Abstract
Osteosarcoma is the most common primary malignant bone tumour. Currently osteosarcoma classification is based on histological appearance. It was the aim of this study to use a more systematic approach to osteosarcoma classification based on gene expression analysis and to identify subtype specific differentially expressed genes. We analysed the global gene expression profiles of ten osteosarcoma samples using Affymetrix U133A arrays (five osteoblastic and five non-osteoblastic osteosarcoma patients). Differential gene expression analysis yielded 75 genes up-regulated and 97 genes down-regulated in osteoblastic versus non-osteoblastic osteosarcoma samples, respectively. These included genes involved in cell growth, chemotherapy resistance, angiogenesis, steroid- and neuropeptide hormone receptor activity, acute-phase response and serotonin receptor activity and members of the Wnt/ß-catenin pathway and many others. Furthermore, we validated the highly differential expression of six genes including angiopoietin 1, IGFBP3, ferredoxin 1, BMP, decorin, and fibulin 1 in osteoblastic osteosarcoma relative to non-osteoblastic osteosarcoma. Our results show the utility of gene expression analysis to study osteosarcoma subtypes, and we identified several genes that may play a role as potential therapeutic targets in the future.
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumour in children and adolescents. The introduction of multiagent chemotherapy followed by surgical resection and postoperative chemotherapy has improved the long-term survival of patients with osteosarcoma from only 20% to nearly 70% during the last 30 years [1]. However, there is still a large number of patients whose tumours respond poorly to chemotherapy and who are at high risk for local recurrence and metastasis. These patients do not benefit from the improvements [2] achieved so far and still die early. The ability to identify a high-risk group among osteosarcoma patients would be of major importance in the development of new and risk-adapted strategies.
Osteosarcoma is classified as a malignant mesenchymal neoplasm in which the tumour produces defective, immature bone (osteoid). Despite this simple definition, the clinical behaviour of osteosarcoma is highly heterogeneous in many aspects.
Some osteosarcoma patients can be cured by local therapy without any further adjuvant therapy, whereas others are resistant to chemotherapeutic drugs and present with widespread distant metastasis at the time of diagnosis. The histomorphological findings of each tumour also show a great variety of characteristics.
The predominant cell type in most osteosarcoma is osteoblastic, while others show more fibroblastic–fibrohistocytic and chondroblastic features. Furthermore, osteosarcoma is one of the most frequent tumours associated with other malignancies or hereditary syndromes such as Li-Fraumeni-, Werner-, or Rothmund-Thomson syndrome. This pronounced heterogeneity raises the question whether osteosarcoma is a single entity at all.
The biological and clinical significance of osteosarcoma subtypes are controversial in literature because data based upon large enough controlled randomised trials recognising osteosarcoma subtypes as separate entities are lacking.
Currently most osteosarcomata are categorised on the basis of morphological and histological criteria as common, chondroblastic, small cell, teleangiectatic, fibroblastic, osteoclast rich, anaplastic, and others.
The prognostic relevance of histological subtypes of osteosarcoma has received little attention and remains a controversial issue [3–6]. Previous studies have shown that the histological subtype of osteosarcoma is a predictive factor for response to chemotherapy [7, 8] and correlates with disease-free [9, 10] and overall survival [3]. Furthermore, a non-common subtype of osteosarcoma raises the possibility of an individual belonging to a family with hereditary cancer syndrome, reflecting a possible genetic background for malignancy [11]. So far the treatment options for most patients with osteosarcoma are not different between either of these histological subtypes. There is an urgent need to identify markers that distinguish subtypes of osteosarcoma and which may have therapeutic and prognostic implications.
The development of advanced technologies, including serial analysis of gene expression has provided the means to identify global gene expression patterns for a large number of tumour and normal tissue samples. These approaches have been used to characterise genes whose altered expression is important in the development and behaviour of subtypes of tumours. Furthermore, gene expression array profile with bioinformatics analysis can be used to identify the molecular signature of an individual patient’s tumour. Subsequent pathway analysis of the resulting gene lists can reveal distinct signalling events which might account for the biological properties attributed to each tumour type.
The aim of this study was to present a comprehensive genomic analysis of osteosarcoma and to better characterise the molecular expression profiles of different sub-types of osteosarcoma.
We applied a microarray-based gene expression profiling approach on ten OS samples to identify molecular signatures that distinguish osteosarcoma subtypes. Elucidation of such molecular expression signatures may be useful in predicting the clinical behaviour of osteosarcoma as well as identifying candidate cellular pathways that can be targets for future therapeutic approaches.
Materials and methods
Patients and total RNA isolation
The study included tissue specimens from ten patients who underwent open biopsy for definite diagnosis of osteosarcoma and before receiving preoperative chemotherapy. All tumour samples were classified by two experienced pathologists. Total RNA was extracted from frozen tissue samples using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The concentration, purity, and integrity of RNA samples were determined by UV absorbance at 260 nm and electrophoresis [12].
cRNA synthesis and gene expression profiling
Total RNA from ten osteosarcoma samples was isolated as described above. Total RNA was repurified with RNeasy MinElute kit per manufacturer’s instructions (Qiagen, Valencia, CA). The total RNA (5 µg) was then used for GeneChip analysis. Preparation of cRNA, hybridisation to human U133A GeneChips (Affymetrix, Santa Clara, CA, USA) and scanning of the arrays were carried out according to manufacturer’s protocols (https://www.affymetrix.com) as previously published [13].
