2-FITC and DAPI, and then examined by laser confocal scanning system as described in [1].
Western Blot
Twenty microgram of total cell extracts in RIPA buffer were loaded on SDS-PAGE gels, electrotransferred to a nitrocellulose membrane (Biotech) and then incubated with anti-FLAG M2 or GP854 serum. Protein bands were visualized using ECL Western blotting detection kit (Amersham). β-actin was used as loading control.
Wound Healing Assay
Cells cultured in medium with 10% FCS to confluent monolayers were incised in the central area with a sterile plastic tip. After removal of detached cells with PBS and addition of 1% FCS fresh media for another 12 h, snapshots of the wound area were taken at 6, 8 and 10 h with a CANON powershot A85 camera. Each condition was photographed in three separate fields and the same field at each time point was used.
In Vitro Proliferation Assay
2 × 103 cells/well from pFLAG or pFLAG-MDGA1 cell lines were seeded on 96 well plates. Adherent cells were stained at different times with crystal violet (0.2% in 2% ethanol) for 20 min and quantified with 1% sodium dodecyl sulfate. Absorbance was read at 590 nm in a BioRad microplate reader (Benchmark).
Transwell Migration Assay
Cell migration assays were performed by using Transwell chambers with 8 μm pore polycarbonate filters (Corning). 100 μl of cells (5 × 105 cells/ml) in serum-free medium were added to the upper chamber and 500 μl of 10% FCS medium in the lower. Migration was allowed to occur for 16 h at 37°C, after that, cells from both upper and lower chambers were counted on a CASY-1 cell counter (Schärfe System GmbH). For quantification, the percentage of migrated cells (migration rate) was calculated as follow: rate of migration (%) = number of migrated cells/(number of migrated cells + number of non-migrated cells) × 100. Results were expressed as a percentage of migration relative to control cells. The transwell assays for each condition were performed in triplicate and the average values obtained are shown.
Adhesion to Extracellular Matrix Proteins Assay
The assay was performed using the Cytomatrix Screening cell adhesion Kit (Chemicon, Temecula, CA) containing 96-well plates coated with fibronectin, vitronectin, laminin, collagen-I or collagen-IV. One-hundred microliter of cells (6 × 105 cells/ml) in serum-free medium were seeded onto coated substrate and incubated at 37°C for 1 h. Cells were washed three times with PBS to remove non adherent cells, and adherent cells were stained with crystal violet (0.2% in 2% ethanol) for 30 min at room temperature. Solubilization buffer (100 μl of a 50:50 mixture of 0.1 M NaH2PO4 (pH 4.5) and 50% ethanol) was added to each well. Cell adhesion was quantified by measuring the absorbance at 595 nm in a BioRad microplate reader (Benchmark). Results were expressed as a percentage of adhesion relative to control cells.
Cell-Cell Adhesion Assays
Cell adhesion assays were performed by using vibrant cell adhesion kit (Molecular Probes, Eugene, OR). 5 × 106 cells/ml were resuspended in serum free medium and incubated with 5 μM calcein AM for 30 min at 37°C. Calcein AM is nonfluorescent but, once loaded into cells, it is cleaved by endogenous esterases to produce highly fluorescent calcein. After incubation, non-incorporated calcein dye was removed by three washes with prewarmed serum free medium. 5 × 105 of the calcein-labeled cells were added to a confluent monolayer of MDCK cells seeded in a 96-well microtiter plate. After 1 h incubation at 37°C, non adherent calcein-labeled cells were removed carefully by washing with prewarmed PBS and wells were overlaid with PBS prior to quantification. Relative fluorescence intensity of the adherent calcein-labeled cells was measured in a fluorescence microplate reader (FL600 BioTek), using a standard fluorescein filter with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Results were expressed as a percentage of adhesion relative to control cells.
siRNA Assay
Custom SMARTpool siRNA oligonucleotides targeting MDGA1 and control (non-targeting siRNA oligonucleotides pool) were purchased from Dharmacon RNA Technologies. Cells were transfected with 100 mM of either siRNA MDGA1 or non-targeting siRNA pool using Oligofectamine reagent (Invitrogen). Forty-eight hour after transfection cells were harvested and adhesion assays were performed.
