Abstract
Space competition between corals and seaweeds is an important ecological process underlying coral-reef dynamics. Processes promoting seaweed growth and survival, such as herbivore overfishing and eutrophication, can lead to local reef degradation. Here, we present the case that increasing concentrations of atmospheric CO2 may be an additional process driving a shift from corals to seaweeds on reefs. Coral (Acropora intermedia) mortality in contact with a common coral-reef seaweed (Lobophora papenfussii) increased two- to threefold between background CO2 (400 ppm) and highest level projected for late 21st century (1140 ppm). The strong interaction between CO2 and seaweeds on coral mortality was most likely attributable to a chemical competitive mechanism, as control corals with algal mimics showed no mortality. Our results suggest that coral (Acropora) reefs may become increasingly susceptible to seaweed proliferation under ocean acidification, and processes regulating algal abundance (e.g. herbivory) will play an increasingly important role in maintaining coral abundance.
Keywords: Carbon dioxide, coral reefs, coral–algal competition, macroalgae, ocean acidification
Introduction
Corals and macroalgae are among the dominant benthic groups on coral reefs, and their relative abundance and competition are often used as indicators of reef status (Mumby et al. 2007). Environmental and anthropogenic processes that drive the competitive interactions, particularly space competition, between corals and seaweeds (fleshy macroalgae) have profound consequences for the dynamics and resilience of reef communities. Well-known processes that can shift reef communities from coral- to seaweed-dominated regimes include the loss of herbivore grazing through overfishing or disease (Hughes et al. 2007), eutrophication via enhanced algal growth (Lapointe 1999) and coral mortality by releasing macroalgae from space competition with corals (Diaz-Pulido et al. 2009). Increasing concentrations of atmospheric carbon dioxide, CO2, and associated ocean acidification is a growing threat to coral reefs globally (Caldeira & Wickett 2003; Hoegh-Guldberg et al. 2007), as it can lead to reduced growth rates (e.g. calcification) of corals and other marine calcifiers (Kleypas et al. 2006; Anthony et al. 2008). Seaweeds show varying responses to ocean acidification, including positive, neutral or negative effects (Israel & Hophy 2002; Diaz-Pulido et al. 2007; Hall-Spencer et al. 2008; Wu et al. 2008), but the role of CO2 enrichment in driving coral–algal interactions and the consequence for reef resilience is unknown (Harley et al. 2006; Hoegh-Guldberg et al. 2007).
Here, we test the hypothesis that ocean acidification shifts the competitive interaction between corals and seaweeds in favour of the seaweeds. We build this hypothesis on the following two premises: First, high pCO2 leads to declining aragonite saturation state (Caldeira & Wickett 2003), which, as a functional consequence, leads to reduced rates of coral growth (Langdon & Atkinson 2005; Kleypas et al. 2006; Silverman et al. 2009), potentially lowering their ability for space competition. Second, growth of subtidal seaweeds is likely to increase as a function of pCO2 (Kübler et al. 1999; Diaz-Pulido et al. 2007; Hall-Spencer et al. 2008), enhancing the seaweed’s ability to overgrow and kill neighbouring corals (e.g. see Jompa & McCook 2002). To analyze this hypothesis experimentally, we compared the survival profiles of the branching coral Acropora intermedia (Brook) grown in isolation and in competition with the brown fleshy seaweed Lobophora papenfussii (Taylor) Farghaly in a flow-through aquarium system with controlled CO2-dosing regimes for an 8-week period. The coral genus Acropora plays fundamental ecological, geological and physical roles in the Great Barrier Reef (GBR) as it is the most abundant hard coral group and a major reef builder in the region (Hutchings et al. 2008). Species of the macroalgal genus Lobophora are ecologically important in both shallow and deep reefs due to their high abundance, and have also been implicated in the degradation of Caribbean (Mumby et al. 2007) and GBR (Diaz-Pulido et al. 2009) reefs. Under normal undisturbed conditions, the seaweed commonly grows at the dead base of coral branches, but away (c. > 5–10 cm) from the healthy coral tissue. The algae’s ability to grow beyond the base of healthy coral branches is limited, due to competitive inhibition by the corals or the removal of algal tissue by herbivores. However, the interaction between branching corals and the seaweed Lobophora is competitive, with both corals and seaweeds inhibiting each other (Jompa & McCook 2002; Diaz-Pulido et al. 2009). In this study, we show that increased pCO2/reduced pH, representing the ocean-acidification range projected for this century, enhances the ability of seaweeds to kill corals, potentially shifting the competitive interaction between corals and seaweeds in favour of seaweeds.
