Abstract
Background:
Correction of nasolabial creases through minimally invasive procedures is increasingly being sought by patients. Injecting non-animal stabilized hyaluronic acid filler is a highly effective method to achieve an optimal and persistent cosmetic result
Aims:
To evaluate the efficacy, persistence and safety of Restylane and Perlane (Q-Med, Sweden) for correction of nasolabial folds in Indian patients
Materials and Methods:
Thirty Indian patients with mild, moderate and severe nasolabial folds (based on Wrinkle Assessment Scale) were recruited in the study after informed consent for correction of their folds with Restylane or Perlane or both. Injections were administered in a single sitting after global assessment of the patient's face using Wrinkle assessment scale (WAS).Optimal filling was performed by using appropriate techniques and its safety and efficacy assessed independently by the investigator as well as by patients at immediately, 3, 6 and 9 months post-procedure. Any adverse reactions were noted.
Results:
Twenty two females and 8 males (age range 45-55 years, mean age 52 years) were recruited in the study. An optimum cosmetic correction was obtained in all patients. The efficacy increased with time and was greatest at 3 months after the treatment. Grade 2 improvement was maintained at 9 months in mild and moderate folds, and grade 3 improvement for severe folds. Minor post injection side effects like erythema at puncture site, needle marks and bruising were seen.
Conclusion:
Restylane and Perlane are safe and effective dermal fillers for correction of nasolabial creases and offer immediate effect.
Keywords: Hyaluronic acid filler, nasolabial folds, wrinkles
INTRODUCTION
Nasolabial crease is a giveaway sign of aging and results from either cheek ptosis or loss of volume or muscle hyperactivity. Nasolabial fold in an individual may reflect a single or more than one of the above contributing factors. Using dermal fillers is a treatment of choice for nasolabial fold correction through a minimally invasive technique. Hyaluronic acid (HA) fillers are the most widely used fillers now. HA, a biopolymer is a naturally occurring substance, which exhibits no species or tissue specificity. It is an essential and one of the predominant component of the extracellular matrix of all animal tissues and has a uniform structure throughout nature. HA is highly hydrophilic, that is, it attracts water, and this property helps it fill and correct volume defect of larger volume relative to its mass. In vitro, it has been shown to form gels at even low concentrations. This biopolymer is composed of d-glucoronic and n-acetyl-d-glucosamine and is cross-linked for stability in tissues. The fact that HA exhibits no tissue or species specificity, is crucially important in minimizing the risk of potential immunologic reactions or transplantation rejection. Hyaluronic acid fillers are thus superior to collagen fillers with minimal allergy and immunogenicity reports.[1–3]
The HA source for the available fillers is either avian, found primarily in rooster combs, or bacteria-sourced, mainly from the synthetic fermentation of the Staphylococcus equine bacterium. The latter source has become more popular recently because of potential allergy to the avian source owing to a high avian protein content, and also because the bacterial-derived HA products are more pure, more viscous and not derived from an animal source.
Restylane is a non-animal stabilized HA, known as NASHA, produced by the fermentation of equine streptococci. It is cross-linked with 1, 4-butanediol diacrylate (BDDA), with 1% degree of cross linking. Restylane has HA concentration of 20 mg/ml. Restylane has a particle size of 400microns and is most appropriate for mid-to-deep dermal injections; Perlane has a particle size of 1000 microns and is used for deep dermal or superficial subcutis injections.[1–3] We hereby present our experience of use of Restylane and Perlane in Indian patients for correction of nasolabial folds.
MATERIALS AND METHODS
Thirty patients with Fitzpatrick phototype IV to VI were recruited in the study after informed consent for correction of bilateral nasolabial folds with NASHA fillers, Restylane and/or Perlane (Q-Med, Sweden). None of the patients were on drugs like aspirin, NSAID's, anticoagulants or herbal supplements that increase the risk of post injection bruising.