Bioinformatic analysis
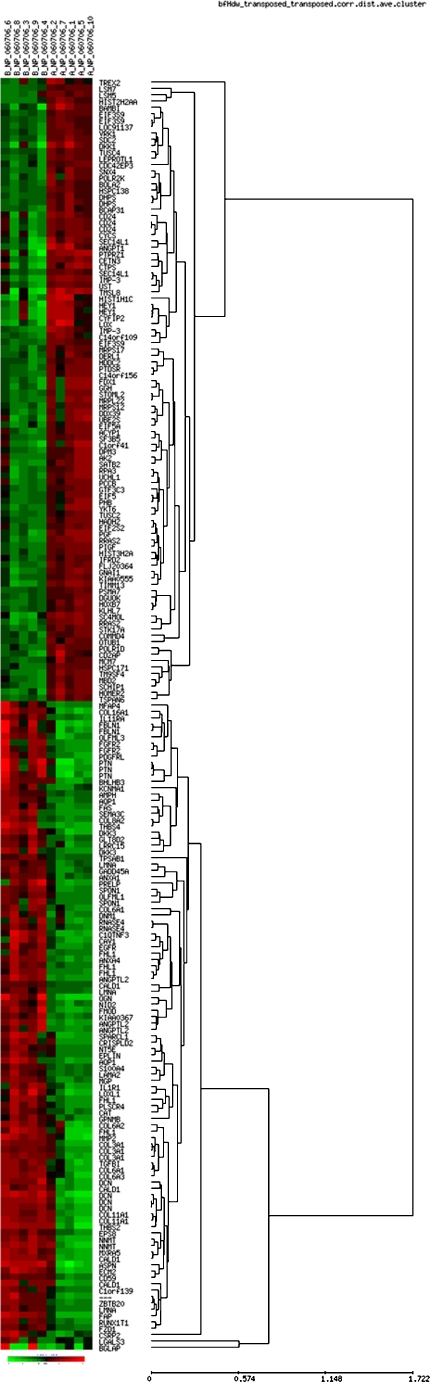
RMA signal extraction, normalisation and filtering was performed as described by Bioconductor (http://www.bioconductor.org) [14]. A non-specific filter was applied prior to hypothesis testing in order to remove genes of low informational content. The filtering criteria for the exemplary data sets required the expression level to be higher than 100 in more than 20% of the samples and the interquartile range (IQR) across the samples on the log base 2 scale to be at least 0.5. To identify genes differentially expressed between the two conditions, we performed a statistical comparison using the limma package implemented in the Bioconductor suite (www.bioconductor.org), which estimates the fold change between predefined sample groups by fitting a linear model and using an empirical Bayes method to moderate the standard errors of the estimated log-fold changes for each probe set. A multiple testing correction based on the false discovery rate (FDR) was performed to produce adjusted p-values [15]. Identification of significantly enriched pathways and gene groups was performed using the methods outlined in a previous publication [16]. For the purpose of visualisation, genes were clustered using a hierarchical cluster algorithm with average linkage and Spearmans rank correlation distance, as provided by the software EPCLUST (http://ep.ebi.ac.uk/EP/EPCLUST/). Results were visualised with the help of heatmaps and dendrograms. The heatmaps show colour-coded expression levels (red high expression, black medium expression, and green low expression) as seen in Fig. 1.
Fig. 1.
Heat map and supervised hierarchical clustering of genes that distinguish osteoblastic from non-osteoblastic osteosarcoma patients. Each row represents a gene, and patient samples are depicted in columns. Red indicates genes that are expressed at higher levels. Green indicates genes that are expressed at lower levels compared with mean expression
Real-time PCR
RNA was extracted using Tri-Reagent (Sigma) according to the manufacturers’ protocols. cDNA was synthesised as previously described and PCR amplification was performed as previously described. Primer pairs were selected to span exon boundry sequences to avoid signal detection from human genomic DNA and were purchased from Applied Biosystems. Primer assays used were: Hs00919202_m1 (angiopoietin 1), Hs00400446_m1 (IGFBP3), Hs01070066_g1 (ferredoxin 1), Hs01002399_m1 (BMP), Hs01072200_m1 (decorin), and Hs00972625_m1 (fibulin 1). Human B2M (beta-2-microglobulin, NM_004048.2, Applied Biosystems) was used as endogenous control.
For each PCR, 6 µl cDNA (diluted 1:3 in nuclease-free water), 25 µl Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 900 nM forward primer, 900 nM reverse primer, 200 nM probe and nuclease-free water were added to a final volume of 50 µl. Amplification and detection were carried out in a DNA Thermal Cycler 480 (Perkin-Elmer). Cycling conditions were as follows: depending on the primers, 25–35 cycles were carried out at 94°C for 1 min, 68°C for 2 min, 72°C for 2 min, with an extension of 5 s with each subsequent cycle.
Results
Identification of differentially expressed genes between osteoblastic and non-osteoblastic osteosarcoma based on gene-expression profiles
Considering the difficulties in determining the histological subtype in osteosarcoma biopsies, we examined genes whose expression differed between the histological subclasses. Two patient groups were compared: group A which included exclusively osteoblastic osteosarcoma samples (five cases) and group B including non-osteoblastic osteosarcoma samples (five cases).
We used Affymetrix Gene Chip arrays containing more than 20,000 genes to generate gene expression profiles for ten osteosarcoma samples. We detected 172 genes differentially expressed between the five osteoblastic and five non-osteoblastic osteosarcoma samples (Table 1). Of these, 75 were significantly up-regulated and 97 significantly down-regulated in osteoblastic versus non-osteoblastic osteosarcoma.
Table 1.