Materials and methods
Experimental design and CO2 system
We tested the combined effects of ocean acidification and algal–coral competition on coral survivorship and growth through simultaneous manipulation of competitors and CO2 levels in an orthogonal, multifactorial experiment carried out on Heron Island Research Station (HIRS), southern GBR. The experiment was conducted during May and June, the Austral autumn/winter, of 2009. We used branches of the coral A. intermedia and thalli of the seaweed L. papenfussii, which are both abundant in reef fronts and upper slopes on GBR reefs. Seaweeds and corals were collected from the reef slope at Coral Gardens (23°26.698′ S, 151°54.533′ E), Heron Reef. The competitor treatment consisted of three manipulation groups: (i) isolated live coral branches, 10-cm long; (ii) pairs of competing corals and seaweeds, in which seaweeds (thalli of c. 5 cm × 5 cm planar area) were attached to the base of the coral branch; and (iii) isolated seaweeds, attached to dead coral. An additional isolated control group involved live coral branches with algal mimics (latex thalli) attached to the coral base, and was used to partly discern whether interaction outcomes were due to mechanical or biological/chemical effects on the coral. Due to space and CO2-treated seawater restrictions, the algal mimic treatment was conducted only for the 400-ppm control treatment. Both live and dead coral branches were suspended from their apical corallites using thin monofilament line to minimize coral and algal contact with other surfaces. Coral and algal specimens were acclimatized in tanks with running seawater during 10 days prior to the experiment.
The CO2 experiment involved four CO2-dosing regimes to cover the range of historical and potentially future ocean-acidification conditions. We used CO2 target values of 300 (pH target: 8.10–8.20), 400 (pH: 8.00–8.10), 560 (pH: 7.85–7.95) and 1140 ppm (pH: 7.60–7.70), representing pre-industrial, present-day, and projected mid-century and late-century CO2 levels under the A1FI scenario by the IPCC (Meehl et al. 2007; Meinshausen et al. 2009; Solomon et al. 2009). The CO2 control system (Aquatronica-AEB Technologies, Cavriago, Italy) used computer-operated solenoid valves to regulate the amount of CO2 injected into the seawater, based on pH levels measured continuously in 200-L mixing tanks (one mixing tank per CO2 treatment) using Mettler-Toledo polarographic sensors (see Anthony et al. 2008, for details). Sumps were connected to the outdoor flow-through aquarium system at HIRS, which uses unfiltered seawater pumped directly from the reef flat. To dampen diurnal pH fluctuations (7.9–8.4) of the pH from the reef flat (caused by benthic carbon fluxes), and better simulate the more stable pH regime on the outer reef slope where both corals and macroalgae were collected, low and high pH extremes of the control were prevented by dosing CO2 when pH exceeded 8.2 and scrubbing CO2 (see below) when pH fell below 8.0. CO2 scrubbing was used to generate a pre-industrial CO2 regime (c. 300 ppm), using the procedure described in Reynaud et al. (2003). Briefly, air was filtered through a column (2 m tall × 5 cm diameter) of soda lime before being injected into the water in the mixing tank for the pre-industrial treatment (and for the control tank when pH dropped below 8). Individual experimental tanks (10-L plastic aquaria) were randomly assigned to treatments and were continuously fed with water from the treated sump with a flow of 2–3 L min−1. Each tank had a small powerhead for water circulation. Each CO2 level had two tanks per treatment combination, each with 10 subsamples and a total of 160 seaweeds and 180 corals. To mimic the light field at the native habitat (6 ± 3 m depth) of both corals and algae, shade screens were used to reduce the natural sunlight to average noon levels of 320 (min: 100, max: 1063) μmol m−2 s−1. Tanks were cleaned every 2–3 days of filamentous algae and inspected for the presence of mesograzers.
Water chemistry analyses
Samples for water chemistry analyses were collected during the course of the experiment (see details in Table 1). Total dissolved inorganic carbon (TCO2) was measured coulometrically and total alkalinity (TA) analyses were made by open-cell potentiometric titration (Dickson et al. 2007), using seawater samples collected from the sumps every 6 h during a 24-h period. The pCO2, aragonite saturation state (ΩArag) and pH on the total seawater scale were calculated for 24–25 °C from the TCO2 and TA values (Lewis & Wallace 1998). Dissolved organic carbon (DOC) was measured by high-temperature combustion (680 °C) using a Shimadzu TOC-5000A carbon analyzer (Shimadzu Corporation, Kyoto, Japan), and dissolved inorganic nutrients were determined by standard wet chemical methods using a segmented flow analyzer. DOC and nutrients data were determined using seawater from the experimental tanks collected during the day.