Baseline photographs of the bilateral nasolabial folds of patients were taken. Global assessment of the patient's face, particularly with respect to photo aging, volume changes and the effects of gravity were carried out using the Wrinkle assessment scale (WAS; range 1-3). WAS is a photographic five-grade subjective test for efficacy analysis used to grade the nasolabial folds. By correlating the grade of wrinkle in the reference photograph with the wrinkle in the patient's face, a grading from 1 to 3 was assigned. The severity of folds was graded as:
Mild
Moderate
Severe
Treatment was carried out under surface anesthesia. In all the 30 cases, topical anesthesia using lidocaine with prilocaine under occlusion was applied 60 minutes before procedure. Patients were placed in a sitting but relaxed position. Use of serial puncture, linear threading, fanning and layering techniques individually (Restylane or Perlane) or in combination for mid to deep dermal correction was employed. The most common technique used was linear threading; fanning was preferred at the base of the nasolabial fold. Layering with Perlane and Restylane was done for patients with severe nasolabial fold creases. Post-injection gentle molding of the filler in the fold was done by massaging through ultrasonic gel both externally and intraorally. After the procedure, patients were instructed to avoid exposure to heat or cold, physical exertion or further massaging of the area for 6 hours. Any adverse reactions were also noted and analysed. All patients were reviewed at 2 weeks interval for further touch up to obtain optimum correction.
Safety and effectiveness was assessed by the physician at 3, 6 and 9 months post-injection. Clinical evaluation of persistence of correction with global assessment using Wrinkle assessment scale (WAS) was done. The physician and patient independently compared the preoperative photograph with the treated face and answered the question: “How would you describe the degree of improvement?” Possible responses were graded as follows: (1) very much improved (2) moderately improved (3) somewhat improved (4) mild improvement or (5) minimal improvement.
>80% persistence of correction
60 to 80% persistence of correction
40 to 60% persistence of correction
20 to 40% persistence of correction
0 to 20% persistence of correction
RESULTS
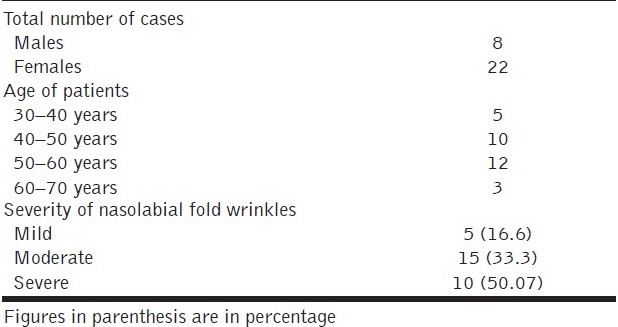
Of the 30 cases, 22 were females and 8 were males. Most patients were in the age group of 45 to 55 years with the mean age being 52 years. The youngest patient was 38 years and the oldest was 68 years old [Table 1]. Of the 30 cases, 16.6 % had mild, 53.33% had moderate and 30.07% had severe nasolabial fold lines.
Table 1.
Demographics of study patients
Restylane was used in 15 patients, Perlane was used in 10 patients and Restylane with Perlane in 5 patients [Figures 1–6]. The volume needed for bilateral correction was as follows: Restylane® mean 1.0 ml (range 0.3-2.8 ml), Perlane® mean 1.0 ml (range 0.5-2.0 ml).
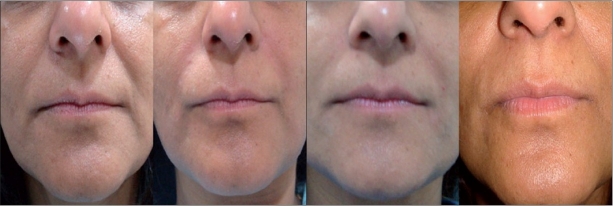
Figure 1.
1a: Mild NL folds, 45 year old female; Before (left) and immediately after (right) Restylane
1b: Mild NL folds, 45 year old female; after 3 months (left) and 9 months (right) of Restylane
Figure 6.
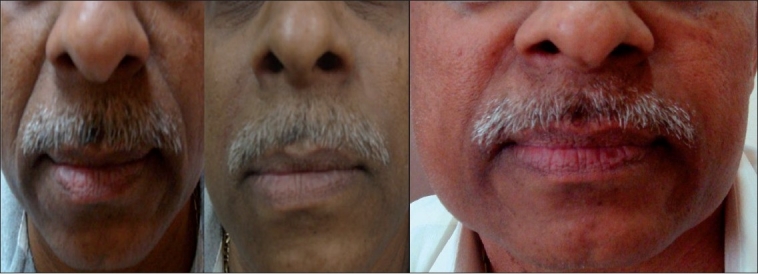
6a: Moderate NL folds, 58 year male; Before (left) and 3 months after (right) Restylane
6b: Moderate NL folds, 58 year male; After 9 months of Restylane (NL- nasolabial folds)
Figure 2.
