Abstract
This was a 13-week, multicentre, randomised, parallel, double-blind study. One hundred men and women volunteers aged ≥40 years with knee osteoarthritis (KOA) were randomised to once daily enzymatic hydrolysed collagen (EHC) 10 g or glucosamine sulphate (GS) 1.5 g for 90 consecutive days. Follow-up took place after two weeks and after one, two and three months. Primary [visual analogue scale (VAS), Western Ontario and McMaster Universities (WOMAC Index)] and secondary outcomes variables, assessed at weeks two, four, eight and 12, were KOA pain intensity measured by quadruple visual analogue scales in the target knee, the WOMAC total score index, patient’s and investigator’s global assessments of disease activity, joint assessment, use of rescue medication (ibuprofen 400 mg tablets) and assessment of Quality of Life index (SF-36 Questionnaire). Safety and tolerability were also evaluated. Clear improvement was observed in both joint pain and symptoms in patients with KOA treated with EHC (Colatech®) and significant differences were observed. Mean reductions from baseline for EHC 10 g daily and GS 1.5 g, respectively, were KOA pain intensity reduction in the target knee for Colatech® (p < 0.05): WOMAC index decrease ≤ 15 points at the last visit (day 90) for Colatech® in 16 patients (34.04%) (p < 0.05) and for glucosamine in six patients (13.04%); total score index for painful joints: Colatech® 1.6 (p < 0.05) and glucosamine 1.8; total score index for swollen joints: Colatech® 0.5 (p < 0.05) and glucosamine 0.7; patient’s global assessment of efficacy as the sum of improvement good + ideal: 80.8% for Colatech® and 46.6% for glucosamine (p < 0.05). EHC (Colatech®) showed superior improvement over GS in the SF-36 Questionnaire in the Physical Health Index (42.0 for Colatech and 40.0 for glucosamine). The incidence of adverse events was similar in both groups. Both EHC and GS were well tolerated.
Introduction
Osteoarthritis is a disease with a huge public-health impact [18] that has been treated with negative attitudes among physicians and low expectations of the value of treatment [8, 12, 13]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common symptomatic treatment but have major adverse effects [3] and might even worsen the osteoarthritis process [7, 9, 13, 19]. Despite the fact that inestimable resources have been dedicated to developing many nonsteroidal anti-inflammatory agents, scarce currency has been given to the notion that progression of osteoarthritis could be retarded by nutritional products [7, 11, 13, 14], although several reports and clinical trials are changing this situation [8]. Several recent studies have shown that orally administered enzymatic hydrolysed collagen (EHC) (Colatech®) is absorbed and distributed to joint tissues [11] and has analgesic and anti-inflammatory properties [1, 2, 11, 16]. Also, glucosamine sulphate (GS) has wide support as an effective treatment for osteoarthritis [6, 10, 15, 17, 20]. These trials have identified some benefit in using orally administered Colatech® or GS when compared with NSAIDs or placebo. They may offer comparatively safer alternatives to NSAIDs for management of osteoarthritis. Further study is warranted to define the differences, if any, in efficacy and safety between Colatech® and GS.
Material and methods
Study design
This was a parallel, double-blind, randomised, clinical trial comparing Colatech® versus GS. All patients underwent a treatment period of three months (Colatech® 10 g qd vs. GS 1.5 g qd).
Patients
Power calculations based on published data for patients with knee osteoarthritis (KOA) indicated that far less than 100 patients [1, 21] were required in each treatment group to detect a 20-mm difference in visual analogue scale (VAS) overall pain score with 90% power at 1% significance (two-sided) [10]. Twenty millimetres has been identified as the minimum clinically important difference in pain between treatment groups in KOA studies [3, 4]. The sample size was increased to 50 patients per group to allow a decrease until 25 to 40%. One hundred community volunteers with KOA participated, and 93 completed the trial.
