Abstract
Background
More than 800 hospitals and 586 intensive care units (ICUs) in Germany currently participate in a nationwide surveillance system for nosocomial infections (Krankenhaus-Infektions-Surveillance-System, KISS), which collects data on the frequency of nosocomial infections and pathogens and on the appearance of pathogens of special epidemiological importance.
Methods
Data were collected from ICUs regarding lower respiratory tract infections, primary sepsis, and urinary tract infections and on the temporal relation of these types of infection to the use of specific medical devices (invasive ventilation, central venous catheters, and urinary catheters). On the basis of these data, device-associated infection rates (number of infection per 1000 device days) were calculated for different types of ICUs. KISS also collected data on all ICU patients colonized or infected with selected multidrug-resistant organisms (MDRO) and on all hospitalized patients with methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile-associated diarrhea (CDAD).
Results
Device-associated infection rates ranged from 0.9 to 9.6 per 1000 device-days, depending on the type of infection and the type of ICU. An extrapolation from these figures yields an estimate of 57 900 ICU-acquired infections occurring in Germany each year. The most common MDRO in ICU patients is MRSA. The frequency of MRSA has remained stable in recent years, but that of other MDROs among ICU patients is rising. Hospitalized patients are twice as likely to acquire CDAD as they are to acquire MRSA.
Conclusion
Nosocomial infections are common in the ICU. The percentage of ICU patients with MDRO is low, but rising. Future preventive strategies must address this development.
Nosocomial infections are one of the most common complications of hospitalization and lead to increased morbidity and mortality (1, 2). These infections prolong hospitalization, require more extensive diagnostics and treatment, and are associated with additional costs (3, 4). Infection with multidrug-resistant pathogens can also further complicate treatment. Greater emphasis must be placed on the prevention of nosocomial infections and containment of multidrug-resistant pathogens both to improve patient safety and to optimize the use of increasingly limited financial resources. It has been shown both nationally and internationally that reporting and analyzing nosocomial infections with subsequent changes to infection control measures (surveillance) can prevent nosocomial infections (5– 7). Implementation of a surveillance system has therefore been regulated in the German Infection Protection Act §23. It is essential that comparable data from other facilities is available to improve the effectiveness of surveillance systems (8). A nationwide surveillance system for the most important nosocomial infections, the Krankenhaus-Infektions-Surveillance-System or KISS, was established in 1996 to ensure that such reference data is available across Germany. KISS is a project run by the German National Reference Center for the Surveillance of Nosocomial Infections (Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen, NRZ) and has developed into a national network with more than 800 voluntarily participating hospitals. KISS is made up of several modules and provides surveillance methods and reference data on various infection-related endpoints. Nosocomial infections, the frequency of patients with epidemiologically important pathogens (methicillin-resistant Staphylococcus aureus [MRSA], vancomycin-resistant enterococci [VRE], extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae [ESBL], and Clostridium difficile-associated diarrhea [CDAD]) and additional indicators of hygiene management such as consumption of antibiotics and hand disinfectants are all monitored. The software required to manage the data is provided to hospitals free of charge. KISS uses data that is collected during routine care of patients for the most part to gain the necessary information to optimize the management of infection prevention. The data also provide important information about the epidemiology of nosocomial infections and important pathogens in Germany. This report presents epidemiological data from KISS about nosocomial infections and their pathogens and about patients with multidrug-resistant organisms (MDROs) in ICUs and data about the frequency of patients with MRSA and CDAD in hospitals.