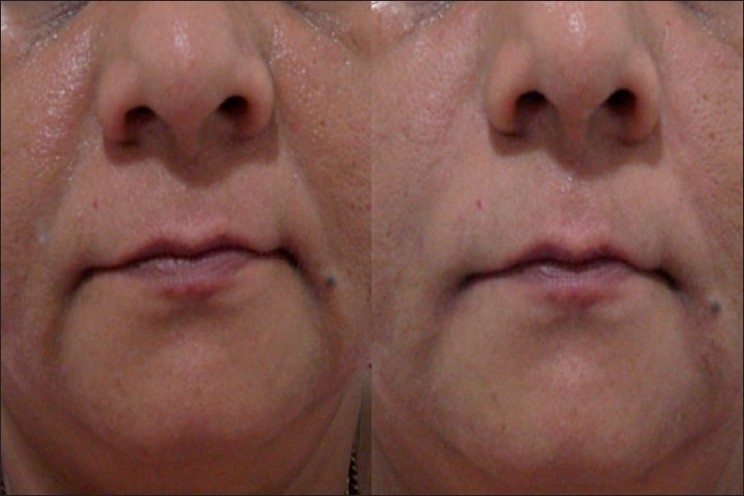
Moderate NL folds, 52 year old female; Before (left) and 3 months after (right) Perlane
Figure 3.
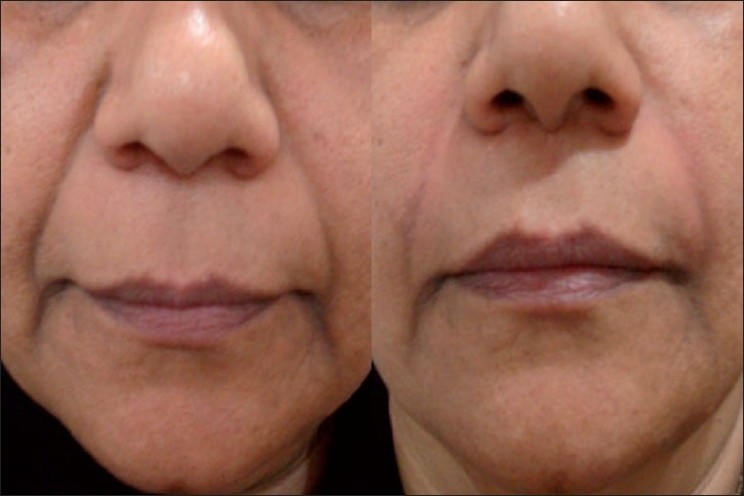
Moderate NL folds, 55 year old female; Before (left) and immediately after (right) Restylane
Figure 4.
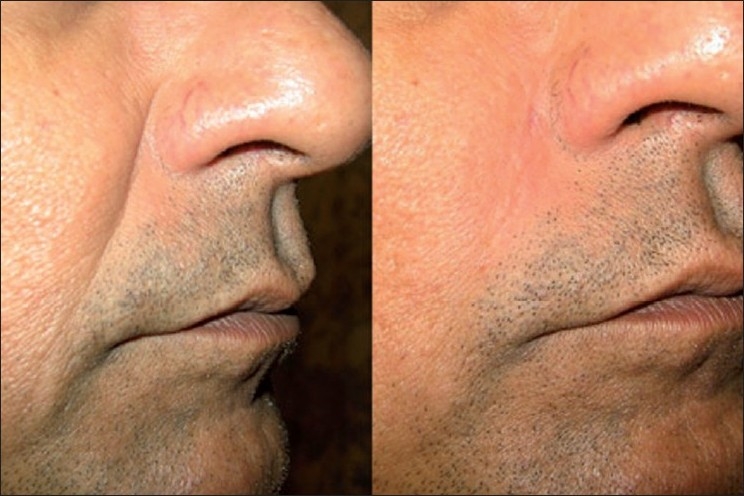
Moderate NL folds, 50 year old male; Before (left) and immediately after (right) Restylane
Figure 5.