Genes upregulated in osteoblastic versus non-osteoblastic osteosarcomas
| Gene symbol | Mean (A) | Mean (B) | Fold change (A/B) | Gene title |
|---|---|---|---|---|
| TMSL8 | 3,403 | 612 | 5.56 | Thymosin-like 8 |
| PTPRZ1 | 1,680 | 430 | 3.90 | Protein tyrosine phosphatase, receptor-type |
| LOX | 4,386 | 790 | 5.55 | Lysyl oxidase |
| ANGPT1 | 1,989 | 409 | 4.86 | Angiopoietin 1 |
| HIST1H1C | 2,442 | 492 | 4.97 | Histone 1, H1c |
| DKK1 | 2,386 | 606 | 3.94 | Dickkopf homolog 1 (Xenopus laevis) |
| CYFIP2 | 3,521 | 978 | 3.60 | Cytoplasmic FMR1 interacting protein 2 |
| BAMBI | 6,453 | 1,865 | 3.46 | BMP and activin membrane-bound inhibitor homolog |
| HEY1 | 4,315 | 1,203 | 3.59 | Hairy/enhancer-of-split related with YRPW motif 1 |
| CTPS | 1,314 | 453 | 2.90 | CTP synthase |
| SEC14L1 | 391 | 123 | 3.17 | SEC14-like 1 (S. cerevisiae) |
| IMP-3 | 327 | 102 | 3.20 | IGF-II mRNA-binding protein 3 |
| TIMM13 | 1,998 | 634 | 3.15 | Translocase of inner mitochondrial membrane13 homolog |
| SC4MOL | 1,214 | 428 | 2.84 | Sterol-C4-methyl oxidase-like |
| C1orf41 | 748 | 241 | 3.11 | Chromosome 1 open reading frame 41 |
| RRAS2 | 767 | 256 | 3.00 | Related RAS viral (r-ras) oncogene homolog 2 |
| UCHL1 | 3,664 | 1,213 | 3.02 | Ubiquitin carboxyl-terminal esterase L1 |
| HOMER2 | 870 | 290 | 3.00 | Homer homolog 2 (Drosophila) |
| UBE2S | 2,351 | 821 | 2.86 | Ubiquitin-conjugating enzyme E2S |
| RRAS2 | 291 | 103 | 2.83 | Related RAS viral (r-ras) oncogene homolog 2 |
| PGF | 869 | 321 | 2.71 | Placental growth factor |
| FDX1 | 2,028 | 705 | 2.88 | Ferredoxin 1 |
| IMP-3 | 253 | 87 | 2.90 | IGF-II mRNA-binding protein 3 |
| CETN3 | 631 | 243 | 2.59 | Centrin, EF-hand protein, 3 |
| GGH | 1,231 | 437 | 2.82 | Gamma-glutamyl hydrolase |
| GNAI1 | 423 | 161 | 2.63 | Guanine nucleotide binding protein (G protein) |
| CD24 | 1,850 | 767 | 2.41 | CD24 antigen |
| SATB2 | 180 | 71 | 2.54 | SATB family member 2 |
| TM9SF4 | 585 | 223 | 2.63 | Transmembrane 9 superfamily protein member 4 |
| CD2AP | 406 | 145 | 2.79 | CD2-associated protein |
| TREX2 /IP1 | 320 | 114 | 2.80 | Three prime repair exonuclease 2 |
| DDX39 | 1,150 | 453 | 2.54 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 39 |
| YKT6 | 362 | 143 | 2.52 | SNARE protein Ykt6 |
| CYCS | 6,205 | 2,743 | 2.26 | Cytochrome c, somatic |
| PIGF | 453 | 186 | 2.44 | Phosphatidylinositol glycan, class F |
| SCHIP1 | 562 | 239 | 2.36 | Schwannomin interacting protein 1 |
| EIF5A | 1,766 | 765 | 2.31 | Eukaryotic translation initiation factor 5A |
| AK2 | 1,083 | 469 | 2.31 | Adenylate kinase 2 |
| UST | 430 | 197 | 2.18 | Uronyl-2-sulfotransferase |
| RPA3 | 2,553 | 1,108 | 2.30 | Replication protein A3, 14 kDa |
| HIST2H2AA | 1,130 | 480 | 2.35 | Histone 2, H2aa |
| LEPROTL1 | 643 | 285 | 2.25 | Leptin receptor overlapping transcript-like 1 |
| MRPL22 | 952 | 394 | 2.42 | Mitochondrial ribosomal protein L22 |
| POLR2K | 1,483 | 628 | 2.36 | Polymerase (RNA) II (DNA directed) |
| FLJ20364 | 366 | 163 | 2.25 | Hypothetical protein FLJ20364 |
| CDC42EP3 | 984 | 463 | 2.13 | CDC42 effector protein (Rho GTPase binding) 3 |
| SNX4 | 264 | 116 | 2.28 | Sorting nexin 4 |
| HIST3H2A | 1,145 | 524 | 2.18 | Histone 3, H2a |
| STK17A | 692 | 318 | 2.18 | Serine/threonine kinase 17a (apoptosis-inducing) |
| SDC2 | 4,912 | 2,350 | 2.09 | Syndecan 2 |
| MCM7 | 846 | 363 | 2.33 | MCM7 minichromosome maintenance deficient 7 |
| MRPS12 | 667 | 308 | 2.17 | Mitochondrial ribosomal protein S12 |
| PTDSR | 347 | 159 | 2.19 | Phosphatidylserine receptor |
| LSM5 | 1,092 | 522 | 2.09 | LSM5 homolog, U6 small nuclear RNA associated |
| BCAP31 | 3,018 | 1,350 | 2.24 | B-cell receptor-associated protein 31 |
| DPM3 | 814 | 378 | 2.16 | Dolichyl-phosphate mannosyltransferase polypeptide 3 |
| C14orf156 | 1,924 | 893 | 2.15 | Chromosome 14 open reading frame 156 |
| ACYP1 | 342 | 166 | 2.06 | Acylphosphatase 1, erythrocyte (common) type |
| PHB | 1,214 | 566 | 2.15 | Prohibitin |
| STOML2 | 1,086 | 496 | 2.19 | Stomatin (EPB72)-like 2 |
| MRPS17 | 887 | 426 | 2.08 | Mitochondrial ribosomal protein S17 |
| PCCB | 681 | 338 | 2.01 | Propionyl Coenzyme A carboxylase, beta polypeptide |
| C14orf109 | 342 | 160 | 2.14 | Chromosome 14 open reading frame 109 |
| EIF3S9 | 814 | 388 | 2.10 | Eukaryotic translation initiation factor 3 |
| KIAA0555 | 323 | 158 | 2.05 | Jak and microtubule interacting protein 2 |
| MBD2 | 1,114 | 549 | 2.03 | Methyl-CpG binding domain protein 2 |
| HDDC2 | 1,785 | 880 | 2.03 | HD domain containing 2 |
| VRK1 | 517 | 259 | 2.00 | Vaccinia related kinase 1 |
| DERL1 | 1,509 | 736 | 2.05 | Der1-like domain family, member 1 |
| DHPS | 468 | 228 | 2.05 | Deoxyhypusine synthase |
| BOLA2 | 1,132 | 553 | 2.05 | BolA-like 2 (E. coli) |
| EIF5 | 821 | 411 | 2.00 | Eukaryotic translation initiation factor 5 |
| TSPAN6 | 613 | 304 | 2.02 | Tetraspanin 6 |
| HOXB7 | 724 | 357 | 2.02 | Homeo box B7 |
| TUSC2 | 534 | 266 | 2.01 | Tumor suppressor candidate 2 |
Selected genes with increased expression in osteoblastic versus non-osteoblastic osteosarcoma samples
Genes are ranked in order of fold change and are listed with their gene symbol ID, mean expression osteoblastic osteosarcoma patients (mean A) and non-osteoblastic osteosarcoma patients (mean B), and with their gene description
Several genes involved in growth, maturation and signalling (TMSL8, ANGPT1, PGF, IMP-3, DKK1, BAMBI, and RRAS2) were expressed in higher levels in osteoblastic osteosarcoma. Genes involved in regulation of gene expression (histone 1, histone 2, histone 3, centrin, and C1orf41) were also expressed in increased levels. Furthermore, several genes implicated in cell cycle and metabolism (SEC14L1, UBE2S, Ferredoxin 1, GGH, Cytochrome c, EIF5A, and prohibitin) and cell–cell interaction/kinase activation (lysyl oxidase, CTP synthase, CD24, CD2AP, adenylate kinase 2, SNX4, syndecan 2, ACYP1 and UCHL1) had increased expression in osteoblastic osteosarcoma compared with non-osteoblastic osteosarcoma. Genes with >2-fold overexpression are presented in Table 1.