Statistical Analysis
Data were statistically analyzed with a two-tailed Student’s t-test. A P-value of <0.05 was considered a statistically significant difference. Error bars indicate standard deviations.
Results
Characterization of MDGA1 Expressing Cell Lines
In order to investigate the role of MDGA1 in migration and cellular adhesion, polyclonal stable cell lines were generated by transfecting MDCK cells with the following constructs: pFLAG-MDGA1, pFLAG-IgGPI, pFLAG-MAMGPI (Fig. 1), or the empty vector (EV) pFLAG, and selected with neomycin (G418). All resistant clones were collected in order to analyse the expression of MDGA1 or the truncated proteins IgGPI and MAMGPI. MDGA1 gene expression was detected by Northern blot analysis as a nearly 4 kb mRNA in stable MDGA1-transfected cells (Fig. 2a). Nevertheless, no expression was detected in non-transfected and control (EV) cells, suggesting a non-endogenous MDGA1 expression in MDCK cells (Fig. 2a). MDGA1 expression was also confirmed by RT-PCR (Fig. 2b) and confocal microscopy analysis (Fig. 2c). Finally, expression of MDGA1 or the truncated proteins (IgGPI, MAMGPI) was analysed by Western blotting (Fig. 2d). A protein form of over 140 kDa for MDGA1 was detected in MDCK cells as described previously by us in a human cancer cell line [1] and in rat brain [2]. In this case, anti-FLAG antibody signal has been lower than signal obtained with GP854 antibody. Nevertheless, the absence of both bands in control (EV) lanes fully confirms the identity of MDGA1 protein in the corresponding lanes (Fig. 2d). Different possibilities could explain the faint band with the anti-FLAG antibody, among them the fact that only one copy of the Flag epitope (8 amino acids) is located at the N-terminal of the protein, meanwhile GP854 recognizes different epitopes spanning the whole MAM domain (165 amino acids) of MDGA1 protein. Moreover, bands of around 113 kDa and 28 kDa for IgGPI and MAMGPI proteins respectively, were also detected by Western blot (Fig. 2d).
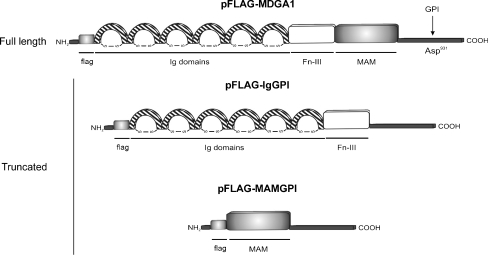
Fig. 1.
Structural organization of MDGA1 and truncated proteins. MDGA1 full length (pFLAG-MDGA1) contains an N-terminal signal peptide followed by flag sequence, six immunoglobulin-like (Ig) domains, one single fibronectin type III (FnIII) domain, a MAM (Meprin, A5 protein, receptor protein-tyrosine phosphatase μ) domain and a potential cleavage site (Asp931) for GPI (GlycosylPhosphatidylInositol) in the C-terminal anchoring the protein to the cell membrane. In the truncated proteins we have removed either the MAM domain (pFLAG-IgGPI) or the six Inmunoglobulin and the FnIII domains (pFLAG-MAMGPI)
Fig. 2.