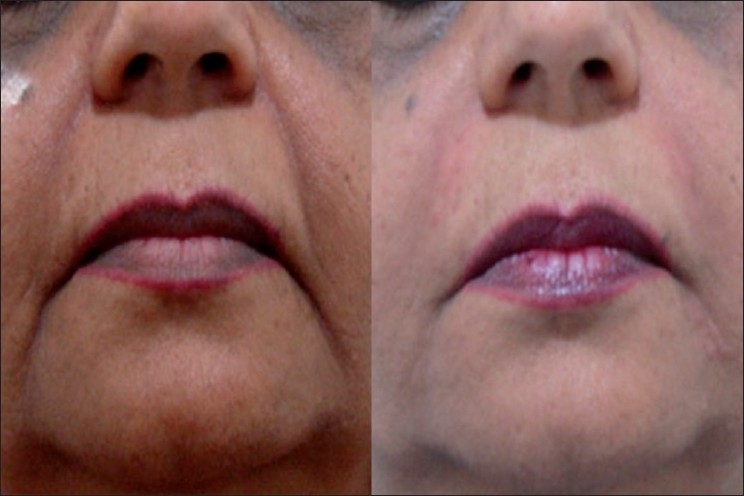
Severe NL folds, 56 year old female; Before (left) and immediately after (right) Restylane and Perlane
At 2 weeks after injection, assessment by the investigator revealed 100% correction for patients who had mild folds, >80% correction of the moderate folds and severe nasolabial wrinkles [Table 2]. Of the 30 patients, 8 (26.6%) patients needed a touch up at 2 weeks.
Table 2.
WAS scale improvement post-injection (percentage) - persistence of correction by investigator and the patient
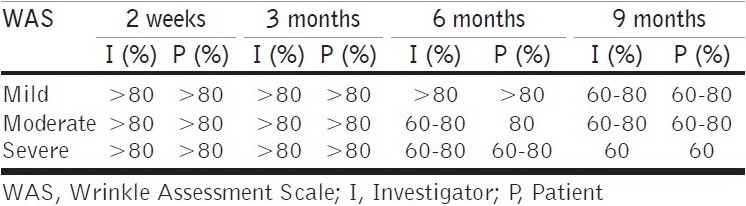
At 3 months post-baseline, the investigator noted persistence of correction as >80% for all patients. At 6 months post-injection, >80% persistence of correction was seen in patients with mild folds and 60 - 80% persistence for moderate and severe degree of nasolabial folds, as noted by the investigator. At 9 months post-injection, maintenance of correction was noted by investigator as 60 - 80% in patients with mild folds and moderate folds, and 40-60% in patients with deeper folds [Figure 7]. The patients independently assessed the persistence of correction and reported marginally better persistence of correction than those reported by the investigator [Figures 7 and 8.
Figure 7.
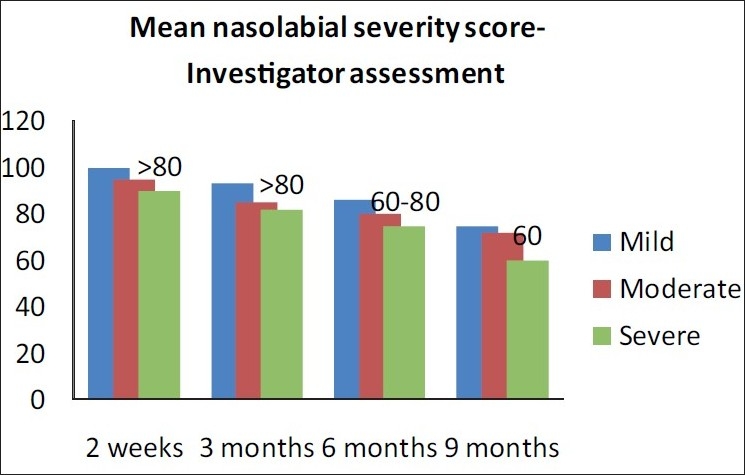
Wrinkle assessment scale (WAS) improvement post-injection (percentage) - persistence of correction by investigator
Figure 8.