Method
Nosocomial infections and ICU patients with MDRO
Surveillance of nosocomial infections in intensive care units (ICUs) is conducted in the ICU-KISS module (9). The most important basic data (patients, patient-days, patient-days with the use of particular medical procedures (devices such as invasive ventilation, central venous catheters [CVC], and urinary catheters [UC])) are collected by the participating ICUs in the form of midnight census statistics. All ICU patients are prospectively observed for development of a nosocomial infection of the lower respiratory tract (LRTI), primary bloodstream infection (BSI) and urinary tract infection. Diagnosis is based on specific definitions which, depending on the type of infection, include combinations of microbiological and/or radiological findings and clinical signs of infection (10). Other variables such as the date of infection, temporal association of the infection with devices (ventilation or central venous catheter or urinary catheter), and verified pathogens are documented for every nosocomial infection. Patients with MRSA, VRE and ESBL can also be monitored. Patients known to be infected with MDROs or whose MDROs are detected during treatment are included. Colonization with MDROs or pre-existing infection with MDROs are also recorded. Conducting routine screening examinations to determine the presence of MDROs is not prescribed by KISS but is done in accordance with the internal specifications of the particular ICU. When an MDRO first manifests the probable acquisition of the pathogen is classified as “pre-existing” (MDRO known upon admission or verified with the first 48 hours) or “not known upon admission/acquired nosocomially in the ICU” (initial verification more than 48 hours after admission to the ICU). (Definition of the time interval to differentiate between pre-existing/nosocomial has recently been changed to: pre-existing = verification on day 1 of hospitalization [day of admission], day 2 or 3 of hospitalization; nosocomial = verified after day 3 of hospitalization).
The frequency of patients with MDRO is indicated as the prevalence (patients with MDRO/100 patients) and the frequency of acquisition of MDRO in the ICU is indicated as incidence density (patients with nosocomial MDRO/1000 patient-days).
Attendance at an introductory course which explains the methodology for surveillance and provides training in diagnosing nosocomial infections based on the definitions is a prerequisite for participating in ICU-KISS. Participants must repeat the diagnostic training once a year. The validity of the infection reports and the representativeness of the ICU-KISS data were reviewed in two studies (11, 12).
Surveillance is conducted in about 60% of the ICUs by infection control personnel and in about 30% of the ICUs by the senior physician or the ward physician responsible for infection control and in the remaining 10% by other personnel. The basic data from an ICU, the infection data, and the MDROs are entered into a web-based computerized system. Data are exchanged with KISS via this system and at any time wards can generate analyses of their own data, which include a comparison with the reference data provided by KISS (13).
Utilization rates for the ICUs (number of ventilator-days, CVC-days, urinary catheter-days/100 patient-days) are calculated to characterize the ICU in terms of the frequency of utilization of ventilation, CVCs, and urinary catheters. The infections are standardized to device-days by calculating the device-associated infection rates, which express, for example, how many CVC-associated bloodstream infections (BSI) per 1000 CVC-days have occurred (number of CVC-associated BSI/number of CVC-days multiplied by 1000). The UC-associated urinary tract infection rate and a ventilator-associated respiratory tract infection rate are calculated similarly.
The infection rates for different types of ICUs are reported separately (interdisciplinary, surgical, internist ICU, etc.) to obtain a better overview of the different infection risks for different patient populations. Interdisciplinary ICUs are also differentiated on the basis of hospital size (< 400 beds and 400 beds or more). The annually updated reference data always refer to the previous 5-year period in order to ensure that the reference data has a high degree of statistical certainty.
Extrapolation of the frequency of nosocomial infections in ICUs in Germany
The data generated by KISS will be used to extrapolate the annual occurrence of nosocomial infections in intensive care units in Germany. The reference utilization rates for CVC, urinary catheters, ventilation and device-associated infection rates for ICU KISS for 2005 to 2009 and the basic data from the hospitals provided by the German Federal Statistical Office for 2008 were used to extrapolate the frequency of nosocomial infections in ICUs in Germany for 2008 (13, 14).
Patients with epidemiologically important pathogens in the hospital
The frequency of the occurrence of MRSA and CDAD in the entire hospital is collected in the KISS modules MRSA-KISS and CDAD-KISS respectively (15). Pre-existing and nosocomial infections are differentiated using the same time limits as described for the ICU-KISS module for MDRO surveillance. The frequency of the occurrence of patients with MRSA and CDAD is determined for each calendar year and is based on the number of patients and patient-days for the calendar year of the hospital. To express the frequency, the prevalence of MRSA (number of cases of MRSA/100 treatment cases) or CDAD (number of CDAD cases/100 treatment cases) is calculated for both modules. The frequency of nosocomially acquired MRSA or nosocomial occurrence of CDAD is expressed as the incidence density (number of nosocomial MRSA or nosocomial CDAD/1000 patient-days).