Methods
At the screening visit (day –7), patients who satisfied the inclusion and exclusion criteria were required to discontinue their NSAID therapy and to demonstrate a pain flare. The flare criteria (based upon a 100 mm quadruple VAS) required at least moderate pain (20–40 mm) and an increase preferably of ≥ 15 mm compared with screening values in the second visit (day 0), although these were not exclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria were male or female patients aged 40 years or older with early stages of degenerative and post-traumatic KOA. A diagnosis according to the American College of Rheumatology (ACR) was required, and the patient had to be in functional class I, II, or III of at least one knee. Pain or discomfort should have been experienced in the affected knee(s) on most days for the previous three months. If the patient had bilateral symptomatic KOA, he or she was asked to specify the worst knee at baseline, and this knee was evaluated throughout the study period. Exclusion criteria were association with a further concurrent rheumatic medical disorder/arthritic disease that could confound or interfere with evaluation of efficacy. These included inflammatory arthritis (e.g., controlled and uncontrolled rheumatoid arthritis), systemic lupus, spondyloarthropathy, polymyalgia, rheumatica, and others.
Objective
The objective was to compare the analgesic effectiveness, safety and tolerability of orally administered Colatech® 10 g qd vs. orally administered GS 1.5 g qd over a 90-consecutive-day period.
Procedures
One hundred patients, 25 in each of four hospitals, were recruited from eligible patients. They were randomly and blindly assigned to treatment, and 93 of them completed the trial (47 in the Colatech® group and 46 in the GS group). All 100 patients were the object of different measurements on days –7, 0, 15, 30, 60, and 90. All participants were forbidden to continue their existing NSAID medication, if any, on the initial visit (day –7). There was a washout period of 7 days. On Day 0 (baseline visit) and on the following visits at the end of each month, patients were receiving 30 tablets of ibuprofen 400 mg each. Use of ibuprofen tablets was recorded at each follow-up assessment.
Treatment procedures
During a run-in period of one week (week 0), patients considered eligible were told to discontinue all analgesics, anti-inflammatory drugs, and other treatment modalities for arthralgia and KOA. At the beginning (day 0), patients who still met the eligibility criteria were randomised into two groups (Colatech® and GS) and treated for 12 weeks. The study duration was three months for each patient. On Day –7 (visit 1), informed consent was obtained, and the investigator assessed the patient’s eligibility to enter the study, verifying that the patient was satisfying the inclusion and exclusion criteria. The following data were recorded during this and all subsequent visits, i.e., two (day 0), three (day 15), four (day 30), and five (day 60): personal and demographic data, medical history, medication history, physical examination and vital signs, pain intensity (quadruple VAS), joint assessment, Western Ontario and McMaster Universities (WOMAC) index [5], Short-Form (SF)-36 Quality of Life Questionnaire, and extraction of 5 ml blood sample in order to obtain laboratory data and laboratory analysis. The following routine hematological analyses were performed: total red blood cell (RBC) count, haemoglobin, haematocrit, total white blood cell (WBC) count, neutrophils, eosinophils, basophils, lymphocytes, monocytes, platelet count, glucose, urea nitrogen, creatinine, uric acid, potassium, sodium, chloride, calcium, total bilirubin, serum glutamic oxaloacetic transaminase [SGOT; aspartate aminotransferase (AST)], serum glutamic pyruvic transaminase [SGPT; alanine aminotransferase (ALT)], ASLO (antistreptolysin O), latex, and C-reactive protein (CRP). All unused clinical trial medication was counted by the investigators for assessment of compliance, and any concomitant medication was recorded in the Case Report Form indicating the period, dosage and administration route.
Primary and secondary efficacy endpoints
VAS were scored from 0 to 100 mm, where 0 indicates no pain and 100 the worst pain imaginable. The primary outcome measurements were the quadruple VAS overall assessment of pain in the affected knee, the evolution evaluated by the WOMAC osteoarthritis severity index [3] for femorotibial KOA, and quality of life evaluated by the SF-36 Questionnaire. The secondary efficacy endpoints were self-assessment, the investigator’s overall opinion, and reduction of the use of rescue medication.
Tolerability and safety
Spontaneously reported adverse events were recorded throughout the study. Vital signs were monitored at every visit. Laboratory investigations including haematology and blood chemistry were performed on days –7 and 90. For all adverse events, intensity, relation to test drug, and any action taken were recorded.