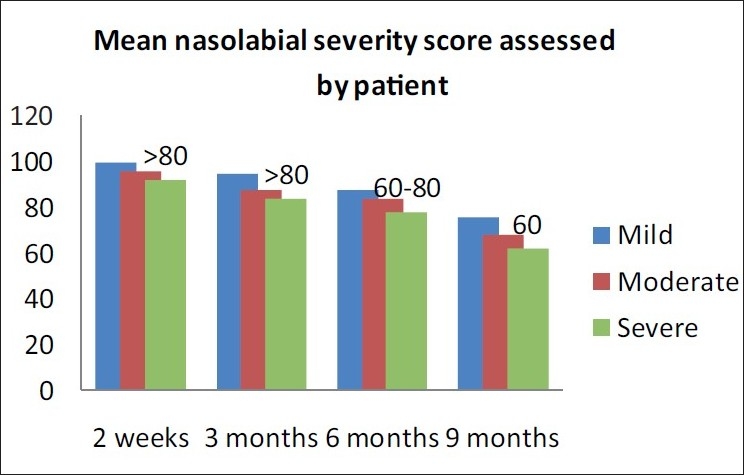
Wrinkle assessment scale (WAS) improvement post-injection (percentage) - persistence of correction by the patient
Transient post-injection effects like erythema at puncture site, needle marks were noted in 80% patients and bruising was seen in 8% patients [Figure 9]. No delayed hypersensitivity was seen in any patient. No hypertrophic scarring or keloidal formation was seen.
Figure 9.
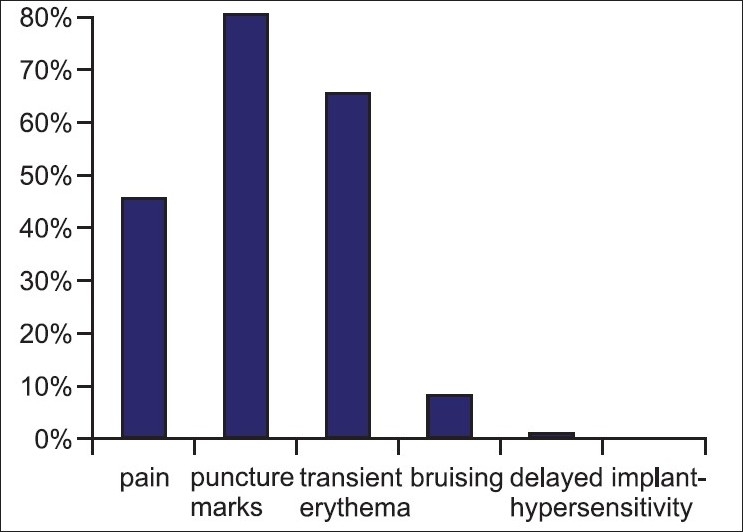
Adverse effects seen after HA fillers in Indian patients
DISCUSSION
In this study we investigated the safety and efficacy of Restylane and Perlane in the correction of nasolabial folds in Indian patients. Restylane and Perlane were chosen because of their easy availability in India and global reports on their safety and effectiveness. In all patients optimal correction was achieved. The efficacy of this augmentation persisted with time and was greatest at 3 months after the last treatment. Duration of the correction was evaluated; WAS showing grade 2-3 persistence of improvement at 9 months.
Persistence of correction was more in younger individuals (aged 45-50 years) as they had mild folds than the elderly patients who had moderate to deep folds. Sustained result was seen in all patients upto 6 months and in some patients upto 9 months.
The duration of wrinkle correction is linked to the physical properties and cross linking of the filler.[5,6] Duranti et al have found moderate to marked improvement in 78% of their subjects at 8 months post-injection.[7] Also, a study by Olenius (1998) reported, 82% and 69% correction at 12 weeks and 26 weeks, respectively.[8] A comparative study by Carruthers between Restylane/Perlane and Hylafoam found Restylane/Perlane to have more durable aesthetic result and acceptable tolerability.[9] In a review on safety, efficacy and durability by Dover et al., 248 subjects treated with NASHA fillers, efficacy and durability was rated high and the safety of each NASHA product was demonstrated at volumes well beyond the volume range listed on the product insert.[10]
There is some evidence suggesting that HA fillers may stimulate de novo collagen synthesis in the skin,[3] enhancing its properties as a filler. In a study by Wang et al., eleven patients received 3 intradermal injections of Restylane into photo damaged forearm on one side and saline into the other forearm. Punch biopsy samples were obtained at 4 and 14 weeks after injection, and collagen synthesis was assessed using immunohistochemical analysis, polymerase chain reaction and electron microscopy. There was significantly more type 1 collagen formation at the HA injection site. Authors thereby postulated that mechanical stretching of fibroblasts by the HA induced collagen production. This could explain why patients often report improvements in their appearance up to 12 months after receiving HA fillers.[11] Thus Restylane® has been used for the past several years by numerous injectors with acceptable clinical results. Restylane® does last longer with most of the above studies indicating correction can be maintained from 6 to 12 months.