In contrast, 97 genes had reduced expression in osteoblastic osteosarcoma patients compared with non-osteoblastic osteosarcoma patients. There was an overrepresentation of members of genes involved in collagen synthesis (COL3A1, COL6A1, COL8A2, COL11A1, COL6A2, COL6A3, and COL16A1) and extracellular matrix (ECM2, MMP2, MGP, and SPON1). Table 2 lists the names and biological functions of genes expressed in reduced levels with a fold difference >2.
Table 2.
Genes down-regulated in osteoblastic versus non-osteoblastic osteosarcomas
| Gene symbol | Mean (A) | Mean (B) | Fold change (A/B) | Gene title |
|---|---|---|---|---|
| AMPH | 120 | 245 | −2.03 | Amphiphysin |
| LMNA | 680 | 1,367 | −2.01 | Lamin A/C |
| ANXA1 | 2,275 | 4,603 | −2.02 | Annexin A1 |
| S100A4 | 3,170 | 6,728 | −2.12 | S100 calcium binding protein A4 |
| FZD1 | 231 | 484 | −2.09 | Frizzled homolog 1 |
| C1orf139 | 234 | 503 | −2.15 | Chromosome 1 open reading frame 139 |
| CD59 | 1,155 | 2,364 | −2.05 | CD59 antigen p18-20 |
| CAT | 976 | 2,187 | −2.24 | Catalase |
| OLFML1 | 341 | 728 | −2.14 | Olfactomedin-like 1 |
| LMNA | 1,019 | 2,242 | −2.20 | Lamin A/C |
| NT5E | 130 | 290 | −2.23 | 5′-nucleotidase, ecto (CD73) |
| GLT8D2 | 381 | 842 | −2.21 | Glycosyltransferase 8 domain containing 2 |
| LAMA2 | 94 | 221 | −2.35 | Laminin, alpha 2 |
| RNASE4 | 92 | 209 | −2.28 | Ribonuclease, RNase A family, 4 |
| EPLIN | 865 | 2,040 | −2.36 | Epithelial protein lost in neoplasm beta |
| GADD45A | 251 | 556 | −2.21 | Growth arrest and DNA-damage-inducible, alpha |
| KCNMA1 | 378 | 925 | −2.44 | Potassium large conductance calcium-activated |
| LGALS3 | 2,364 | 5,638 | −2.38 | Lectin, galactoside-binding, soluble, 3 (galectin 3) |
| RUNX1T1 | 140 | 341 | −2.43 | Runt-related transcription factor 1; translocated to, 1 |
| EPS8 | 393 | 918 | −2.33 | Epidermal growth factor receptor pathway substrate 8 |
| GPNMB | 2,309 | 5,634 | −2.44 | Glycoprotein (transmembrane) nmb |
| COL3A1 | 9,038 | 20,742 | −2.30 | Collagen, type III, alpha 1 |
| SPON1 | 45 | 121 | −2.70 | Spondin 1, extracellular matrix protein |
| DNM1 | 175 | 456 | −2.61 | Dynamin 1 |
| KIAA0367 | 172 | 457 | −2.65 | KIAA0367 |
| IL11RA | 125 | 341 | −2.72 | Interleukin 11 receptor, alpha |
| COL6A1 | 390 | 1,075 | −2.76 | Collagen, type VI, alpha 1 |
| COL8A2 | 282 | 859 | −3.04 | Collagen, type VIII, alpha 2 |
| LRRC15 | 1,522 | 4,094 | −2.69 | Leucine rich repeat containing 15 |
| ZBTB20 | 407 | 1,149 | −2.82 | Zinc finger and BTB domain containing 20 |
| SPON1 | 304 | 873 | −2.87 | Spondin 1, extracellular matrix protein |
| FAS | 137 | 421 | −3.08 | Fas (TNF receptor superfamily, member 6) |
| CRISPLD2 | 563 | 1,553 | −2.76 | Cysteine-rich secretory protein LCCL |
| MGP | 2,104 | 6,137 | −2.92 | Matrix Gla protein |
| PLSCR4 | 191 | 557 | −2.92 | Phospholipid scramblase 4 |
| EGFR | 111 | 361 | −3.26 | Epidermal growth factor receptor |
| NID2 | 233 | 736 | −3.17 | Nidogen 2 (osteonidogen) |
| RNASE4 | 112 | 358 | −3.21 | Ribonuclease, RNase A family, 4 |
| IL1R1 | 293 | 914 | −3.12 | Interleukin 1 receptor, type I |
| DKK3 | 89 | 321 | −3.61 | Dickkopf homolog 3 |
| MFAP4 | 143 | 590 | −4.12 | Microfibrillar-associated protein 4 |
| CSRP2 | 589 | 2,004 | −3.40 | Cysteine and glycine-rich protein 2 |
| ANGPTL2 | 114 | 411 | −3.61 | Angiopoietin-like 2 |
| FHL1 | 157 | 557 | −3.55 | Four and a half LIM domains 1 |
| COL3A1 | 6,245 | 18,135 | −2.90 | Collagen, type III, alpha 1 |
| COL6A1 | 2,820 | 8,359 | −2.96 | Collagen, type VI, alpha 1 |
| CALD1 | 417 | 1,254 | −3.01 | Caldesmon 1 |