Statistical analysis
A single sample of evaluable patients was defined and used for both the efficacy analysis and the safety and tolerability analysis. Using the intention-to-treat (ITT) criterion, this sample included all randomised patients who started treatment and returned for at least one visit, providing the information required to calculate the main efficacy variables, pain score in VAS. Qualitative variables were compared between groups using a chi-square test. Quantitative variables were compared using a nonparametric Mann–Whitney U test. In addition, changes in the scales during the study were reported and compared using generalised linear models (GLM) for repeated measures. These models test the time effect and the group effect and the interaction between both. For analysis of the effect of the different centres on the main efficacy variables, a binary logistic regression model was adjusted (p < 0,05). Statistical processing of results was performed using software SAS version 9.1.3. Two-tailed statistical tests were used. Results were considered to be statistically significant when the alpha error value was <5% (p < 0.05). For the primary variable (improvement as assessed by a ≥20-mm decrease in VAS), results were also evaluated for a 1% alpha error.
Results
A total of 105 patients were recruited into this study, of whom five were excluded. The remaining 100 patients were randomised into the Colatech® and GS groups. Four patients withdrew due to failure in the follow-up and transportation problems (n = 2), and another one had a domestic accident during the study. Thus, each group comprised 47 (in the EHC group) and 46 (in the GS group) completers. The two treatment groups were not significantly different in demographic data, e.g., sex, age, weight, height, duration of KOA, location of KOA, base-line data, vital signs, or toxic habits . The radiographic findings at entry were no different between the two groups. During the study, the rates of compliance with EHC medication and the GS group were similar. As a few patients withdrew from the trial, the results were not substantially affected, whether the statistical method was performed by an ITT analysis or analysis on available completers.
Quadruple VAS
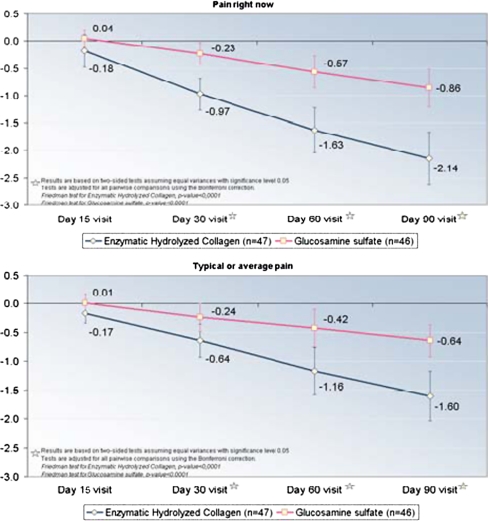
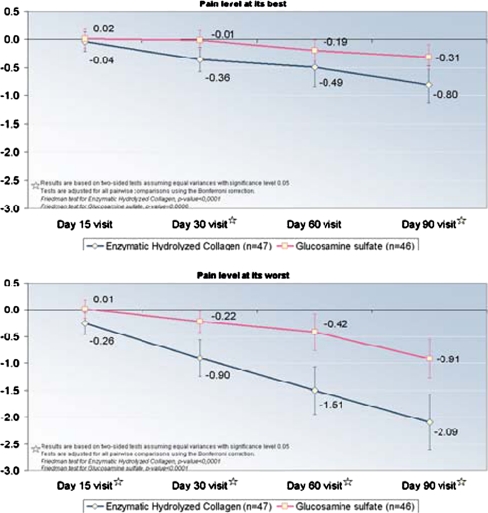
The quadruple VAS measurements (pain right now, average pain, pain at its best, and pain at its worst) that assessed pain at the end of weeks two, four, eight and 12 after the start of the treatments showed that the pain significantly decreased when compared with their own baseline values and that Colatech® was statistically more active (p < 0.05) than GS at two weeks (pain right now) (p < 0.05) and at four (p < 0.05), eight (p < 0.05), and 12 (p < 0.05) weeks in all evaluated VAS (Table 5, and Fig. 1). Analgesic efficacy of study treatments, assessed by VAS, showed significant (p < 0.05) differences between groups during and after three months of treatment. In the group treated with Colatech®, 68% of patients showed a clear improvement in pain assessment (score improvement of ≥20 mm ) compared with 37% of patients treated with GS (chi-square test, p < 0.05). (Table 1)
Table 5.