Expression of MDGA1 and truncated proteins in stable cell lines. MDGA1 expression in MDCK cells was analysed by a Northern-blot. β actin was used as loading control; b RT-PCR. GAPDH was used as positive control c Confocal microscopy. DAPI cell nucleus staining (a). MDGA1 expression using anti-Flag M2-FITC-conjugated monoclonal antibody was detected in the cell membrane (b). Merged images (c). d Expression of MDGA1 by Western blot using anti-FLAG and anti-MAM (GP854) antibodies (see Materials and Methods). MDGA1 was expressed as a 140 kDa protein in the anti-FLAG blot (indicated by an arrow) and in the GP854 blot. Truncated proteins IgGPI and MAMGPI were detected as an around 113 kDa (indicated by an arrow) and an around 28 kDa bands respectively. In both cases immunoblot was performed using anti-FLAG antibody. In all cases β-actin was used as a loading control
MDGA1 Increases Cell Motility
Cell motility of MDGA1-expressing cells and the control (EV) cell line was assessed by different migration assays. Firstly, we performed wound healing assays that provide qualitative information about the migration properties of a determined cell line. This assay shows the ability of cells to migrate into a wound made in confluent culture. In order to perform this assay, confluent cell monolayers of stable MDGA1-expressing cells and control (EV) cells were wounded with a plastic tip, and migration of cells into the cell-free area was analysed after 6, 8 and 10 h. A quantification of the cell-free area has been indicated (Fig. 3a). As shown in this figure, an increased migration of MDGA1 cells was observed as compared to control (EV) cells. Increased motility of these cells, observed at the different time points, suggests that MDGA1 expression increases the ability of MDCK cells to migrate.
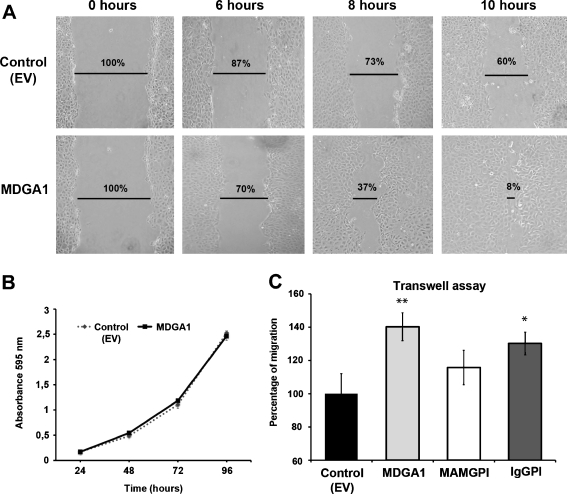
Fig. 3.
MDGA1 increases cell motility in MDCK cells. a Wound healing assay was performed in MDGA1 and control (EV) cells. Snapshots of the healing were taken at 0, 6, 8 and 10 h, showing MDGA1 cells exhibited an increase in migration (lower lane) compared to control (EV) cells (upper lane). Cell-free area percentages are indicated. b No differences were found in the proliferation rate between MDGA1 and control (EV) cells. c MDGA1 or IgGPI expression increases cell migration rates in Transwell assays compared to control (EV) cells. Values represent mean ± S.D. Significant values are indicated by asterisks
An increased proliferation rate in one of the cell lines might mask different migration behaviours. In order to avoid possible misinterpretation of our data, proliferation assays were performed in both cell lines showing no difference in growth (Fig. 3b) demonstrating that the differences found in the wound healing assays were due to increase migration.
Wound healing assays are useful for comparing the migration rates of different cell lines but are difficult to quantify. Alternatively, Transwell chamber assays have been developed in order to determine migration rates. In this case, MDGA1 protein domains involved in migration were investigated as well. For this purpose, migration assays by Transwell chambers were performed in MDGA1, MAMGPI and IgGPI MDCK cells. Cell migration was expressed as migration percentage of the control (EV) cells (Fig. 3c). Our results show that cell migration levels were significantly increased by 40% (p = 0,002) in MDGA1 cells (Fig. 3c). In addition, IgGPI increased migration by 30% (p = 0,005) and a non-significant increase was obtained in MAMGPI cells (Fig. 3c). Since the highest migration rate was obtained for MDGA1 expressing cells, we suggest that both domains, Ig and MAM, are necessary to achieve a full migration response mediated by MDGA1 in MDCK cells.