Results
Nosocomial infections and their infectious agents in ICUs
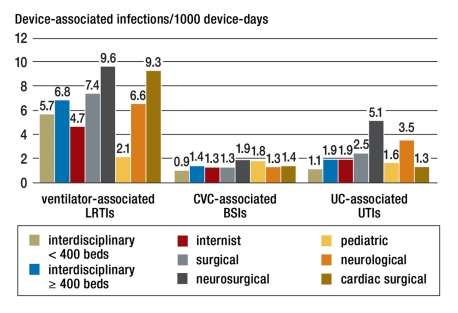
From January 2005 to December 2009 586 intensive care units supplied ICU-KISS with data. Data from a total of 1 651 941 intensive care patients with 5 876 973 ICU-days were entered into the reference data. Figure 1 shows the number of ICUs that participated stratified by ICU type. The device-associated infection rates for the different types of ICU are shown in Figure 2.
Figure 1.
Number of intensive care units (ICUs) according to ICU type in the ICU-KISS reference data 2005–2009
Figure 2.
Device-associated infection rates stratified by ICU type; LRTI, lower respiratory tract infections; BSI, bloodstream infections; UTI, urinary tract infections
A pathogen could be identified for 13 745 (88%) of the total 15 607 ventilator-associated LRTIs, for 5067 (100%) of the CVC-associated BSI cases, and for 9065 (97%) of the 9357 UC-associated urinary tract infections that developed in the ICU. The Table shows the proportion of nosocomial infections for which the specified pathogen could be identified as the infectious agent for the five most common pathogens for each type of infection according to the type of ICU. Large differences could be determined in some cases between different types of ICU. For example, in pediatric ICUs MRSA is identified as the pathogen in less than 1% of ventilator-associated LRTIs while in neurological ICUs the proportion of MRSA infections among ventilator-associated LRTIs was almost 14%.
Table. The most common pathogens for nosocomial infections (NI) in ICUs; proportion of nosocomial infections (in %) with the relevant pathogen for ventilator-associated infections of the lower respiratory tract, CVC-associated bloodstream infections, and UC-associated urinary tract infections; total and stratified by ICU type.
| ICU type NI with pathogen (%) | All ICUs | Interdisciplinary < 400 beds | Interdisciplinary ≥ 400 beds | Internal | Surgical | Neurosurgical | Pediatric | Neurological | Cardiac surgical |
| Ventilator-associated infections of the lower respiratory tract | |||||||||
| S. aureus (total) | 20.6 | 19.8 | 22.6 | 19.1 | 19.8 | 32.6 | 11.2 | 31.8 | 8.7 |
| – of which MRSA | 7.2 | 9.6 | 8.7 | 6.1 | 6 | 5.2 | 0.9 | 13.6 | 2.7 |
| P. aeruginosa | 17.7 | 21.8 | 19.7 | 16.4 | 17.2 | 10.4 | 16.4 | 10.3 | 13.3 |
| Klebsiella spp. | 12.3 | 10.2 | 13.7 | 10.6 | 12.7 | 14.1 | 12.1 | 11.2 | 12.1 |
| E. coli | 12.2 | 10 | 14.6 | 10.1 | 12.4 | 14.8 | 6 | 7.6 | 11.6 |
| Enterobacter spp. | 5.8 | 5.8 | 9.1 | 6.7 | 9.4 | 11 | 11.2 | 9.7 | 11.1 |
| CVC-associated BSI | |||||||||
| CNS | 32.1 | 26.1 | 29.3 | 32.9 | 33.6 | 41 | 34.3 | 42 | 38.2 |
| S. aureus (total) | 8.7 | 11.1 | 9.1 | 9.2 | 7.8 | 9.5 | 11.2 | 5 | 4.5 |
| – of which MRSA | 5.8 | 8.6 | 5.7 | 5.2 | 6 | 2.2 | 1.4 | 4 | 4.9 |
| Enterococcus | 18.5 | 16.9 | 20.2 | 24 | 17.4 | 11.7 | 13.3 | 13 | 17.4 |
| Klebsiella spp. | 5.2 | 7.4 | 6 | 4.2 | 4.2 | 3.7 | 6.3 | 6 | 4.5 |
| Candida albicans | 5.6 | 8.5 | 4.8 | 3.9 | 6.5 | 2.9 | 2.8 | 3 | 5.9 |
| UC-associated urinary tract infection | |||||||||
| E. coli | 27.8 | 27.2 | 26.6 | 25.1 | 28.3 | 35.4 | n.a. | 25.8 | 22.5 |
| Enterococcus | 26.5 | 27.4 | 27.6 | 26.3 | 27.6 | 22.8 | n.a. | 25.5 | 14.9 |