Activity of enzymatic hydrolysed collagen (EHC) (Colatech®) and glucosamine sulfate (GS) on joint improvement assessment
| Glucosamine sulphate | Enzymatic hydrolyzed collagen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Colatech®) | ||||||||||
| M | SD | Me | Range | No. | M | SD | Me | Range | No. | |
| Visit 2 (day 0) | ||||||||||
| Total painful joints2 | 2.4 | 1.5 | 2.0 | 0.0–7.0 | 46 | 2.8 | 1.9 | 2.0 | 0.0–7.0 | 47 |
| Total swollen joints1,2 | 1.1 | 1.1 | 1.0 | 0.0–4.0 | 46 | 0.9 | 1.0 | 1.0 | 0.0–4.0 | 47 |
| Score index, painful joints1,2 | 3.4 | 2.1 | 3.0 | 0.0–9.0 | 46 | 4.5 | 3.1 | 4.0 | 0.0–11.0 | 47 |
| Score index, swollen joints2 | 1.3 | 1.3 | 1.0 | 0.0–5.0 | 46 | 1.3 | 1.5 | 1.0 | 0.0–8.0 | 47 |
| Visit 3 (day 15) | ||||||||||
| Total painful joints2 | 2.3 | 1.5 | 2.0 | 0.0–7.0 | 46 | 2.7 | 1.7 | 2.0 | 0.0–7.0 | 47 |
| Total swollen joints1,2 | 1.0 | 1.1 | 1.0 | 0.0–4.0 | 46 | 1.1 | 1.1 | 1.0 | 0.0–4.0 | 47 |
| Score index, painful joints1,2 | 3.3 | 2.1 | 3.0 | 0.0–8.0 | 46 | 4.1 | 2.7 | 4.0 | 0.0–11.0 | 47 |
| Score index, swollen joints2 | 1.3 | 1.4 | 1.0 | 0.0–5.0 | 46 | 1.4 | 1.5 | 1.0 | 0.0–8.0 | 47 |
| Visit 4 (day 30) | ||||||||||
| Total painful joints2 | 2.4 | 1.3 | 2.0 | 0.0–6.0 | 46 | 2.3 | 1.7 | 2.0 | 0.0–7.0 | 47 |
| Total swollen joints1,2 | 1.1 | 1.1 | 1.0 | 0.0–3.0 | 46 | 0.9 | 1.0 | 1.0 | 0.0–4.0 | 47 |
| Score index, painful joints1,2 | 3.2 | 1.6 | 3.0 | 0.0–7.0 | 46 | 3.1 | 2.4 | 3.0 | 0.0–9.0 | 47 |
| Score index, swollen joints2 | 1.2 | 1.3 | 1.0 | 0.0–4.0 | 46 | 1.1 | 1.3 | 1.0 | 0.0–7.0 | 47 |
| Visit 5 (day 60) | ||||||||||
| Total painful joints2 | 2.1 | 1.0 | 2.0 | 0.0–5.0 | 46 | 1.9 | 1.5 | 1.0 | 0.0–6.0 | 47 |
| Total swollen joints1,2 | 1.0 | 1.0 | 1.0 | 0.0–4.0 | 46 | 0.6 | 0.9 | 0.0 | 0.0–4.0 | 47 |
| Score index, painful joints1,2 | 2.6 | 1.1 | 3.0 | 0.0–5.0 | 46 | 2.3 | 1.9 | 2.0 | 0.0–8.0 | 47 |
| Score index, swollen joints2 | 1.1 | 1.1 | 1.0 | 0.0–4.0 | 46 | 0.7 | 1.0 | 0.0 | 0.0–4.0 | 47 |
| Visit 6 (day 90) | ||||||||||
| Total painful joints2 | 1.8 | 0.9 | 2.0 | 0.0–4.0 | 46 | 1.6 | 1.3 | 1.0 | 0.0–5.0 | 47 |
| Total swollen joints1,2 | 0.6 | 0.8 | 0.5 | 0.0–3.0 | 46 | 0.5 | 0.8 | 0.0 | 0.0–4.0 | 47 |
| Score index, painful joints1,2 | 2.0 | 1.1 | 2.0 | 0.0–4.0 | 46 | 1.7 | 1.4 | 1.0 | 0.0–6.0 | 47 |
| Score Index, swollen joints2 | 0.7 | 0.9 | 0.5 | 0.0–3.0 | 46 | 0.5 | 0.8 | 0.0 | 0.0–4.0 | 47 |
M median, ME median range, SD standard deviation, No. number of joints, GLM generalized linear model
1Statistically significant differences were found for the follow-up values between the two groups (glucosamine vs. Colatech®) (GLM, repeated measures; p < 0.05).
2Statistically significant differences were found for the follow-up values in each group for each treatment (GLM, repeated measures; p < 0.05).