MDGA1 Reduces Cell-Matrix Protein Adhesion
Cell Adhesion Molecules (CAMs) mediate cell-cell and/or cell-matrix interactions. In order to investigate MDGA1 or the truncated proteins adhesion to extracellular matrix proteins, cells expressing MDGA1, IgGPI or MAMGPI were seeded onto wells coated with any of the following cell-matrix proteins: collagen I, collagen IV, fibronectin, vitronectin or laminin. After 1 h incubation, cells adhered to matrix components were fixed and stained, and relative cell adhesion was determined by absorbance readings. Results were expressed as a percentage of adhesion relative to control (EV) cells. As shown in Fig. 4a, MDGA1-expressing cells showed a decreased adhesion to all ECM proteins analysed, especially to collagen IV (44% decrease, p = 0,002) and laminin (37% decrease, p = 0,003). Moreover, cells expressing the truncated proteins (IgGPI and MAMGPI) also showed a decrease in adhesion to all ECM proteins analysed, especially to collagen IV. Nevertheless, this decrease was lower in these cases than those obtained in MDGA1-expressing cells, especially when vitronectin was assayed.
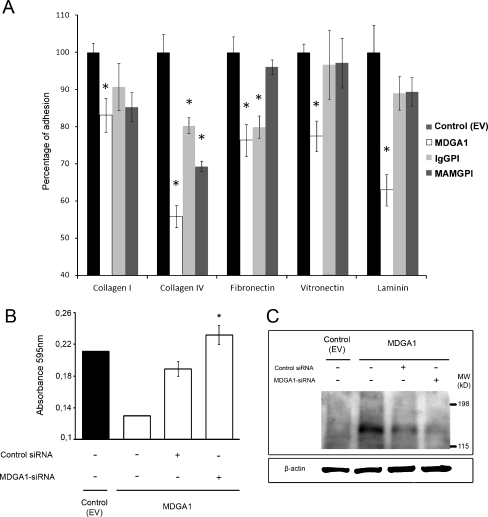
Fig. 4.
MDGA1 reduces adhesion to cell-matrix proteins in MDCK cells. a Adhesion to cell-matrix proteins of cells expressing MDGA1 and IgGPI or MAMGPI truncated proteins were analysed in coated collagen type I and IV, fibronectin, vitronectin and laminin wells, as described in Materials and Methods. Relative cell adhesion was expressed as a percentage of control (EV) cells. Values represent mean ± S.D. Significant values are indicated by asterisks. b, c siRNA untransfected control (EV) cells, siRNA untransfected MDGA1-expressing cells and MDGA1-expressing cells transfected with either siRNA control or siRNA against MDGA1 were harvested 48 h after transfection, and then cells were used in adhesion to collagen IV assay (b) or immunodetection by Western blot (c). β actin was used as loading control (c)
In addition, MDGA1 silencing was performed by siRNA (Fig. 4b) in order to confirm that MDGA1 mediates the adhesion decrease observed above. Adhesion to collagen type IV was analysed as this was the most significant decrease from our previous results. With this aim, siRNA untransfected control (EV) cells, siRNA untransfected MDGA1-expressing cells as well as MDGA1-expressing cells transfected with either siRNA control or siRNA against MDGA1 were collected and seeded in collagen IV coated wells. Then relative adhesion to this matrix protein was analysed as described above. The results of this experiment demonstrate that, although transient transfection of siRNA control in MDGA1-expressing cells affect to their capability to adhere to collagen IV, the reduction in MDGA1 levels by transfection with specific siRNA against MDGA1 induced a statistically significant increase in adhesion to collagen IV (p = 0,008), as compared to MDGA1-expressing cells transfected with siRNA control (Fig. 4b). This result support that MDGA1 mediates the observed decrease in adhesion to collagen type IV (Fig. 4a). A direct correlation between suppression of MDGA1 protein expression by siRNA and recovery of adhesion to collagen IV is observed in Fig. 4c.