| BHLHB3 | 289 | 1,007 | −3.48 | Basic helix-loop-helix domain containing, class B, 3 |
| SEMA3C | 54 | 205 | −3.77 | Sema domain, immunoglobulin domain |
| CALD1 | 96 | 321 | −3.35 | Caldesmon 1 |
| COL6A2 | 1,754 | 5,247 | −2.99 | Collagen, type VI, alpha 2 |
| AQP1 | 638 | 2,785 | −4.36 | Aquaporin 1 (channel-forming integral protein) |
| DCN | 3,851 | 13,005 | −3.38 | Decorin |
| PDGFRL | 236 | 944 | −4.01 | Platelet-derived growth factor receptor-like |
| FGFR2 | 65 | 329 | −5.10 | Fibroblast growth factor receptor 2 |
| NNMT | 382 | 1,268 | −3.32 | Nicotinamide N-methyltransferase |
| ANXA4 | 221 | 856 | −3.87 | Annexin A4 |
| MXRA5 | 831 | 2,852 | −3.43 | Matrix-remodelling associated 5 |
| TGFBI | 2,013 | 6,936 | −3.45 | Transforming growth factor, beta-induced, 68 kDa |
| OLFML3 | 717 | 2,831 | −3.95 | Olfactomedin-like 3 |
| PRELP | 467 | 2,287 | −4.89 | Proline/arginine-rich end leucine-rich repeat protein |
| COL6A3 | 3,161 | 10,536 | −3.33 | Collagen, type VI, alpha 3 |
| COL11A1 | 3,111 | 9,944 | −3.20 | Collagen, type XI, alpha 1 |
| SPARCL1 | 734 | 3,136 | −4.28 | SPARC-like 1 (mast9, hevin) |
| CALD1 | 1,283 | 4,535 | −3.54 | Caldesmon 1 |
| CAV1 | 602 | 2,814 | −4.68 | Caveolin 1, caveolae protein, 22 kDa |
| DCN | 2,351 | 10,377 | −4.41 | Decorin |
| FMOD | 503 | 2,694 | −5.36 | Fibromodulin |
| FBLN1 | 271 | 1,686 | −6.21 | Fibulin 1 |
| ANGPTL2 | 705 | 3,621 | −5.14 | Angiopoietin-like 2 |
| C1QTNF3 | 259 | 1,428 | −5.51 | C1q and tumor necrosis factor related protein 3 |
| DKK3 | 402 | 1,922 | −4.78 | Dickkopf homolog 3 (Xenopus laevis) |
| NNMT | 545 | 1,979 | −3.63 | Nicotinamide N-methyltransferase |
| ANGPTL2 | 198 | 1,114 | −5.61 | Angiopoietin-like 2 |
| THBS2 | 914 | 2,833 | −3.10 | Thrombospondin 2 |
| MMP2 | 1,670 | 7,241 | −4.34 | Matrix metallopeptidase 2 |
| THBS4 | 298 | 2,250 | −7.54 | Thrombospondin 4 |
| COL16A1 | 253 | 1,509 | −5.97 | Collagen, type XVI, alpha 1 |
| ECM2 | 187 | 954 | −5.11 | Extracellular matrix protein 2 |
| FHL1 | 988 | 4,692 | −4.75 | Four and a half LIM domains 1 |
| DCN | 1,864 | 8,437 | −4.53 | Decorin |
| FAP | 244 | 1,376 | −5.65 | Fibroblast activation protein, alpha |
| LOXL1 | 185 | 1,272 | −6.87 | Lysyl oxidase-like 1 |
| FBLN1 | 183 | 1,777 | −9.71 | Fibulin 1 |
| PTN | 424 | 3,781 | −8.92 | Pleiotrophin (heparin binding growth factor 8) |
| DCN | 380 | 2,379 | −6.26 | Decorin |
| ASPN | 1,415 | 9,796 | −6.92 | Asporin (LRR class 1) |
| OGN | 78 | 1,027 | −13.20 | Osteoglycin (osteoinductive factor, mimecan) |
| BGLAP | 178 | 5,425 | −30.54 | Bone gamma-carboxyglutamate (gla) |
Selected genes with lower expression in osteoblastic versus non-osteoblastic osteosarcoma samples
Genes are ranked in order of fold change and are listed with their gene symbol ID, mean expression osteoblastic osteosarcoma patients (mean A) and non-osteoblastic osteosarcoma patients (mean B), and with their gene description
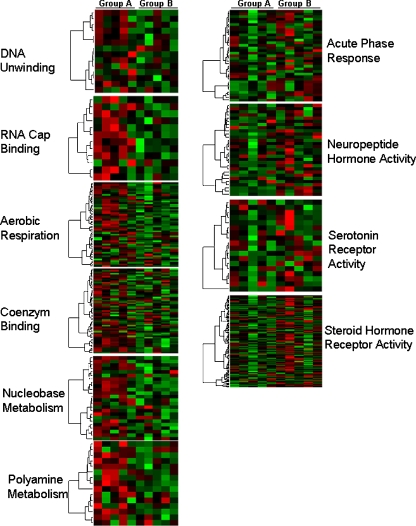
To determine whether particular functional categories of genes were highly enriched in one of the groups we identified gene ontology functional categories that were statistically significant among the list of differentially regulated genes. Genes with increased expression in osteoblastic osteosarcoma were linked to nucleobase- and polyamine metabolism and aerobic respiration. Genes expressed in reduced levels in osteoblastic osteosarcoma included genes involved in steroid- and neuropeptide hormone receptor activity, acute-phase response and serotonin receptor activity. Additional functional and pathway classification of the differentially expressed genes is shown in Fig. 2.