One must distinguish between true adverse outcomes and transient injection related reactions that resolve over time. Examples of the true adverse effects include allergic reactions, granuloma formation, vascular occlusion or compromise, Tyndall effect (bluish tint resulting from injections that are too superficial), overcorrection or persistent lumpiness and infection. Examples of transitory reactions include needle marks, bruising, erythema or edema, acneiform eruptions, transient lumpiness and sterile abscesses.[8,12–15] Studies by Duranti and Olenius[7,8] reported injection-related reactions, including treatment-site erythema, hyperpigmentation and pain from the injection itself in 13% of patients. In this study, the observed side effects were mild, temporary and transient. Post-injection effects like erythema at puncture site, needle marks were noted in most of the patients and transient bruising was seen in 8% of the patients. Complications like erythema and puncture marks were treated with topical steroid antibiotic combination creams. Bruising did not require active therapy and spontaneously resolved in a week. No lumpiness at the injection site, delayed hypersensitivity, infections, acneiform eruptions, allergenic adverse reactions and granuloma formation were seen in this study, none of the patients had pigmentary altercations at the injection sites.Temporary biodegradable filler has less potential for major side effects as stated in IADVL dermatosurgery taskforce guidelines, and this is substantiated by this study.[15]
While only a few studies on the use of Restylane for skin of colour are reported, the study by Odunze[16] showed that the use of Restylane in people with darker skin (Type IV to VI) was as safe and effective as in people with fair skin. In addition, the study highlighted the importance of minimizing the number of needle punctures to the skin, in order to limit the risk of hyperpigmentation. There were subtle points emphasized, such as the use of ice before and during treatment, the use of hydrogen peroxide instead of alcohol to clean the skin and pre-treating darker skin with skin bleaching agents.[16]
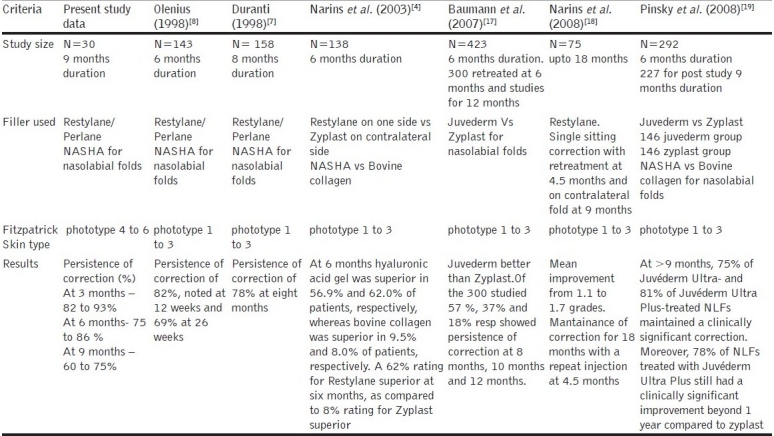
More recent studies by Narins and Pinsky et al., have validated the long term persistence of correction upto 9 months. A comparison of results in different studies (including the present study) is shown in Table 3.
Table 3.