MDGA1 Mediates Cell-Cell Adhesion
The effect of MDGA1 on cell-cell adhesion was also investigated. For this aim, MDGA1-expressing cells and control (EV) cells were labelled with calcein AM. This molecule is non-fluorescent but, once loaded into cells, it is cleaved by endogenous esterases to produce highly fluorescent calcein, without affecting the cell adhesion process. Calcein-labelled cells were added either to a monolayer of confluent MDCK cells expressing MDGA1 or to control (EV) cells, which had been seeded the day before in microtiter plates. Following co-incubation, non-adherent cells were removed and relative fluorescence intensity of the adherent cells was determined. Relative cell adhesion was expressed as a percentage of control (EV) cells. As shown in Fig. 5, cell adhesion capacity was significantly increased in cells expressing MDGA1 as compared to control (EV) cells, when MDGA1 monolayer was used (33% increase, p < 0,001). Similar results were obtained when adhesion assay was performed by adding calcein-labelled cells to a control (EV) monolayer substrate. Altogether, our results indicate that MDGA1 increases cell-cell adhesion independent of the cell monolayer used suggesting that MDGA1 mediates heterophilic cell-cell interactions.
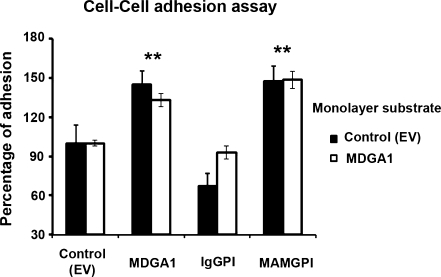
Fig. 5.
MDGA1 increases cell-cell adhesion. Cells expressing MDGA1, IgGPI or MAMGPI protein, and control (EV) cells were labelled with calcein, and after it they were seeded onto a monolayer of confluent MDCK cells expressing MDGA1 or to control (EV) cells. Following co-incubation, non-adherent cells were removed and relative fluorescence intensity of the adherent cells was determined. Relative cell adhesion was expressed as a percentage of control (EV) cells. Values represent mean ± S.D. Significant values are indicated by asterisks
We also examined the adhesion capacity of MDGA1 truncated forms (IgGPI and MAMGPI) in order to analyse which protein domain was involved in MDGA1- mediated adhesion (Fig. 5). When MAMGPI-expressing cells were used in adhesion assays, a significant cell-cell adhesion increase was also obtained compared to control (EV) cells with the two monolayer substrates assayed (48% increase, p = 0,001). Nevertheless, the rates of adhesion in IgGPI-expressing cells were lower or similar than those obtained in the control (EV) cells (Fig. 5). Altogether, these results suggest that MAM seems to be the domain implicated in MDGA1-mediated cell-cell adhesion.
Discussion
The main goal of the present study has been to investigate the implication of human MDGA1 in cell migration and cellular adhesion. MDCK cells have been widely used in migration and adhesion experiments by different groups [27, 28], and more importantly these cells do not display endogenous MDGA1 expression. By contrast MDGA1 expression was detected when cells were transfected with p-FLAG-MDGA1, p-FLAG-IgGPI and p-FLAG-MAMGPI. In addition, no difference in proliferation between control (EV) and MDGA1-expressing cells was found, avoiding misinterpretations of our results. All together, let us to consider MDCK cells a suitable candidate cell line for our studies. For this purpose, polyclonal stable MDCK cells expressing MDGA1 protein or the truncated forms, IgGPI and MAMGPI, were used in migration and adhesion in vitro assays. We analysed migration capacity by wound healing assays, finding that MDCK cells expressing MDGA1 have an increased cell motility compared to control (EV) cells. Our results are in agreement with previous studies in developing mice brain which indicate expression of MDGA1 is correlated with migrating neurons [2, 5]. Quantitative migration assays by Transwell chambers were also performed in order to characterize the MDGA1 functional domains. In this case we compared the migration capacity of MDGA1, IgGPI and MAMGPI expressing cells. Our results revealed that cell migration levels were significantly increased in MDGA1 and IgGPI cells. This suggests that both domains, Ig and MAM, are involved in the increase of the migratory behaviour of MDCK cells. The absence of a cytoplasmic domain in MDGA1 suggests that it might function as a member of an unidentified protein complex, therefore interaction between MDGA1 and other protein(s) might activate a signal transduction pathway inducing cell migration. It has been reported that the ciliary neurotrophic factor receptor (CNTFR), another GPI protein, is able to transduce survival and/or neuronal differentiation signals [29, 30], as it is associated to the transmembrane proteins LIF and gp130 [31] forming a protein complex whose activation induces intracellular signals through JAK kinases [32]. Thy-1 represents another GPI protein whose association with αMβ2 integrin induces adhesion and migration in leukocytes [33]. NCAM (Neural Cell-Adhesion Molecule), one of the best-studied members of the IgSF, which is involved in cellular migration and cell-cell adhesion, interacts with FGFR (Fibroblast Growth Factor Receptor) probably through its Ig domains, and N-cadherin interacts with FGFR through its extracellular cadherin domain. The signal transduction pathways that are activated by the N-CAM-FGFR-N-cadherin complex ultimately lead to the activation of β1-integrin mediated cell-matrix adhesion and neurite outgrowth [34].