Fig. 2.
Functional and pathway classification of the differentially expressed genes. Columns represent the gene expression levels of osteoblastic osteosarcoma patients (group A) and non-osteoblastic osteosarcoma patients (group B). Gene expression profile through pathway analysis demonstrates that genes involved in nucleobase- and polyamine metabolism and aerobic respiration were up-regulated in osteoblastic osteosarcoma patients (group A). Genes involved in steroid- and neuropeptide hormone receptor activity, acute-phase response and serotonin receptor activity were upregulated in non-osteoblastic osteosarcoma patients (group B). Each row represents a gene, and patient samples are depicted in columns. Red indicates genes that are expressed at higher levels. Green indicates genes that are expressed at lower levels compared with mean expression
Real-time PCR validation of microarray data
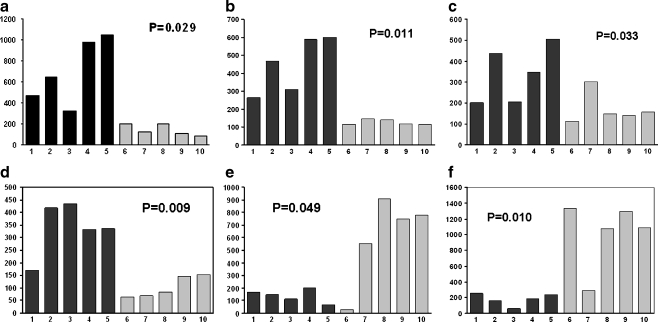
To confirm the results obtained using microarrays, we performed real-time PCR on six selected genes. These genes included angiopoietin 1, IGFBP3, ferredoxin 1, BMP, decorin, and fibulin 1. We used RNA from the same ten tumour samples that were used for microarray analysis.
As shown in Fig. 3, RT-PCR analysis performed on five osteoblastic and five non-osteoblastic osteosarcoma demonstrated significant expression differences. This result indicates that the RT-PCR results are highly consistent with the microarray data.
Fig. 3.
Real-time (RT)-PCR for angiopoietin (a), IGFBP-3 (b), ferredoxin 1 (c), BMP (d), decorin (e), and fibulin 1 (f) was performed on total RNA extracted from tumour biopsies deriving from the malignant tissue of five patients with osteoblastic osteosarcoma and from five patients with non-osteoblastic osteosarcoma. Differences in gene expression levels between the two groups were analysed by Student’s t test. Primers and cycling conditions for each of the amplified genes are described in Materials and Methods
Discussion
Microarray technology has provided the means for studying the molecular basis of tumours by examining thousands of genes simultaneously. Using whole genome expression profiling of osteosarcoma samples, we showed that conventional, osteoblastic osteosarcoma are clearly distinct from other osteosarcoma subtypes.
This is consistent with the distinct clinicopathological aspects of different osteosarcoma subtypes [10]. The subtype of osteosarcoma seems to be a predictive factor for response to chemotherapy [17] and tends to be associated with disease-free and overall survival [4, 8].
Although classification of osteosarcoma based on morphological appearance of the tumour is an important prognostic factor, histological subclassification can be difficult even among experienced pathologists. Therefore there is a need to develop new objective methods of osteosarcoma subclassification.
The results of our study using microarray expression signature suggest that osteosarcoma can be classified into two groups based on gene expression profiles, which showed a strong association with histomorphological subtype. Several genes involved in the formation of extracellular matrix showed a clearly distinct expression pattern. For example, the collagen types 3, 6, 11, and 16 were down-regulated in the osteoblastic osteosarcoma subgroup. This is in accordance with previous studies where the histological appearance of osteosarcoma specimens has been linked to differences in collagen expression [18]. Furthermore, our comparison of differentially expressed genes within these clusters identified several genes with important implications concerning the origin and clinical behaviour of osteosarcoma and genes that may be targeted for novel therapeutics.
Differential expression of genes encoding for growth factors and receptors
We found a significantly different expression of transforming growth factor, beta-induced (TGFBI) between osteoblastic and non-osteoblastic osteosarcoma. Transforming growth factor, beta-induced (TGFBI) is an extracellular matrix molecule initially cloned from human adenocarcinoma cells treated with TGF-β. Transforming growth factor-beta (TGF-ß) isoforms play an important role in the regulation of cell development and growth. Osteosarcoma expression of TGF-ß isoforms is related to tumour grade and disease progression [19], and it is a key molecule triggering the expression of extracellular matrix components that play an essential role in tumour cell behaviour [20]. Furthermore, we found a strong decrease of the expression of fibulin 1, a secreted glycoprotein, in osteoblastic osteosarcoma. The fibulins modulate cell morphology and growth and play a role in adhesion and invasion of sarcoma cells [21].
Moreover, the two osteosarcoma subgroups showed a different expression level of heparin binding growth factor 8, pleiotrophin (PTN). PTN modulates cell growth and proliferation of various tumours and has been linked to chemoresistance in osteosarcoma cells [22]. Another component that modulates proliferation, cell adhesion, and migration is Syndecan-2. The Syndecans are cell surface heparan sulphate proteoglycans that can induce apoptosis [23] and sensitise osteosarcoma cells to the cytototoxic effect of chemotherapeutics [24]. Furthermore, in our setting, the expression level of fibroblast growth factor receptor 2 (FGFR2) was significantly different in both osteosarcoma subgroups. Fibroblast growth factor receptor 2 (FGFR2) plays an essential role in bone morphogenesis, and inherited mutations of the FGFR2 gene result in skeletal dysplasias [25]. Loss of heterozygosity of FGFR2 has been found in high grade osteosarcoma [26], and rearrangement of FGFR2 was reported in rat osteosarcoma cells [27]. The clinical relevance of FGFR2 expression in human osteosarcoma is yet to be determined.
Expression of genes involved in chemotherapy resistance
The success of conventional chemotherapy in osteosarcoma has been limited by drug resistance mechanisms [9, 28]. Therefore one of the most important prognostic factors in osteosarcoma is the response to preoperative chemotherapy. The administration of more intensified chemotherapy to poor responders has failed to improve survival in this patient group in several clinical trials [17]. Therefore it has been suggested that there may be an immanent genetic difference between responsive and non-responsive tumours [29].