Simplified comparision of various studies on nasolabial fold correction
CONCLUSION
Restylane and Perlane are safe and effective temporary methods to correct moderate to severe nasolabial creases and offers immediate effect, good product longevity and patient satisfaction for Indian patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Narins RS, Bowman PH. Injectable skin fillers. Clin Plast Surg. 2005;32:151–62. doi: 10.1016/j.cps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Narins R, Brandt FS, Lorenc ZP, Maas CS, Monheit GD, Smith SR, et al. A randomized multicenter study of safety and efficacy of dermicol P35 and non animal stabilized hyaluronic acid gel for correction of nasolabial folds. Dermatol Surg. 2007;33:13–21. doi: 10.1111/j.1524-4725.2007.33364.x. [DOI] [PubMed] [Google Scholar]
- 3.Brandt FS, Cazzaniga A. Hyaluronic acid gel fillers in management of aging face. Clin Interv Aging. 2008;3:153–9. doi: 10.2147/cia.s2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–95. doi: 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]
- 5.Falcone SJ, Berg RA. Crosslinked hyaluronic acid dermal fillers: a comparison of rheological properties. J Biomed Mater Res A. 2008;87:264–71. doi: 10.1002/jbm.a.31675. [DOI] [PubMed] [Google Scholar]
- 6.Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther. 2008;10:35–42. doi: 10.1080/14764170701774901. [DOI] [PubMed] [Google Scholar]
- 7.Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation.A clinical and histological study. Dermatol Surg. 1998;24:1317–25. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 8.Olenius M. The first clinical study using a new biodegradable implant for the treatment of the lips, wrinkles, and folds. Aesthetic Plast Surg. 1998;22:97–101. doi: 10.1007/s002669900172. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers A, Carey W, De Lorenzi C, Remington K, Schachter D, Sapra S. Randomized, Double - Blind Comparision of the efficacy of two Hyaluronic acid derivatives, RESTYLANE perlane and Hylaform, in the treatment of Nasolabial Folds. Dermatol Surg. 2005;31:1591–8. doi: 10.2310/6350.2005.31246. [DOI] [PubMed] [Google Scholar]
- 10.Dover JS, Rubin MG, Bhatia AC. Review of the efficacy, durability, and safety data of two nonanimal stabilized hyaluronic acid fillers from a prospective, randomized, comparative, multicenter study. Dermatol Surg. 2009;35:322–31. doi: 10.1111/j.1524-4725.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–63. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Alam M, Dover JS. Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg. 2007;120:98S–105S. doi: 10.1097/01.prs.0000248859.14788.60. [DOI] [PubMed] [Google Scholar]
- 13.Moers-Carpi M, Vogt S, Santos BM, Planas J, Vallve SR, Howell DJ. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatol Surg. 2007;33:S144–51. doi: 10.1111/j.1524-4725.2007.33354.x. [DOI] [PubMed] [Google Scholar]
- 14.Schanz S, Schippert W, Ulmer A, Rassner G, Fierlbeck G. Arterial embolisation caused by injection of hyaluronic acid (Restylane) Br J Dermatol. 2002;146:928–9. doi: 10.1046/j.1365-2133.2002.04707.x. [DOI] [PubMed] [Google Scholar]
- 15.Vedamurthy M. IADVL Dermatosurgery task Force. Indian J Dermatol Venereol Leprol. 2008;74:S23–7. Standard guidelines for the use of dermal fillers. [PubMed] [Google Scholar]
- 16.Odunze M, Cohn A, Few JW. Restylane and people of color. Plast Reconstr Surg. 2007;120:2011–6. doi: 10.1097/01.prs.0000287330.94038.63. [DOI] [PubMed] [Google Scholar]
- 17.Baumann LS, Shamban AT, Lupo MP, Monheit GD, Thomas JA, Murphy DK, et al. Comparision of smooth gel hyaluronic acid dermal fillers with cross linked bovine collagen: A multicenter, double masked, randomized, within- subject study. Dermatol Surg. 2007;33:128–35. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- 18.Narins RS, Dayan SH, Brandt FS, Baldwin EK. Persistence and improvement of nasolabial fold correction with nonanimal-stabilized hyaluronic acid 100,000 gel particles/ml filler on two retreatment schedules: Results up to 18 months on two treatment schedule. Dermatol Surg. 2008;34:S2–8. doi: 10.1111/j.1524-4725.2008.34236.x. [DOI] [PubMed] [Google Scholar]
- 19.Pinsky MA, Thomas JA, Murphy DK, Walker PS, Juvéderm vs. Zyplast Nasolabial Fold Study Group.Juvéderm injectable gel: a multicenter, double-blind, randomized study of safety and effectiveness. Aesthet Surg J. 2008;28:596–7. doi: 10.1016/j.asj.2007.09.005. [DOI] [PubMed] [Google Scholar]