| P. aeruginosa | 14.2 | 14.8 | 14.7 | 11.2 | 15.2 | 13.2 | n.a. | 13.6 | 12.2 |
| Candida albicans*1 | 8.7 | 7 | 10 | 12.5 | 7.8 | 4.7 | n.a. | 10.4 | 6.3 |
| Klebsiella spp. | 8.1 | 8 | 8.5 | 7.2 | 7.8 | 9.2 | n.a. | 9.3 | 9.5 |
| Enterobacter spp. | 5 | 4.9 | 5.2 | 3.3 | 4.9 | 7.3 | n.a. | 4.3 | 7.7 |
*1as sole pathogen; CNS, coagulase-negative staphylococci; n.a., no data available due to insufficient quantity of data (< 100 infections)
Frequency of nosocomial infections in intensive care units
The data generated by KISS on device-utilization frequency and on the frequency of development of nosocomial infections during use of the devices enable extrapolations to be made of the annual occurrence of nosocomial infections in intensive care units in Germany. According to statements from the German Federal Statistical Office in 2008 there were a total of 7 042 898 patient-days in ICUs (14). From KISS it can be calculated that there were 2.9 million ventilator-days (ICU-KISS ventilation rate 41%), 4.8 million CVC-days (CVC utilization rate 68%), and 5.7 million UC-days (UC utilization rate 81%). Across all ICU types in KISS infection rates per 1000 device-days were calculated for ventilator-associated respiratory tract infections (6.53), CVC-associated BSI (1.26), and for UC-associated urinary tract infections (1.97). From the total days of risk in ICUs and the infection frequency during such days of risk, the result is annually approximately 18 900 ventilator-associated infections of the lower respiratory tract, 6000 CVC-associated cases of BSI, and 11 200 urinary catheter-associated urinary tract infections, giving a total of 36 100 device-associated infections in intensive care units. The proportion of device-associated infections amongst these nosocomial infection types (78%) and the proportion these types of infection make up of all nosocomial infections (80%) is known from a representative investigation of the prevalence of nosocomial infections in Germany (16). Calculations based on these 36 100 device-associated infections yield an estimated total number of 57 900 infections that develop each year in intensive care units in Germany.
Occurrence of patients with MDRO in ICUs
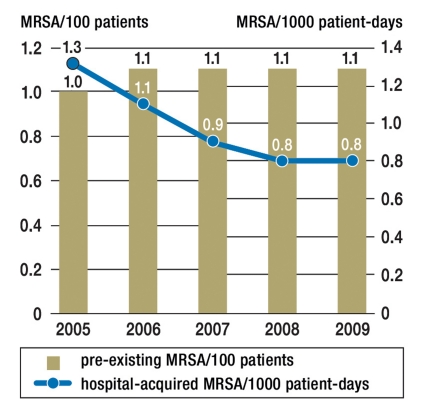
In the period from 2005 to 2009 345 intensive care units recorded data about patients with MDROs. Among the 946 485 patients treated in the ICUs in this period (with 3 456 110 patient-days), MRSA was detected in 13 468 patients, ESBL in 3466 patients, and VRE in 1260 patients upon admission or during treatment in the ICU. Among 751 ICU patients on average one patient was therefore colonized or infected with VRE. On the other hand, one patient is colonized or infected with ESBL amongst 273 ICU patients and every 70th ICU patient in Germany is colonized or infected with MRSA. Figure 3 shows the opposing trend in the development over time of admission prevalence of MRSA in ICUs and the incidence density of nosocomial ICU-acquired MRSA, resulting in a high, but stable, level of occurrence of patients with MRSA in ICUs (Figure 4). In contrast, the frequency of patients with VRE and with ESBL in particular increased from 2005 to 2009 (Figure 4).