Table 1.
Summary of values for water chemistry parameters for CO2 treatment levels
| Treatments | pH | pCO2 (ppm) | TA (μmol kg−1) | TCO2 (μmol kg−1) | ΩArag | DOC (mg L−1) | NH4 (μmol L−1) | NO2 (μmol L−1) | NO3 (μmol L−1) | PO4 (μmol L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 300 | 8.12 (0.02) | 305 (11) | 2193 (13) | 1882 (16) | 3.37 (0.06) | 0.65 (0.02) | 0.037 (0.023) | 0.038 (0.005) | 0.450 (0.020) | 0.237 (0.007) |
| 400 | 8.02 (0.02) | 402 (20) | 2170 (27) | 1918 (29) | 2.78 (0.07) | 0.68 (0.01) | 0.020 (0.020) | 0.032 (0.009) | 0.460 (0.058) | 0.238 (0.010) |
| 560 | 7.85 (0.01) | 564 (12) | 2208 (22) | 2012 (17) | 2.26 (0.07) | 0.69 (0.01) | 0.072 (0.045) | 0.036 (0.010) | 0.524 (0.019) | 0.254 (0.007) |
| 1140 | 7.63 (0.02) | 1140 (52) | 2212 (20) | 2123 (24) | 1.32 (0.04) | 0.71 (0.03) | 0.070 (0.045) | 0.033 (0.002) | 0.545 (0.024) | 0.252 (0.007) |
pH, pCO2 (partial pressure CO2), TA (total alkalinity), TCO2 (total dissolved inorganic carbon) and ΩArag (aragonite saturation state) values are means of eight replicates (SEM). DOC (dissolved organic carbon), NH4, NO2, NO3 and PO4 are means of six replicates (SEM).
Response variables and data analyses
A census of coral survivorship was conducted daily throughout the course of the experiment. Coral branches were considered dead when at least half of the branch was devoid of tissue. Dead corals were subsequently removed from the experimental tanks. Growth of both corals and macroalgae was measured to quantify the level of competition. At the beginning of the experiment, a marker (plastic cable tie, 1 mm thick) was tied to the bottom part of each coral branch or rubble as a reference point for growth measurements. Coral growth (as linear extension) was measured as the increase in the distance from the coral tip to the cable tie. Algal growth (as marginal growth) was measured as the extension of the blade margin relative to the reference point (Jompa & McCook 2002). Photographs of each specimen were taken at the beginning and end of the experiment, and growth estimates were made using ImageJ software (http://rsbweb.nih.gov/ij/).
Coral survival was analyzed using an exponential failure-time (hazard) model with CO2 and tanks as factors (Muenchow 1986). Effects of CO2 and competition on coral and seaweed growth were analyzed using a factorial, nested anova, with competitor and CO2 levels as fixed factors, and tanks as replicates. Specimens were nested within tanks. However, because the tank factor was non-significant (P> 0.25, data not shown), tanks were pooled in subsequent analyses and specimens were used as replicates (Underwood 1997), hence increasing the power of the analysis. Data from the latter analyses are presented here, followed by post hoc Student–Newman–Keuls (SNK) tests. To minimize the risks of overlooking small CO2 effects (i.e. Type II errors), CO2 effects on seaweed growth were specifically tested within the isolated seaweed treatment (in the absence of the corals) using a one-way anova. Some algal and coral specimens were lost during the course of the 8-week experiment, reducing the degrees of freedom in the analyses. Data were tested for homogeneity of variance (Cochran’s test), outliers and normality of residuals.
Results
Elevated pCO2 strongly increased mortality rates of corals in contact with seaweeds. In the highest pCO2 treatment (1140 ppm), very high mortality rates (5–10% per day) set in after 1 week and the entire coral population in this treatment died within 3 weeks (Fig. 1). In contrast, for the low pCO2 treatment (300 ppm, pre-industrial), high coral mortality set in after 3 weeks and continued at a slower rate than at the high CO2 level. Corals in the 400 and 560 ppm treatments showed high initial mortality rates (after 2–3 weeks), but ended with 20–30% survival after 8 weeks, similar to that of the pre-industrial group. The proportional hazard model indicated that the CO2 level was significantly correlated with coral mortality (P< 0.001), although the absolute coral mortality rate (in the treatment in contact with seaweeds) of all but the highest level of CO2 was similar at week 8. Isolated coral branches (control corals with no seaweeds) showed < 5% mortality, regardless of the CO2 level. Mortality of corals with algal mimics (thin latex thalli) attached was also negligible (< 5%, Fig. 1). Coral mortality caused by the presence of seaweeds occurred at the interface between the algal thalli and the coral (Fig. 2) and was not preceded by tissue bleaching. Following the onset of coral tissue necrosis in the contact zone, tissue disintegration progressed up the branch at a rate of 2–3 cm day−1.