On the other hand, when we investigated the adhesion of MDGA1-expressing cells to ECM proteins, a significant decrease in adhesion was obtained with all ECM proteins analysed, with the greatest decrease (up to 44%) observed with collagen IV, the main component of extracellular matrix proteins. Likewise, cells expressing the truncated proteins (IgGPI and MAMGPI) showed a decreased in adhesion to all ECM proteins assayed, again with the greatest decrease in adhesion to collagen IV. These results suggest that the Ig and MAM domains are involved in adhesion of MDGA1 to ECM proteins, being necessary the presence of both domains together to produce a higher decrease in adhesion than any of these domains alone. In addition, we have shown that a reduction of MDGA1 expression results in an increased adhesion to collagen IV. Loss of function studies using RNA interference (siRNA) targeting different sequences of human MDGA1, indicated a direct correlation between suppressing MDGA1 expression and recovery of adhesion to collagen IV. Taken together, our results indicate that MDGA1 also plays a role in cell-matrix adhesion.
MDGA1 is furthermore involved in cell to cell adhesion since MDGA1-expressing cells exhibited increased adhesion to cell monolayers. In our hands, adhesion results were similar when MDGA1 cells were added either to a monolayer of cells expressing MDGA1 or to control (EV) cells. This observation supports the idea that MDGA1 might interact with another protein in the neighbouring cells through a heterophilic interaction. Moreover, our results indicate that MDGA1 may interact with this protein through its MAM domain, as cells expressing MAMGPI truncated protein exhibited a significant increase in cell-cell adhesion, compared to IgGPI expressing cells lacking the MAM domain. In the developing chicken nervous system it has been reported that MDGA1 is associated with axon-rich regions mainly through its MAM domain, suggesting that MDGA1 might function as an axon guidance molecule [4]. Furthermore, these authors report that this interaction is at least in part heterophilic in concordance with our results. We agree with Fujimura et al. [4] that our results do not exclude a potential homophilic interaction among MDGA1 molecules present in different cells that might not be detected by the calcein assay we used. In other proteins, including neuropilin-1 and PTPμ, homophilic interaction mediated by the MAM domain present in the cell surface of these proteins has been reported [15, 25]. We consider that the apparent presence of heterophilic association through the MAM domain suggests that MDGA1 might function in an unidentified protein complex. Fujimura et al. has also reported that MDGA1 interacts with differentiating muscle through its N-terminal region, which contains Ig-repeat domains. Our results indicate that these domains do not seem to be implicated in MDCK cell-cell adhesion, but we cannot discard that MDGA1 Ig domains may be involved in interactions with other cell lineages, different from MDCK cells. To this aim, identification of protein(s) which interact with MDGA1 and increase cell-cell adhesion will be considered in our future studies.
In our previous studies expression analysis of MDGAs in human tissues revealed that these genes are expressed in brain [3]. Consistent with this, in the last few years both MDGA1 and MDGA2 have been implicated in human neurodevelopmental syndromes. MDGA1 has been proposed as a new schizophrenia susceptibility gene involved in neuronal migration [35], meanwhile MDGA2 has been postulated as a novel autism susceptibility gene that shows a high similarity to contactin 4 (CNTN4), which has also been linked to this disease [36]. Moreover, MDGA1 expression has been found to be altered in tumor tissues [3, 37]. Future studies to investigate the implication of the MDGAs in these human diseases should be promising.