Figure 3.
MRSA prevalence upon admission to ICU (MRSA patients/100 patients) as bars; incidence density of ICU-acquired MRSA (patients with nosocomial MRSA/1000 patient-days) as a line for the period from 2005 to 2009
Figure 4.
Total MDRO prevalence (patients with MRSA/ESBL/VRE per 100 patients) during ICU hospitalization for the period from 2005 to 2009
Patients with MRSA and CDAD in hospitals
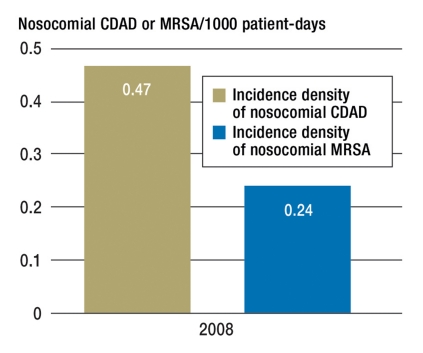
Fifty-nine hospitals with 1 565 084 patients registered data with KISS on the frequency of patients with CDAD and 184 hospitals with 3 283 136 patients registered data on the frequency of the occurrence of patients with MRSA for 2008. This yields an average of 96 CDAD cases and 134 MRSA cases per hospital per year. This corresponds to a hospital prevalence of 0.35 for CDAD and 0.75 for MRSA. Of the cases of patients with MRSA treated each year in hospitals, 75% are cases of pre-existing MRSA colonization/infection in which the MRSA was either acquired during previous hospitalization, from other hospitals or medical facilities or via other routes. The ratio for CDAD, however, is almost reversed with a proportion of 70% for nosocomial infections. During hospitalization the risk of acquiring CDAD is almost twice as high as the risk of acquiring MRSA for the first time (Figure 5).
Figure 5.
Incidence density of nosocomial CDAD and MRSA per 1000 patient-days in hospitals for 2008
Discussion
The primary aims of KISS were to provide a uniform surveillance method and comparable values for internal quality management. The data collected as part of KISS were used in the present study to draw conclusions about the frequency of nosocomial infections and the occurrence of epidemiologically important pathogens in Germany.
Between 2005 and 2009 586 ICUs recorded data about nosocomial infections in KISS. Extrapolations based on the KISS data about nosocomial infections yield a frequency of 57 900 newly acquired infections each year in intensive care units in Germany. In different studies reductions of between 11% and 55% could be achieved by introducing different infection control strategies, revealing a prevention potential of significant magnitude (17).
The infection rates stratified by ICU type reveal large differences in some cases. The differences in the infection frequencies reflect the risk structures of the different populations in the ICUs. For example, an increased risk of pneumonia is known for thoracic procedures or a decline in vigilance that is manifested in comparably higher ventilator-associated LRTI rates in cardiac surgery and neurosurgery ICUs. The age structure of the patients treated in different types of ICU also impacts the level of infection rates as revealed in the comparably low infection rates for urinary tract infections and infections of the lower respiratory tract in pediatric ICUs. These differences highlight the importance of separately reporting comparative data for the individual types of ICU. The world’s largest surveillance system for nosocomial infections, the NHSN in the U.S., which uses an almost identical surveillance method to that of KISS, also stratifies the infection rates generated in the system by the type of intensive care unit (18). Differences in the infection frequencies can also be seen here when comparing the individual types of ICU. The maximum values for U.S. infection rates for CVC-associated BSI and urinary catheter-associated urinary tract infections are higher than in the German KISS. These are, however, determined in types of ICU that are not represented in the ICU-KISS (such as serious burns victims). When infection rates for identical ICU types in the U.S. and Germany are compared, however, the differences are less remarkable. For example, the CVC-associated BSI rate is 1.3 for neurological ICUs in KISS and 1.4 CVC-associated BSI cases/1000 CVC-days in the U.S.