No statistical significant differences were found for the follow-up values in each visit between two groups (GLM, repeated measures; p < 0.05)
Fig. 1.
Analgesic activity of enzymatic hydrolysed collagen (EHC) ( Colatech®) and glutamate sulphate (GS) on the quadruple visual analogue scale (VAS)
Table 1.
Analgesic activity of enzymatic hydrolysed collagen (EHC) (Colatech® ) and glutamate sulphate (GS) on the quadruple visual analogue scale (VAS)
| GS | Colatech® | |||
|---|---|---|---|---|
| VAS decreasea | No. | % | No. | % |
| Clear Improvement (≥ 20 mm) | 17 | 37.0 | 32 | 68.1 |
| (between 10 and 20 mm) | 14 | 30.4 | 8 | 17.0 |
| No Improvement (< 10 mm) | 15 | 32.6 | 7 | 14.9 |
| Overall | 46 | 100.0 | 47 | 100.0 |
aStatistically significant differences were found between the two groups (χ2 test; p < 0.05)
An analysis was performed of the area under the curve (AUC) of differences from the baseline visit for each of the four VAS measurements, comparing the mean area between the two treatment groups using a nonparametric Mann–Whitney test. This calculation was made by estimating the area between the co-ordinate axis and the line drawn by changes in each of the four measures: Pain right now, Typical or average pain, Pain level at its best, and Pain level at its worst. As the lines drawn reflected differences from baseline in each of these measures, the resulting areas were negative, indicating decreases. Thus, lower areas indicate greater decreases (Fig. 1). Table 2 shows the results. Colatech® was inducing statistically significantly better improvement of analgesic activity than GS (p < 0.001) (Table 2)
Table 2.
Analgesic activity of enzymatic hydrolysed collagen (EHC) (Colatech® ) and glucosamine sulphate (GS) on the quadruple visual analogue scale (VAS). Area under the curve (AUC)
| GS | Colatech® | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | ME | Range | No. | M | SD | ME | Range | No. | |
| Pain right nowa | −1.2 | 2.5 | −0.6 | −8.6–2.4 | 46 | −3.8 | 3.5 | −3.9 | −10.5–2.9 | 47 |
| Typical or average paina | −1.0 | 2.7 | −0.3 | −9.1–4.0 | 46 | −2.7 | 3.3 | −2.1 | −11.8–5.3 | 47 |
| Pain level at its besta | −0.3 | 1.9 | 0.0 | −5.0–2.9 | 46 | −1.3 | 2.6 | −0.5 | −9.1–5.0 | 47 |
| Pain level at its worsta | −1.0 | 2.5 | −0.8 | −8.1–3.4 | 46 | −3.7 | 3.8 | −3 | −13.8–1.8 | 47 |
M median, SD standard deviation, ME median range
aStatistically significant differences were found between the two groups (Mann-Whitney test; p < 0.05)
With the ITT analysis, the WOMAC index presented a greater reduction (p < 0.05) in the Colatech® group than in the GS group. The health status score of the WOMAC scale showed significant differences between patient groups at 90 days of treatment (p < 0.05). A ≥15-point improvement in WOMAC scores was seen in 34.8% of patients treated with Colatech® compared with 13.3% of patients treated with GS (chi-square test; p < 0.05). (Table 3).
Table 3.
Activity of enzymatic hydrolysed collagen (EHC) (Colatech®) and glucosamine sulphate (GS) on improvement in health status assessed by the Western Ontario and McMaster Universities (WOMAC) index
| GS | Colatech® | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| WOMAC Index decreasea | 45 | 100.0 | 46 | 100.0 |
| ≥ 15 (decrease more or equal 15 points) | 6 | 13.3 | 16 | 34.8 |
| < 15 (decrease less to 15 points) | 39 | 86.7 | 30 | 65.2 |
aStatistically significant differences were found between the groups (χ2 test; p < 0.05)
SF-36 questionnaire
Table 4 shows the results obtained at the start (day –7) and at the end (day 90) of the study. The results demonstrate that both Colatech® and GS, administrated during 90 consecutive days at a dose of 10 g qd and 1.5 g qd, respectively clearly improved the quality of life. The absolute differences between evaluation values at the start and end of the study were always better in Colatech® group than in the GS group. In such important aspects as general health (Colatech® 3.7 vs. GS 2.9), vitality (Colatech® 4.1 vs. GS 3.4), social function (Colatech® 9.3 vs. GS 4.1), physical function (Colatech® 12.2 vs. GS 7.0), physical work (Colatech® 24.8 vs. GS 13.6), emotional function (Colatech® 6.8 vs. GS 2.2) and mental health (Colatech® 3.5 vs. GS 1.7), we can prove that treatment with Colatech® always induced a better improvement in the different investigated parameters.