Figure 1.
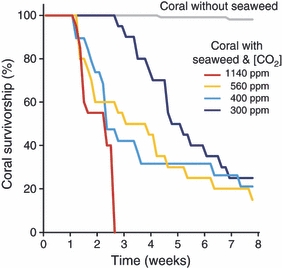
Coral mortality as a function of time, CO2 level and seaweed competition. The initial population size for each treatment group was 20 corals. The curve of coral without seaweed is the average of the survivorship rate of isolated corals (with no algal contact) across all CO2 treatments and in contact with plastic seaweeds.
Figure 2.
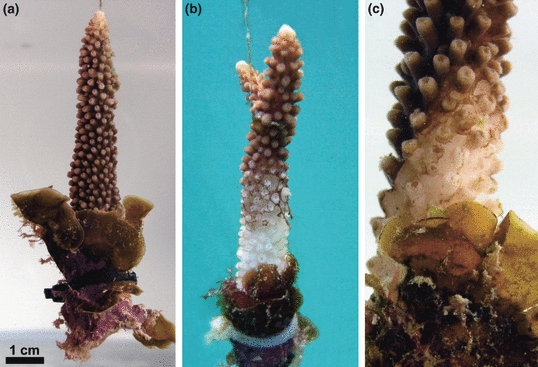
Coral mortality caused by seaweeds in experimental coral–algal pairs exposed to high CO2 levels. (a) Competing corals and seaweeds of the species Acropora intermedia and Lobophora papenfussii at the beginning of the experiment. (b) and (c) Coral branches with advanced tissue mortality caused by the seaweed. Coral tissue disintegration occurred rapidly in the high CO2 treatment. Coral white areas show bare coral skeleton devoid of coral tissue.
The rate of marginal extension of seaweed thalli was significantly affected by both competitor and pCO2 treatments. Seaweed growth rate was higher in the absence of live corals (i.e. algal controls growing on dead coral rubble) compared with the treatment where the alga was in contact with live coral (P< 0.001; Table 2; Fig. 3). Localized damage to the margin of the algal blades was observed at the interface with the coral. The effect of CO2 on algal growth was significant (P= 0.046; Table 2), but smaller than the effect of the competitor (Fig. 3). SNK post hoc tests in the overall analyses indicated that the algal growth rate was lower in the highest pCO2 treatment than at any other CO2 level (Table 2), whereas there was no significant difference between growth rates at 300, 400 and 560 ppm pCO2. Because the presence of the competing coral reduced algal growth rates (potentially masking small CO2 effects), pCO2 effects on seaweed growth were specifically tested within the isolated seaweed treatment. Results of this analysis were significant (P= 0.006) and SNK post hoc test (Table 2 footnote) showed that growth rates of isolated seaweed thalli at the 400- and 560-ppm CO2 level were significantly higher (24% and 40% respectively) than that in the low pre-industrial CO2 treatment. Algal growth rate, however, declined to pre-industrial levels at the highest pCO2 (Table 2 footnote; Fig. 3). The growth rate of seaweeds in the presence of corals was negligible and did not vary significantly across pCO2 treatments (P= 0.439; Fig. 3). In contrast, the growth rate of corals (expressed as linear extension) in isolation and in the presence of seaweeds declined dramatically and monotonically with increasing pCO2 (P= 0.039), reaching negative values at the highest CO2 concentration (Table 2; Fig. 4). There was no significant difference (P= 0.622) in the growth rates of isolated corals vs. corals in competition.
Table 2.