Interestingly we found a different expression of several genes related to drug resistance including prohibitin, Annexin1, Annexin 4 and gamma-glutamyl hydrolase (GGH) among the two osteosarcoma subgroups. Prohibitin is a potential tumour suppressor protein that plays an essential role in the modulation of drug-induced cell death and significantly reduced chemotherapy resistance in osteosarcoma cells [30]. The annexins are involved in bone resorption and formation and have been linked to drug resistance in osteosarcoma patients [29] and several human cancer cell lines [31]. One of the drugs most commonly used in systemic osteosarcoma therapy is Methotrexate (MTX). Overexpression of gamma-glutamyl hydrolase (GGH) decreases intracellular MTX and thereby impairs anti-tumour activity. Increased expression of GGH has also been shown to be associated with resistance to MTX in sarcoma cell lines [32].
Expression of genes involved in angiogenesis
Malignant proliferating cells depend on supply of nutrients and oxygen. Several genes whose expression is associated with the activation of angiogenesis were differentially expressed between the two subgroups. Among those were angiopoietin (Ang)-1 and Ang-2, decorin and Interleukin 1 receptor. Angiopoietins promote endothelial cell migration, proliferation and capillary formation and have been found to be critical mediators of angiogenesis in several tumours [33]. Differential expression of angiopoietins partially regulated by Interleukin 1 beta was also demonstrated in chondrosarcoma cells [34]. Furthermore, Decorin, an extracellular matrix protein, suppressed angiogenesis and tumour growth in osteosarcoma [35]. Decorin also inhibited cell motility and invasion and the occurrence of pulmonary metastasis in a murine osteosarcoma model [36].
Differential expression of members of the Wnt/ß-catenin pathway
The Wnt/ß-catenin signal transduction pathway promotes new bone formation acting as a positive regulator of osteoblasts. Over-expression of the Wnt pathway inhibitors, the Dickkopf (DKK) protein family members, have been associated with osteolytic metastatic bone disease in prostate carcinoma [37]. In osteosarcoma, Dickkopf (DKK) homolog 1 increased proliferation by activation of the cell cycle. Another member of the Dickkopf family, DKK 3 inhibited invasion and motility of osteosarcoma cells by modulating the Wnt/ß-catenin pathway and plays a possible role in the pathobiology and progression of osteosarcoma [38].
Low expression of Fas by osteoblastic osteosarcoma
The Fas receptor and its ligand belong to the tumour necrosis factor receptor family. Fas plays an important role in tumour cell apoptosis and tumorigenesis and in several clinical studies a decrease of Fas expression correlated with poor prognosis [39]. Furthermore, inhibition of Fas signalling promoted lung metastases growth in a murine osteosarcoma model and was considered as a potential therapeutic target for the treatment of osteosarcoma [40].
Conclusion
Using microarray-based differential expression and gene set analysis, we identified a distinct gene expression pattern of osteoblastic and non-osteoblastic osteosarcoma subgroups. The results of this analysis included genes and gene sets important to osteosarcoma pathogenesis and progression.
We are aware that our study relates to a small sample size; even so, the highly significant results distinguishing the two groups are remarkable. This study could be the basis for further investigations of osteosarcoma gene expression which may lead to the development of an important prognostic tool and the identification of potential targets for the development of new targeted therapy in the future.
Acknowledgments
Conflicts of interest statement None declared.
References
- 1.Nakano H, Tateishi A, Miki H, et al. Hyperthermic isolated regional perfusion for the treatment of osteosarcoma in the lower extremity. Am J Surg. 1999;178:27–32. doi: 10.1016/S0002-9610(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 2.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12:423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari S, Bertoni F, Mercuri M, et al. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol. 2001;12:1145–1150. doi: 10.1023/A:1011636912674. [DOI] [PubMed] [Google Scholar]
- 4.Hudson M, Jaffe MR, Jaffe N, et al. Pediatric osteosarcoma: therapeutic strategies, results, and prognostic factors derived from a 10-year experience. J Clin Oncol. 1990;8:1988–1997. doi: 10.1200/JCO.1990.8.12.1988. [DOI] [PubMed] [Google Scholar]
- 5.Petrilli AS, Gentil FC, Epelman S, et al. Increased survival, limb preservation, and prognostic factors for osteosarcoma. Cancer. 1991;68:733–737. doi: 10.1002/1097-0142(19910815)68:4<733::AID-CNCR2820680412>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Taylor WF, Ivins JC, Unni KK, et al. Prognostic variables in osteosarcoma: a multi-institutional study. J Natl Cancer Inst. 1989;81:21–30. doi: 10.1093/jnci/81.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Ferrari S, Delepine N, et al. Predictive factors of histologic response to primary chemotherapy in osteosarcoma of the extremity: study of 272 patients preoperatively treated with high-dose methotrexate, doxorubicin, and cisplatin. J Clin Oncol. 1998;16:658–663. doi: 10.1200/JCO.1998.16.2.658. [DOI] [PubMed] [Google Scholar]
- 8.Hauben EI, Weeden S, Pringle J, et al. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38:1218–1225. doi: 10.1016/S0959-8049(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 9.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 10.Hauben EI, Bielack S, Grimer R, et al. Clinico-histologic parameters of osteosarcoma patients with late relapse. Eur J Cancer. 2006;42:460–466. doi: 10.1016/j.ejca.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Hauben EI, Arends J, Vandenbroucke JP, et al. Multiple primary malignancies in osteosarcoma patients. Incidence and predictive value of osteosarcoma subtype for cancer syndromes related with osteosarcoma. Eur J Hum Genet. 2003;11:611–618. doi: 10.1038/sj.ejhg.5201012. [DOI] [PubMed] [Google Scholar]