Table 4.
Activity of enzymatic hydrolysed collagen (EHC) (Colatech®) and glucosamine sulphate (GS) on the Short-Form (SF)-36 Questionnaire
| GS | Colatech® | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial (visit 1; day -7) | M | SD | ME | Range | No. | Initial (visit 1; day -7) | M | SD | ME | Range | No. |
| Physical function (0–100)b | 58.2 | 21.5 | 52.5 | 20.0–100.0 | 44 | Physical function (0–100)b | 56.0 | 20.6 | 55.0 | 20.0–100.0 | 47 |
| Physical work (0–100)b | 34.8 | 37.1 | 25.0 | 0.0–100.0 | 46 | Physical work (0–100)b | 36.4 | 34.8 | 25.0 | 0.0–100.0 | 46 |
| Physical pain (0–100)a,b | 53.4 | 14.5 | 52.0 | 22.0–84.0 | 46 | Physical pain (0–100)a,b | 52.5 | 14.8 | 52.0 | 22.0–100.0 | 46 |
| General health (0–100)b | 54.7 | 17.1 | 52.0 | 25.0–97.0 | 45 | General health (0–100)b | 53.1 | 16.1 | 52.0 | 20.0–97.0 | 47 |
| Vitality (0–100)b | 53.7 | 14.2 | 52.5 | 30.0–100.0 | 46 | Vitality (0–100)b | 52.9 | 15.5 | 52.5 | 25.0–100.0 | 46 |
| Social function (0–100)b | 71.5 | 20.1 | 75.0 | 25.0–100.0 | 43 | Social function (0–100)b | 72.3 | 18.0 | 75.0 | 25.0–100.0 | 46 |
| Emotional function. (0–100)b | 90.6 | 22.9 | 100.0 | 0.0–100.0 | 46 | Emotional function (0–100)b | 82.6 | 34.2 | 100.0 | 0.0–100.0 | 46 |
| Emotional health (0–100)b | 68.9 | 12.5 | 68.0 | 44.0–96.0 | 44 | Emotional health (0–100)b | 66.8 | 15.5 | 64.0 | 28.0–100.0 | 45 |
| Physical health indexb | 35.8 | 9.8 | 34.5 | 20.4–56.8 | 39 | Physical health indexb | 36.0 | 7.7 | 34.2 | 23.8–52.8 | 45 |
| Emotional health index | 51.8 | 6.8 | 52.7 | 29.4–64.9 | 39 | Emotional health index | 50.1 | 9.1 | 52.1 | 25.6–66.9 | 45 |
| Final (visit 6 – day 90) | |||||||||||
| Physical function (0–100)b | 65.2 | 23.6 | 75.0 | 15.0–100.0 | 45 | Physical function (0–100)b | 68.2 | 21.4 | 70.0 | 20.0–100.0 | 47 |
| Physical work (0–100)b | 48.4 | 42.0 | 50.0 | 0.0–100.0 | 46 | Physical work (0–100)b | 61.2 | 38.2 | 75.0 | 0.0–100.0 | 47 |
| Physical pain (0–100)a,b | 62.7 | 16.6 | 64.0 | 22.0–84.0 | 46 | Physical pain (0–100)a,b | 67.1 | 17.0 | 74.0 | 22.0–100.0 | 47 |
| General health (0–100)b | 57.6 | 16.0 | 52.0 | 25.0–97.0 | 45 | General health (GH) (0–100)b | 56.8 | 13.7 | 57.0 | 30.0–97.0 | 47 |
| Vitality (0–100)b | 57.1 | 15.7 | 55.0 | 30.0–100.0 | 46 | Vitality (0–100)b | 57.0 | 16.7 | 55.0 | 25.0–100.0 | 47 |
| Social function(0–100)b | 76.1 | 19.8 | 75.0 | 25.0–100.0 | 44 | Social function(0–100)b | 81.6 | 15.2 | 87.5 | 50.0–100.0 | 47 |
| Emotional function (0–100)b | 92.8 | 25.3 | 100.0 | 0.0–100.0 | 46 | Emotional function (0–100)b | 89.4 | 28.7 | 100.0 | 0.0–100.0 | 47 |
| Emotional health (0–100)b | 70.6 | 13.3 | 68.0 | 44.0–100.0 | 45 | Emotional Health (0–100)b | 70.3 | 13.4 | 68.0 | 36.0–100.0 | 47 |
| Physical health indexb | 40.0 | 9.9 | 41.0 | 21.8–56.8 | 41 | Physical health indexb | 42.0 | 8.3 | 44.9 | 24.8–53.1 | 47 |
| Emotional health index | 51.1 | 7.1 | 52.9 | 29.6–61.6 | 41 | Emotional health index | 50.7 | 7.7 | 52.1 | 25.6–66.5 | 47 |
aStatistically significant differences were found between groups for the follow-up values [generalised linear models (GLM)], repeated measures; p < 0.05)
bStatistically significant differences were found for the follow-up values in each group (GLM, repeated measures; p < 0.05). No statistically significant differences were found between groups for the follow-up values in each visit (GLM, repeated measures; p < 0.05).