In ICU-KISS there are large differences in the most common pathogens for nosocomial infections for each type of ICU. An investigation from Spain revealed clear differences in some cases in the distribution of pathogens for ventilator-associated pneumonia across different ICUs (19). The causal pathogens are determined to a large extent by patient-specific factors (underlying disease, duration of ventilation, previous antibiotic therapy) (20). There are only three studies of the pathogen distribution for which data were collected from a large number of ICUs and which took into account the type of ICU (21– 23). KISS is the only source in Germany of such comparative data generated in accordance with a uniform methodology and on such a large scale.
Data on the resistance situation with Staphylococcus aureus, for example, is available from the Paul-Ehrlich-Society (Paul-Ehrlich-Gesellschaft, PEG) (24). The data, however, are microbiological laboratory data on resistance rates based on tested pathogens without reference to population, meaning that direct comparisons between KISS data and that of PEG are scarcely feasible. What can be determined in both systems, however, is that MRSA has now appeared to reach a stable plateau both in terms of resistance rates in the PEG data and in reference to populations in the ICU-KISS (25). On the other hand, the frequency of patients with VRE and ESBL in ICUs has increased. In the hospital the risk of newly acquiring CDAD is higher than that of acquiring MRSA. This reveals problematic areas, the development of which should be very closely monitored. The opportunities for pathogen surveillance in KISS will be expanded from 2012 to stay abreast of these developments.
Key Messages.
The Hospital Infection Surveillance System (KISS, Krankenhaus-Infektions-Surveillance-System) is a surveillance system for internal hygiene quality management with more than 800 hospitals across Germany participating. From the data generated by KISS conclusions can be drawn about the epidemiology of nosocomial infections and of pathogens of particular importance in Germany.
Based on data from 586 ICUs in KISS, the number of newly acquired infections each year in ICUs in Germany is estimated to be 57 900.
The infection rates for nosocomial infections and their pathogens differ greatly between different types of ICU corresponding to the different risk structure of the patients.
The proportion of ICU patients with MRSA has remained constant for several years. On the other hand, there has been a pronounced increase in the proportion of ICU patients with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae (ESBL).
During hospitalization nosocomial Clostridium difficile-associated diarrhea (CDAD) occurs more frequently than newly acquired methicillin-resistant Staphylococcus aureus (MRSA).
Acknowledgments
Translated from the original German by language & letters.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Geffers C, Sohr D, Gastmeier P. Mortality attributable to hospital-acquired infections among surgical patients. Infect Control Hosp Epidemiol. 2008;29:1167–1170. doi: 10.1086/592410. [DOI] [PubMed] [Google Scholar]
- 2.Aranaz-Andres JM, Aibar-Remon C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62:1022–1029. doi: 10.1136/jech.2007.065227. [DOI] [PubMed] [Google Scholar]
- 3.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. Jama. 1994;271:1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 4.Beyersmann J, Gastmeier P, Grundmann H, et al. Use of multistate models to assess prolongation of intensive care unit stay due to nosocomial infection. Infect Control Hosp Epidemiol. 2006;27:493–499. doi: 10.1086/503375. [DOI] [PubMed] [Google Scholar]
- 5.Rioux C, Grandbastien B, Astagneau P. Impact of a six-year control programme on surgical site infections in France: results of the INCISO surveillance. J Hosp Infect. 2007;66:217–223. doi: 10.1016/j.jhin.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Gastmeier P, Schwab F, Sohr D, Behnke M, Geffers C. Reproducibility of the surveillance effect to decrease nosocomial infection rates. Infect Control Hosp Epidemiol. 2009;30:993–999. doi: 10.1086/605720. [DOI] [PubMed] [Google Scholar]
- 7.Geubbels EL, Nagelkerke NJ, Mintjes-De Groot AJ, et al. Reduced risk of surgical site infections through surveillance in a network. Int J Qual Health Care. 2006;18:127–133. doi: 10.1093/intqhc/mzi103. [DOI] [PubMed] [Google Scholar]
- 8.Anonymus Surveillance nosokomialer Infektionen sowie die Erfassung von Erregern mit speziellen Resistenzen und Multiresistenzen. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2000. pp. 887–890. [DOI] [PubMed] [Google Scholar]
- 9.Geffers C, Koch J, Sohr D, et al. Establishment of a national database for ICU-associated infections. First results from the „Krankenhaus-Infections-Surveillance-System“ (KISS) Anaesthesist. 2000;49:732–737. doi: 10.1007/s001010070068. [DOI] [PubMed] [Google Scholar]
- 10.NRZ. Definitionen nosokomialer Infektionen (CDC-Definitionen) http://www.nrz-hygiene.de/dwnld/CDC-Definitionen_Stand04-2008_6Auflage2008.pdf.