Acknowledgments
We are very grateful to Dr. Carmen Rivas (Departamento de Microbiologia. Facultad de Farmacia. UCM) for continuous scientific support and insightful discussions, to Dr. Jesús Cruces (Departamento de Bioquímica. Facultad de Medicina. UAM) for valuable comments and hepful discussions on MDGA1 and to Dr. Miguel Quintanilla (Instituto de Investigaciones Biomédicas. CSIC.) for kindly providing MDCK cells. We also thank Professor Ian Hart (Barts & The London School of Medicine & Dentistry, London, UK) for his hospitality and assistance in the migration assays. A. Diaz-Lopez was a fellowship of Ministerio de Educación y Ciencia of Spain. This work was supported by grants from Fundación Investigación Médica Mútua Madrileña (FMMA) (Spain); Ministerio de Sanidad y Consumo (FIS PI080033) and Red Temática de Investigación Cooperativa de Centros de Cáncer (RTICC) RD06/0020/0021.
References
- 1.Díaz-López A, Rivas C, Iniesta P, et al. Characterization of MDGA1, a novel human glycosylphosphatidylinositol-anchored protein localized in lipid rafts. Exp Cell Res. 2005;307:91–99. doi: 10.1016/j.yexcr.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Litwack ED, Babey R, Buser R, et al. Identification and characterization of two novel brain-derived immunoglobulin superfamily members with a unique structural organization. Mol Cell Neurosci. 2004;25:263–274. doi: 10.1016/j.mcn.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Juan C, Iniesta P, Gonzalez-Quevedo R, et al. Genomic organization of a novel glycosylphosphatidylinositol MAM gene expressed in human tissues and tumors. Oncogene. 2002;21:3089–3094. doi: 10.1038/sj.onc.1205383. [DOI] [PubMed] [Google Scholar]
- 4.Fujimura Y, Iwashita M, Matsuzaki F, Yamamoto T. MDGA1, an IgSF molecule containing a MAM domain, heterophilically associates with axon- and muscle-associated binding partners through distinct structural domains. Brain Res. 2006;1101:12–19. doi: 10.1016/j.brainres.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi A, O’Leary DD. Radial migration of superficial layer cortical neurons controlled by novel Ig cell adhesion molecule MDGA1. J Neurosci. 2006;26:4460–4464. doi: 10.1523/JNEUROSCI.4935-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi A, Hamasaki T, Litwack ED, O’Leary DD. Novel IgCAM, MDGA1, expressed in unique cortical area- and layer-specific patterns and transiently by distinct forebrain populations of cajal-retzius neurons. Cereb Cortex. 2007;17:1531–1541. doi: 10.1093/cercor/bhl064. [DOI] [PubMed] [Google Scholar]
- 7.Pimenta AF, Fischer I, Levitt P. cDNA cloning and structural analysis of the human limbic-system-associated membrane protein (LAMP) Gene. 1996;170:189–195. doi: 10.1016/0378-1119(96)84698-1. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara Y, Kawasaki M, Tani A, et al. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DJ, Kim DS, Clarke GA, et al. A family of glycoproteins (GP55), which inhibit neurite outgrowth, are members of the Ig superfamily and are related to OBCAM, neurotrimin, LAMP and CEPU-1. J Cell Sci. 1996;109:3129–3138. doi: 10.1242/jcs.109.13.3129. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Kaye FJ, Henslee JG, et al. Expression of carcinoembryonic antigen and related genes in lung and gastrointestinal cancers. Int J Cancer. 1992;52:718–725. doi: 10.1002/ijc.2910520509. [DOI] [PubMed] [Google Scholar]
- 11.Ilantzis C, Jothy S, Alpert LC, et al. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Investig. 1997;76:703–716. [PubMed] [Google Scholar]
- 12.Peck D, Walsh FS. Differential effects of over-expressed neural cell adhesion molecule isoforms on myoblast fusion. J Cell Biol. 1993;123:1587–1595. doi: 10.1083/jcb.123.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haenisch C, Diekmann H, Klinger M, et al. The neuronal growth and regeneration associated Cntn1 (F3/F11/Contactin) gene is duplicated in fish: expression during development and retinal axon regeneration. Mol Cell Neurosci. 2005;28:361–374. doi: 10.1016/j.mcn.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Zuellig RA, Rader C, Schroeder A, et al. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992;204:453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]