Other outcome measures
Other outcome measures used for the efficacy study included evaluation of pain and stiffness of other joints affected, the physician’s and patients’ overall opinions on improvement, and the ibuprofen 400 mg tablets used as rescue medication. Joint assessment using pain and swelling index showed changes with statistically significant differences both between the study groups and between patients within each group. The group treated with Colatech® showed significant decreases in Total swollen joints and Score index for painful joints, suggesting improvement compared with the GS group (Table 5).
Efficacy assessment showed significant differences (p < 0.05) between the study groups, with an assessment of good and ideal efficacy favouring the group of patients treated with Colatech®. No significant differences were seen in rescue medication between groups. Mean use of rescue medication was 0.2 tablets/day (SD = 0.3) in the GS, compared with 0.3 tablets/day (SD = 0.4) in the Colatech® group.
Adverse events
The majority of patients in both groups experienced no adverse events (91.3% vs. 87.2% for GS and EHC groups, respectively). All adverse events reported were mild in intensity in both groups. The most common adverse events occurring in the EHC and GS groups were gastrointestinal symptoms, including dyspepsia, diarrhoea, flatulence, and one gastrointestinal disorder (4.3% vs. 10.6%, respectively). The least common adverse events were one case of acute bronchitis, one case of cystitis, and one case of pharyngitis. More than one adverse event occurred in one patient in the GS group. However, the percentages of patients who experienced each adverse event in both groups were not statistically significantly different.
Discussion
As the dosages of EHC and GS were different, this study was designed as a randomised, controlled trial in order to blind completely both patients and physician (double blind). We found that Colatech® significantly improved pain, stiffness, joint function and quality of life over the 12 administration weeks. Althought Colatech® has demonstrated good efficacy in several earlier clinical trials [1, 2, 16], inducing improvement of the joints and general symptomatology such as pain and stiffness, until now, it has never been demonstrated in a double-blind, randomised, comparative trial. In our study, Colatech® demonstrated statistically significantly better analgesic potency (p < 0.05) than GS. It also proved much better than GS in improving stiffness (p < 0.05), tin the WOMAC score (p < 0.05), in the number of affected joints (p < 0.05) and in the SF-36 Questionnaire (p < 0.05). Moreover Colatech® proved better than GS according to the investigator’s and patients’ opinions. It can be argued that the Colatech® regimen was 10 g qd, whereas the GS regimen was 1.5 g qd, but it must be considered that Colatech® is not a drug, but a food. Therefore, if the amount of food is greater, it does not increase the number or the intensity of side effects, and really, there was no statistically significant difference between GS and Colatech® in side effects after 15, 30, 60 or 90 days of consecutive administration. Moreover, there were no statistically significant differences between GS and Colatech® in the amount of ibuprofen tablets (rescue medication) used. These data show that Colatech® treatment is an effective and advantageous alternative to GS.
Conclusion
Colatech® has demonstrated to be efficient in improving clinical status in KOA patients, with significant improvement in pain scores, functional joint status and a better quality of life. It demonstrated clinically better efficacy than GS after 2, 4, 8 and 12 weeks of treatment. Moreover, the quicker, although only slight, onset of action of Colatech®, as well as an approximately equal rate of adverse events to GS, might represent an interesting, adequate and advantageous alternative and play an important role in the management of OA of the knee.