Factorial anovas for the effects of CO2 levels and coral–algal competition on the growth of seaweeds and corals
| Source of variation | d.f. | MS | F | P | Conclusion – SNK test |
|---|---|---|---|---|---|
| Seaweeds | |||||
| CO2 | 3 | 70.08 | 2.74 | 0.046 | (300 = 400 = 560) > 1140* |
| Competition | 1 | 1710.71 | 66.75 | < 0.001 | Isolated alga > alga with coral |
| CO2 × Competition | 3 | 22.86 | 0.89 | 0.447 | n.s. |
| Error | 133 | 25.63 | |||
| Corals | |||||
| CO2 | 3 | 23.021 | 2.863 | 0.039 | 300 = 400 > 560 = 1140 |
| Competition | 1 | 1.963 | 0.244 | 0.622 | n.s. |
| CO2 × Competition | 3 | 3.175 | 0.395 | 0.757 | n.s. |
| Error | 127 | 8.041 | |||
MS, mean square; SNK, Student–Newman–Keuls test; n.s., not significant.
We also specifically tested for CO2 effects on seaweed growth within the isolated seaweed treatment. The results of this test indicated: anova, P= 0.006; SNK: (400 = 560) > 1140, 560 > 300, 400 > (300 = 1140). anova for the effects of CO2 on seaweed growth within the seaweed-coral treatment was not significant (P= 0.439).
Figure 3.
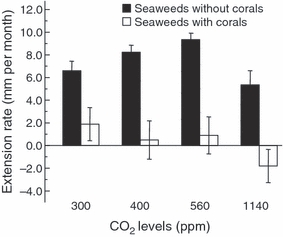
Growth (marginal extension) response of seaweed Lobophora papenfussii to increased CO2 concentrations and presence of coral competitor (Acropora intermedia). Data are means ± SEM; n = 15–20.
Figure 4.
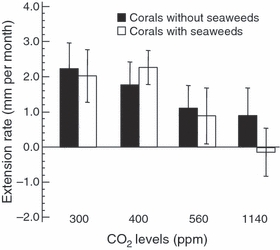
Growth (linear extension) response of coral Acropora intermedia to increased CO2 concentrations and presence of seaweed competitor (Lobophora papenfussii). Data are means ± SEM; n = 14–20.
Discussion
It is now well documented that rising atmospheric CO2 levels and the consequent changes in ocean carbon chemistry (ocean acidification) have profound implications for the physiology of many marine organisms (Raven 2005; Kleypas et al. 2006; Anthony et al. 2008; Pelejero et al. 2010). However, the impacts of ocean acidification on marine ecological interactions are largely unknown (Harley et al. 2006; Doney et al. 2009). The results of this study provide novel empirical evidence that ocean acidification alters the competitive interactions between two species of reef organisms belonging to the most abundant benthic groups on coral reefs: hard corals and benthic algae. Corals and algae compete for space and light, and fleshy macroalgae (seaweeds) may out-compete corals (McCook et al. 2001; Nugues et al. 2004b; Smith et al. 2006), particularly under situations of low herbivory (Jompa & McCook 2002; Rasher & Hay 2010). In our experiments, we demonstrate that ocean acidification enhances the ability of a common seaweed (Lobophora) to kill and potentially out-compete a coral (Acropora). Only corals in contact with live seaweeds showed significant mortality, and mortality was exacerbated by elevated pCO2. This indicates that coral mortality can be attributed to the presence of, and interaction with, seaweeds. Accordingly, increased coral mortality with increasing pCO2 is therefore likely to be a consequence of CO2, enhancing the competitive strength of the seaweeds.
The mechanism by which increased pCO2 enhanced coral mortality at the interface between the seaweed and the coral tissues could not be deduced from our observations or data. However, our experiment may suggest that mortality was caused by a chemical or biological rather than a physical (e.g. shading or abrasion) effect, as coral mortality occurred only when seaweeds, and not inert algal mimic (latex blades), were present. Although the algal mimic treatment was only conducted for the control CO2 level (potentially limiting the interpretation of interactions between algal presence and CO2 level), coral mortality across all CO2 treatments took place before the seaweed could overgrow the corals (as seaweeds showed limited marginal growth when in contact with the corals, possibly caused by inhibition by the coral polyps when still alive). This strongly suggests that coral mortality was caused by a chemical/biological interaction process. We propose two likely mechanisms: First, increased allelochemicals released by the seaweed can kill the coral directly (e.g. McCook et al. 2001; Rasher & Hay 2010). Lobophora has been shown to produce a range of secondary metabolites that are effective as chemical defenses (Rasher & Hay 2010), particularly when plants are stressed (Arnold & Targett 2000), for example by contact with corals (e.g. Nugues et al. 2004a). Further, there is evidence in terrestrial C3-plants that increased CO2 concentrations can lead to increased production of carbon-based allelochemicals due to augmented production of carbohydrates (Bidart-Bouzat & Imeh-Nathaniel 2008). Second, increasing leakage of DOC from the seaweeds can cause an increase in microbial growth on the surface of the corals, as suggested by Smith et al. (2006). However, the DOC level was lower in the algal and coral–algal competition treatment than in that of the isolated corals (anova, P= 0.046), providing no support for this hypothesis.