- 15.Cismasiu VB, Denes SA, Reilander H, et al. The MAM (meprin/A5-protein/PTPmu) domain is a homophilic binding site promoting the lateral dimerization of receptor-like protein-tyrosine phosphatase mu. J Biol Chem. 2004;279:26922–26931. doi: 10.1074/jbc.M313115200. [DOI] [PubMed] [Google Scholar]
- 16.Beckmann G, Bork P. An adhesive domain detected in functionally diverse receptors. Trends Biochem Sci. 1993;18:40–41. doi: 10.1016/0968-0004(93)90049-S. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Gorbea CM, Flannery AV, et al. The alpha subunit of meprin A. Molecular cloning and sequencing, differential expression in inbred mouse strains, and evidence for divergent evolution of the alpha and beta subunits. J Biol Chem. 1992;267:9185–9193. [PubMed] [Google Scholar]
- 18.Takagi S, Hirata T, Agata K. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7:295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- 19.Gao Z, Garbers DL. Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J Biol Chem. 1998;273:3415–3421. doi: 10.1074/jbc.273.6.3415. [DOI] [PubMed] [Google Scholar]
- 20.Brandenberger R, Schmidt A, Linton J, et al. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol. 2001;154:447–458. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimura N, Tezuka Y, Watanabe N, et al. Molecular cloning of POEM: a novel adhesion molecule that interacts with alpha8beta1 integrin. J Biol Chem. 2001;276:42172–42181. doi: 10.1074/jbc.M103216200. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa H. From the discovery of neuropilin to the determination of its adhesion sites. Adv Exp Med Biol. 2002;515:1–12. doi: 10.1007/978-1-4615-0119-0_1. [DOI] [PubMed] [Google Scholar]
- 23.Zondag GC, Koningstein GM, Jiang YP, et al. Homophilic interactions mediated by receptor tyrosine phosphatases mu and kappa. A critical role for the novel extracellular MAM domain. J Biol Chem. 1995;270:14247–14250. doi: 10.1074/jbc.270.24.14247. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/S0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura F, Tanaka M, Takahashi T, et al. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/S0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 26.Sano S, Takashima S, Niwa H, et al. Characterization of teleost Mdga1 using a gene-trap approach in medaka (Oryzias latipes) Genesis. 2009;0:1–9. doi: 10.1002/dvg.20528. [DOI] [PubMed] [Google Scholar]
- 27.Peinado H, Quintanilla M, Cano A. Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 28.Moreno-Bueno G, Peinado H, Molina P, et al. The morphological and molecular features of the epithelial-to-mesenchymal transition. Nat Protoc. 2009;4:1591–1613. doi: 10.1038/nprot.2009.152. [DOI] [PubMed] [Google Scholar]
- 29.Davis S, Aldrich TH, Valenzuela DM, et al. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 30.Sendtner M, Carroll P, Holtmann B, et al. Ciliary neurotrophic factor. J Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- 31.Stahl N, Yancopoulos GD. The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol. 1994;25:1454–1466. doi: 10.1002/neu.480251111. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich PC, Behrmann I, Muller-Newen G, et al. L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetzel A, Chavakis T, Preissner KT, et al. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Immunol. 2004;172:3850–3859. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- 34.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 35.Kähler AK, Djurovic S, Kulle B, et al. Association analysis of schizophrenia on 18 genes involved in neuronal migration: MDGA1 as a new susceptibility gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1089–1100. doi: 10.1002/ajmg.b.30726. [DOI] [PubMed] [Google Scholar]
- 36.Bucan M, Abrahams BS, Wang K, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu XY, Lu Y, Zhao YJ, et al. Cell cycle regulator gene CDC5L, a potential target for 6p12-p21 amplicon in osteosarcoma. Mol Cancer Res. 2008;6(6):937–946. doi: 10.1158/1541-7786.MCR-07-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]