References
- 1.Adam M. Therapie der osteoarthrosis. Welche Wirkung haben gelatinepräparate? Therapiewoche. 1991;41:2458–2461. [Google Scholar]
- 2.Arquer A, Pujol P. Physical exercise in the elderly people. Selección. 1996;5(3):121–128. [Google Scholar]
- 3.Bellamy N, Carette S, Ford PM, et al. Osteoarthritis antirehumatic drug trials. II.Tables for calculating sample size for clinical trials. J Rheumatol. 1992;19:444–450. [PubMed] [Google Scholar]
- 4.Bellamy N, Carette S, Ford PM, et al. Osteoarthritis antirehumatic drug trials. III. Setting the delta for clinical trials-results of a consensus development (Delphi) exercise. J Rheumatol. 1992;19:451–457. [PubMed] [Google Scholar]
- 5.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J Orthop Rheum. 1988;1:95–108. [PubMed] [Google Scholar]
- 6.Hughes R, Carr A. A randomized, double-blind, placebo-controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology. 2002;41:279–284. doi: 10.1093/rheumatology/41.3.279. [DOI] [PubMed] [Google Scholar]
- 7.Jerosch J, Verdonk R, Trč T, Price A, Bailleul F, Dijk NC. Efficacy and safety of a single dose of Hylan G-20 vs. Placebo in patients with knee osteoarthritis pain. Knee Surgery Sport Traumatology Arthroscopy. 2008;16(Suppl 1):27–28. [Google Scholar]
- 8.McAlindon T. Glusoamine for osteoarthritis: dawn of a new era? Lancet. 2001;357:247–248. doi: 10.1016/S0140-6736(00)03606-0. [DOI] [PubMed] [Google Scholar]
- 9.Newman NM, Ling RS. Acetabular bone destruction related to non-steroidal anti-inflammatory drugs. Lancet. 1985;2:11–14. doi: 10.1016/S0140-6736(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 10.Noack W, Fischer M, Forster KK, et al. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthr Cartil. 1994;2(1):51–59. doi: 10.1016/S1063-4584(05)80006-8. [DOI] [PubMed] [Google Scholar]
- 11.Oesser S, Adam M, Babel W, Seifert J. Oral administration of 14ClLabelled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL) J Nutr. 1999;129:1891–1895. doi: 10.1093/jn/129.10.1891. [DOI] [PubMed] [Google Scholar]
- 12.Pavelka K, Trč T, Karpaš K, et al. The efficacy and safety of diacerein bn the treatment of painful osteoarthritis of the knee. Arthritis Rheum. 2007;56:4055–4064. doi: 10.1002/art.23056. [DOI] [PubMed] [Google Scholar]
- 13.Pavelka K, Trč T, Karpaš K, Moster R, Audy P. Treatment of knee osteoarthritis by intraarticular application of Hyalgan: clinical praxis experiences in Czech Republic. Acta Chir Orthop Traum Čech. 2002;69:302–304. [PubMed] [Google Scholar]
- 14.Reginster JY, Gillot V, Bruyere O, Henrotin Y. Evidence of nutriceutical effectiveness in the treatment of osteoarthritis. Curr Rheumatol Rep. 2000;2:472–477. doi: 10.1007/s11926-000-0023-9. [DOI] [PubMed] [Google Scholar]
- 15.Reginster JY, Deroisy R, Rovatti LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 16.Ribas J, Molinero O. Effects of gelatine hydrolysatesin the prevention of sportsmen and women injuries. Arch Med Deport. 1998;XV 66:277–282. [Google Scholar]
- 17.Rindone J, Hiller D, Collacot E, et al. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. West J Med. 2000;172:91–94. doi: 10.1136/ewjm.172.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saase JL, Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271–280. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trč T, Handl M. Hyaluronic acid experiences in treatment of arthritis. Acta Chir Orthop Traum Čech. 1997;64:95–98. [PubMed] [Google Scholar]
- 20.Trentham D, Dynesius-Trentham R, Orav E, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 21.Zeidler H. Epidemiology and NSAID induced gastropathy. J Rheumatol. 1991;28(suppl):2–5. [PubMed] [Google Scholar]