The increase in competitive strength of seaweeds over corals under elevated CO2 was also supported by a comparison of growth patterns for seaweeds and corals. Specifically, the growth of seaweed blades in the absence of corals increased with rising pCO2 (although algal growth declined to pre-industrial levels at the highest pCO2). These results are consistent with studies, particularly on red seaweeds, demonstrating enhanced algal growth rates with moderate enhancement of pCO2 (Gao et al. 1991, 1993; Kübler et al. 1999). However, there is also other evidence indicating that growth of benthic algae does not respond to increased pCO2 because algae are able to use the carbon from the abundant bicarbonate (HCO3−) pool in seawater by a series of cellular CO2-concentrating mechanisms (CCMs; Israel & Hophy 2002; Raven et al. 2005). Although it has been suggested that the Caribbean seaweed Lobophora variegata possesses CCMs (Enríquez & Rodríguez-Román 2006), growth of the experimental seaweed L. papenfussii in this study was enhanced by moderate CO2 enrichment (< 560 ppm). On the other hand, growth rates of corals declined with increased pCO2 (lowered pH), regardless of the competition treatment. Reduction of coral growth may be related to the lowering of the aragonite saturation state (Table 1) associated with a reduction of the amount of carbonate ions available for coral calcification as pH levels decrease. Stress from acidosis and/or damage to the photoprotective mechanisms in corals have also been suggested to occur under ocean-acidification conditions (Anthony et al. 2008; Crawley et al. 2010). The increase in seaweed growth rate in conjunction with a decline in coral growth and enhanced coral mortality as pCO2 increased in this study strongly suggest that corals could become increasingly disadvantaged in space competition with macroalgae as ocean acidification intensifies.
On the other hand, the presence of the coral competitor inhibited the rate of marginal growth of seaweeds. This suggests that the seaweed may have reduced ability to overgrow live coral tissue and that the corals have some potential to reduce algal overgrowth (see also de Ruyter van Steveninck et al. 1988; Nugues et al. 2004a). However, because algae caused significant mortality of coral tissue, particularly at the high pCO2 treatment, any potential inhibition of seaweed growth by coral may be overwhelmed by the negative effect of seaweeds on coral survivorship. It is likely that the seaweeds would have been released from space competition with the coral following coral tissue mortality and grown unrestrictedly if dead coral–algal pairs had not been removed from the tanks immediately after the corals died (to prevent potential water quality deterioration). Therefore, increased algal growth following coral mortality could not be demonstrated in our study. However, previous studies have shown increased algal growth and colonization following coral disturbances (Jompa & McCook 2002; Diaz-Pulido et al. 2009).
The enhanced ability of L. papenfussii to kill A. intermedia and the increase in competitive strength of the seaweed over the corals as a function of CO2 enrichment suggest that future reefs may become more likely to undergo coral–algal phase shifts than predicted previously (Hoegh-Guldberg et al. 2007; Mumby et al. 2007; Maynard et al. 2010). We acknowledge that by using only one species pair, we are not accounting for the high diversity of coral and algal species in tropical reefs, or the variety in mechanisms by which they compete (McCook et al. 2001; Nugues et al. 2004b; Smith et al. 2006; Rasher & Hay 2010). However, as these two genera co-occur on the same reef habitat (although the L. papenfussii generally grow centimetres away from healthy coral tissue) and are widespread on coral reefs globally, our results may have wide implications for a large number of reef habitats. Our findings also suggest that reef community dynamics will be altered by increased mortality risk of adult corals. In particular, high abundance of various types of seaweeds can impede coral population recovery following disturbances and reduce the resilience of the reef ecosystem by hindering coral settlement and recruitment (Diaz-Pulido et al. 2010) and the survival of juvenile corals (Box & Mumby 2007; Birrell et al. 2008). For example, under conditions of reduced herbivory, the alga Lobophora significantly decreased the growth of juvenile corals in the Caribbean (Box & Mumby 2007), whereas in the GBR, the presence of Lobophora around the branches of the coral colonies caused significant coral tissue mortality (Jompa & McCook 2002). Therefore, processes that regulate the abundance and type of seaweeds may be increasingly important under rising CO2. For instance, grazing by herbivores, maintenance of the