- 12.Svoboda M, Thalhammer T, Aust S, et al. Estrogen sulfotransferase (SULT1E1) expression in benign and malignant human bone tumors. J Surg Oncol. 2007;95:572–581. doi: 10.1002/jso.20748. [DOI] [PubMed] [Google Scholar]
- 13.Bilban M, Ghaffari N, Hintermann E, et al. Kisspeptin-10, a KiSS1/metastin-derived dekapeptide, is a physiologic invasion inhibitor of primary human trophoblast. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 14.Bilban M, Heintel D, Scharl T, et al. Deregulated expression of fat and muscle genes in B-cell chronic lymphocytic leukemia with high lipoprotein lipase expression. Leukemia. 2006;20:1080–1088. doi: 10.1038/sj.leu.2404220. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 16.Tian L, Greenberg SA, Kong SW, et al. Discovering statistically significant pathways in expression profiling studies. Proc Natl Acad Sci USA. 2005;102:13544–13549. doi: 10.1073/pnas.0506577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacci G, Forni C, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: intensification of preoperative treatment does not increase the rate of good histologic response to the primary tumor or improve the final outcome. J Pediatr Hematol Oncol. 2003;25:845–853. doi: 10.1097/00043426-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Roessner A, Voss B, Rauterberg J, et al. Biologic characterization of human bone tumors. II. Distribution of different collagen types in osteosarcoma—a combined histologic, immunofluorescence and electron microscopic study. J Cancer Res Clin Oncol. 1983;106:234–239. doi: 10.1007/BF00402614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloen P, Gebhardt MC, Perez-Atayde A, et al. Expression of transforming growth factor-beta (TGF-beta) isoforms in osteosarcomas: TGF-beta3 is related to disease progression. Cancer. 1997;80:2230–2239. doi: 10.1002/(SICI)1097-0142(19971215)80:12<2230::AID-CNCR3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Nikitovic D, Zafiropoulos A, Katonis P, et al. Transforming growth factor-beta as a key molecule triggering the expression of versican isoforms v0 and v1, hyaluronan synthase-2 and synthesis of hyaluronan in malignant osteosarcoma cells. IUBMB Life. 2006;58:47–53. doi: 10.1080/15216540500531713. [DOI] [PubMed] [Google Scholar]
- 21.Qing J, Maher VM, Tran H, et al. Suppression of anchorage-independent growth and matrigel invasion and delayed tumor formation by elevated expression of fibulin-1D in human fibrosarcoma-derived cell lines. Oncogene. 1997;15:2159–2168. doi: 10.1038/sj.onc.1201385. [DOI] [PubMed] [Google Scholar]
- 22.Walters DK, Steinmann P, Langsam B, et al. Identification of potential chemoresistance genes in osteosarcoma. Anticancer Res. 2008;28:673–679. [PubMed] [Google Scholar]
- 23.Modrowski D, Orosco A, Thévenard J, et al. Syndecan-2 overexpression induces osteosarcoma cell apoptosis: implication of syndecan-2 cytoplasmic domain and JNK signaling. Bone. 2005;37:180–189. doi: 10.1016/j.bone.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Orosco A, Fromigué O, Bazille C, et al. Syndecan-2 affects the basal and chemotherapy-induced apoptosis in osteosarcoma. Cancer Res. 2007;67:3708–3715. doi: 10.1158/0008-5472.CAN-06-4164. [DOI] [PubMed] [Google Scholar]
- 25.Wilkie AO, Patey SJ, Kan SH, et al. FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am J Med Genet. 2002;112:266–278. doi: 10.1002/ajmg.10775. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza S, David H, Gaylord GM, Miller CW. Allelic loss at 10q26 in osteosarcoma in the region of the BUB3 and FGFR2 genes. Cancer Genet Cytogenet. 2005;158:142–147. doi: 10.1016/j.cancergencyto.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzi MV, Horii Y, Yamanaka R, et al. FRAG1, a gene that potently activates fibroblast growth factor receptor by C-terminal fusion through chromosomal rearrangement. Proc Natl Acad Sci USA. 1996;93:8956–8961. doi: 10.1073/pnas.93.17.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trieb K, Kotz R. Proteins expressed in osteosarcoma and serum levels as prognostic factors. Int J Biochem Cell Biol. 2001;33:11–17. doi: 10.1016/S1357-2725(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 29.Mintz MB, Sowers R, Brown KM, et al. An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res. 2005;65:1748–1754. doi: 10.1158/0008-5472.CAN-04-2463. [DOI] [PubMed] [Google Scholar]
- 30.Fellenberg J, Dechant MJ, Ewerbeck V, Mau H. Identification of drug-regulated genes in osteosarcoma cells. Int J Cancer. 2003;105:636–643. doi: 10.1002/ijc.11135. [DOI] [PubMed] [Google Scholar]
- 31.Han EK, Tahir SK, Cherian SP, et al. Modulation of paclitaxel resistance by annexin IV in human cancer cell lines. Br J Cancer. 2000;83:83–88. doi: 10.1054/bjoc.2000.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole PD, Kamen BA, Gorlick R, et al. Effects of overexpression of gamma-Glutamyl hydrolase on methotrexate metabolism and resistance. Cancer Res. 2001;61:4599–4604. [PubMed] [Google Scholar]
- 33.Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- 34.Kalinski T, Krueger S, Sel S, et al. Differential expression of VEGF-A and angiopoietins in cartilage tumors and regulation by interleukin-1beta. Cancer. 2006;106:2028–2038. doi: 10.1002/cncr.21848. [DOI] [PubMed] [Google Scholar]
- 35.Grant DS, Yenisey C, Rose RW, et al. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 36.Shintani K, Matsumine A, Kusuzaki K, et al. Decorin suppresses lung metastases of murine osteosarcoma. Oncol Rep. 2008;19:1533–1539. [PubMed] [Google Scholar]
- 37.Hall CL, Bafico A, Dai J, et al. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 38.Hoang BH, Kubo T, Healey JH, et al. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.CAN-03-1952. [DOI] [PubMed] [Google Scholar]
- 39.Chan KW, Lee PY, Lam AK, et al. Clinical relevance of Fas expression in oesophageal squamous cell carcinoma. J Clin Pathol. 2006;59:101–104. doi: 10.1136/jcp.2005.027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koshkina NV, Khanna C, Mendoza A, et al. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res. 2007;5:991–999. doi: 10.1158/1541-7786.MCR-07-0007. [DOI] [PubMed] [Google Scholar]