- 11.Zuschneid I, Rucker G, Schoop R, et al. Representativeness of the surveillance data in the intensive care unit component of the German nosocomial infections surveillance system. Infect Control Hosp Epidemiol. 2010;31:934–938. doi: 10.1086/655462. [DOI] [PubMed] [Google Scholar]
- 12.Zuschneid I, Geffers C, Sohr D, et al. Validation of surveillance in the intensive care unit component of the German nosocomial infections surveillance system. Infect Control Hosp Epidemiol. 2007;28:496–499. doi: 10.1086/512631. [DOI] [PubMed] [Google Scholar]
- 13.NRZ. http://www.nrz-hygiene.de/dwnld/RefDaten/200501_200912_ITS_reference.pdf. Referenzdaten ITS-KISS. [Google Scholar]
- 14.Statistisches, Bundesamt. https://www-ec.destatis.de/csp/shop/sfg/bpm.html.cms.cBroker.cls?cmspath=struktur,vollanzeige.csp&ID=1024819. Grunddaten der Krankenhäuser. [Google Scholar]
- 15.Chaberny IF, Sohr D, Ruden H, Gastmeier P. Development of a surveillance system for methicillin-resistant Staphylococcus aureus in German hospitals. Infect Control Hosp Epidemiol. 2007;28:446–452. doi: 10.1086/513444. [DOI] [PubMed] [Google Scholar]
- 16.Rüden H, Daschner F, Schumacher M. Nosokomiale Infektionen in Deutschland: Erfassung und Prävention (NIDEP-Studie); Teil 1: Prävalenz nosokomialer Infektionen; Qualitätssicherung in der Krankenhaushygiene. Baden-Baden: Nomos Verlagsgesellschaft. 1995 [Google Scholar]
- 17.Harbarth S, Sax H, Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports. J Hosp Infect. 2003;54:258–266. doi: 10.1016/s0195-6701(03)00150-6. quiz 321. [DOI] [PubMed] [Google Scholar]
- 18.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Rello J, Sa-Borges M, Correa H, Leal SR, Baraibar J. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999;160:608–613. doi: 10.1164/ajrccm.160.2.9812034. [DOI] [PubMed] [Google Scholar]
- 20.Rello J, Torres A. Microbial causes of ventilator-associated pneumonia. Semin Respir Infect. 1996;11:24–31. [PubMed] [Google Scholar]
- 21.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in coronary care units in the United States. National Nosocomial Infections Surveillance System. Am J Cardiol. 1998;82:789–793. doi: 10.1016/s0002-9149(98)00450-0. [DOI] [PubMed] [Google Scholar]
- 22.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics. 1999;103 doi: 10.1542/peds.103.4.e39. [DOI] [PubMed] [Google Scholar]
- 24.Kresken M, Hafner D, Schmitz FJ, Wichelhaus TA. Prevalence of mupirocin resistance in clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis: results of the Antimicrobial Resistance Surveillance Study of the Paul-Ehrlich-Society for Chemotherapy, 2001. Int J Antimicrob Agents. 2004;23:577–581. doi: 10.1016/j.ijantimicag.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Paul-Ehrlich-Gesellschaft. Studie 2007. http://www.p-e-g.org/ag_resistenz/main.htm. [DOI] [PubMed]