abundance and species diversity of herbivorous functional groups, and controls on water quality will become more and more critical to reduce macroalgal dominance and maintaining coral reef resilience (Bellwood et al. 2004; Hughes et al. 2007; Mumby et al. 2007; Burkepile & Hay 2008; Rasher & Hay 2010). However, the extent to which high herbivore abundance and diversity can control Lobophora proliferations and phase shifts from Acropora to Lobophora on some coral reefs in the future is uncertain, as recent evidence shows that Lobophora spp. may be much less susceptible to herbivory than other reef seaweeds (Bennett et al. 2010; but see Jompa & McCook 2002). Further, some inshore reefs of the central GBR have experienced persistent coral –Lobophora phase shifts (> 7 years), in the absence of fishing pressure on herbivorous fishes and at moderate water quality (Cheal et al. 2010). This suggests that even ambient levels of grazing may be inadequate to reduce the strength of spatial competition of Lobophora over corals, particularly after disturbances, potentially perpetuating phase shifts under ocean-acidification scenarios. Physical disturbances such as those caused by storm activities and algal seasonality may also play important roles in controlling the abundance of seaweeds in the future (e.g. Diaz-Pulido et al. 2009). Our experiment provides a timely example of how ocean acidification affects not only the physiological processes of reef organisms, but also the ecological interactions between species.
Acknowledgments
We thank D. Bender for help in the field and V. Witt and S. Uthicke from the Australian Institute of Marine Science for the nutrient and irradiance data. This project was supported by the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies and grants from ARC and the Australian Climate Change Science Program. Two anonymous referees provided valuable comments that improved the study.
References
- Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. U.S.A. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold TM, Targett NM. Evidence for metabolic turnover of polyphenolics in tropical brown algae. J. Chem. Ecol. 2000;26:1393–1410. [Google Scholar]
- Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- Bennett A, Verges A, Bellwood DR. Branching coral as a macroalgal refuge in a marginal coral reef system. Coral Reefs. 2010;29:471–480. [Google Scholar]
- Bidart-Bouzat MG, Imeh-Nathaniel A. Global change effects on plant chemical defenses against insect herbivores. J. Integr. Plant Biol. 2008;50:1339–1354. doi: 10.1111/j.1744-7909.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- Birrell CL, McCook LJ, Willis BL, Diaz-Pulido G. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. Annu. Rev. 2008;46:25–64. [Google Scholar]
- Box SJ, Mumby PJ. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 2007;342:139–149. [Google Scholar]
- Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl Acad. Sci. U.S.A. 2008;105:16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, et al. Coral-macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs. 2010;29:1005–1015. [Google Scholar]
- Crawley A, Kline DI, Dunn S, Anthony KRN, Dove S. The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob. Change Biol. 2010;16:851–863. [Google Scholar]
- Diaz-Pulido G, McCook LJ, Larkum AWD, Lotze HK, Raven JA, Schaffelke B, et al. Vulnerability of macroalgae of the Great Barrier Reef to climate change. In: Johnson JE, Marshall PA, editors. Climate Change and the Great Barrier Reef. Townsville: Great Barrier Reef Marine Park Authority & Australian Greenhouse Office; 2007. pp. 153–192. [Google Scholar]
- Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff J, Kline DI, et al. Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE. 2009;4:e5239. doi: 10.1371/journal.pone.0005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O. The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs. 2010;29:203–208. [Google Scholar]
- Dickson AG, Sabine CL, Christian JR. Guide to Best Practices for Ocean CO2 Measurements. Sidney, BC: North Pacific Marine Science Organization (PICES Special Publication, 3); 2007. [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Enríquez S, Rodríguez-Román A. Effect of water flow on the photosynthesis of three marine macrophytes from a fringing-reef lagoon. Mar. Ecol. Prog. Ser. 2006;323:119–132. [Google Scholar]
- Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M. Enhanced growth of the red alga Porphyra yendoensis Ueda in high CO2 concentrations. J. Appl. Phycol. 1991;3:355–362. [Google Scholar]
- Gao K, Aruga Y, Asada K, Kiyohara M. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J. Appl. Phycol. 1993;5:563–571. [Google Scholar]
- Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 2008;454:96–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- Harley CDG, Hughes AAR, Hultgrem KM, Miner BG, Sote CJB, Thornber CS, et al. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli DM, Hoegh-Guldberg O, McCook LJ, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007;17:1–6. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Hutchings PA, Kingsford MJ, Hoegh-Guldberg O. The Great Barrier Reef: Biology, Environment and Management. Collingwood: CSIRO Publishing; 2008. [Google Scholar]
- Israel A, Hophy M. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Glob. Change Biol. 2002;8:831–840. [Google Scholar]
- Jompa J, McCook LJ. Effects of competition and herbivory on interactions between a hard coral and a brown alga. J. Exp. Mar. Biol. Ecol. 2002;271:25–39. [Google Scholar]
- Kleypas JA, Feely RA, Fabry VJ, Langdon C, Sabine CL, Robbins LL. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research. St Petersburg, FL: NSF, NOAA, and the U.S. Geological Survey; 2006. [Google Scholar]
- Kübler JE, Johnston AM, Raven JA. The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ. 1999;22:1303–1310. [Google Scholar]
- Langdon C, Atkinson MJ. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 2005;110:C09S07. [Google Scholar]
- Lapointe BE. Simultaneous top-down and bottom-up forces control macroalgal blooms on coral reefs (Reply to the comment by Hughes et al.) Limnol. Oceanogr. 1999;44:1586–1592. [Google Scholar]
- Lewis E, Wallace DWR. Program Developed for CO2System Calculations. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; 1998. ORNL/CDIAC-105. [Google Scholar]
- Maynard JA, Anthony KRN, Afatta S, Dahl-Taccon N, Hoegh-Guldberg O. Making a model meaningful to coral reef managers in a developing nation: a case study of overfishing and rock anchoring in Indonesia. Conserv. Biol. 2010;24:1316–1326. doi: 10.1111/j.1523-1739.2010.01487.x. [DOI] [PubMed] [Google Scholar]
- McCook LJ, Jompa J, Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs. 2001;19:400–417. [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, et al. Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, et al., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. pp. 747–845. [Google Scholar]
- Meinshausen M, Meinshausen N, Hare W, Raper SCB, Frieler K, Knutti R, et al. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nature. 2009;458:1158–1162. doi: 10.1038/nature08017. [DOI] [PubMed] [Google Scholar]
- Muenchow G. Ecological use of failure-time analysis. Ecology. 1986;67:246–250. [Google Scholar]
- Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- Nugues MM, Delvoye L, Bak RPM. Coral defence against macroalgae: differential effects of mesenterial filaments on the green alga Halimeda opuntia. Mar. Ecol. Prog. Ser. 2004a;278:103–114. [Google Scholar]
- Nugues MM, Smith GW, Hooidonk RJv, Seabra MI, Bak RPM. Algal contact as a trigger for coral disease. Ecol. Lett. 2004b;7:919–923. [Google Scholar]
- Pelejero C, Calvo EVA, Hoegh-Guldberg O. Paleo-perspectives on ocean acidification. Trends Ecol. Evol. 2010;25:332–344. doi: 10.1016/j.tree.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Rasher DB, Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl Acad. Sci. U.S.A. 2010;107:9683–9688. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. Ocean Acidification due to Increasing Atmospheric Carbon Dioxide. London: The Royal Society; 2005. [Google Scholar]
- Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC. Algae lacking carbon-concentrating mechanisms. Can. J. Bot. 2005;83:879–890. [Google Scholar]
- Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pagés C, Jaubert J, Gattuso J-P. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Change Biol. 2003;9:1660–1668. [Google Scholar]
- de Ruyter van Steveninck ED, Van Mulekom LL, Breeman AM. Growth inhibition of Lobophora variegata (Lamouroux) Womersley by scleractinian corals. J. Exp. Mar. Biol. Ecol. 1988;115:169–178. [Google Scholar]
- Silverman J, Lazar B, Cao L, Caldeira K, Erez J. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys. Res. Lett. 2009;36:L05606. doi: 10.1029/2008GL036282. [Google Scholar]
- Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, et al. Indirect effects of algae on coral: algae-mediated, microbe induced coral mortality. Ecol. Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- Solomon S, Plattner GK, Knutti R, Friedlingstein P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl Acad. Sci. U.S.A. 2009;106:1704–1709. doi: 10.1073/pnas.0812721106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood AJ. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- Wu H, Zou D, Gao K. Impacts of increased atmospheric CO2 concentration on photosynthesis and growth of micro- and macro-algae. Sci. China, C, Life Sci. 2008;51:1144–1150. doi: 10.1007/s11427-008-0142-5. [DOI] [PubMed] [Google Scholar]


