Abstract
Various reports of long-term complications with semi-permanent fillers, appearing several years after injections have created some concern about their long-term safety profile. We report a case of foreign body granuloma secondary to dermal filler containing a copolymer of the acrylic hydrogel particles, hydroxyethylmethacrylate and ethylmethacrylate, occurring 2 years after the injection. The foreign body granulomas could not be treated satisfactorily with intralesional steroids, and the patient required a surgical excision of her granulomas. The physical and psychological consequences to such patients can be quite devastating.
Keywords: Acrylic hydrogel particles, dermalive, excisional skin biopsy, foreign body granuloma, semi-permanent fillers
INTRODUCTION
Synthetic biodegradable injectable fillers are widely used as relatively safe injectable alternatives to surgical skin rejuvenation. By combining biodegradable materials like hyaluronic acid with non-biodegradable materials like acrylic hydrogel particles, a longer lasting ‘semi-permanent’ product is created. However, these combination fillers achieve their volume filling effect from the intended host foreign-body response to the microparticles. While this host response increases the longevity of the filler and reduces the risk of migration, it may also potentiate the risk of long-term adverse events. This report documents one such side effect of the semipermanent filler, Dermalive.
CASE REPORT
A 51-year-old female patient in good health presented with nodular swellings over both her nasolabial folds. She reported a history of injection of the cosmetic filler ‘Dermalive’ (Dermatech, Paris, France) at the site of the swelling, 4 years ago at a clinic in Australia. The swellings had appeared 2 years following the filler injection [Figure 1]. She then visited another dermatologist in South-East Asia who suggested that a reinjection of more Dermalive would improve the swelling. The patient then received a reinjection with the same filler at the same site 2 years after the original filler implantation. The patient was not treated with any other filler material. The slightly erythematous palpable indurations had appeared insidiously, and had gradually increased in size and thickness. The swellings worsened in appearance following the second injection. There was no relevant medical history and the patient did not have any autoimmune or allergic diseases. On palpation, there were firm erythematous longitudinal swellings measuring 5 cm × 2 cm along the lines of filler implantation at the nasolabial folds [Figure 2]. The nodules did not become prominent on projection from inside the oral cavity, and did not show transilluminance on throwing light from within the oral cavity using a pen torch. The patient was emotionally devastated about these reactions, and was constantly applying camouflage make-up [Figure 3]. We treated the patient with 3 injections of Triamcinolone (40 mg/ml), followed by 3 more injections of 10 mg/ml Triamcinolone at monthly intervals, with good reduction of the swelling on the left nasolabial fold, but only minimal reduction in the size of the swelling on the right side [Figure 4].
Figure 1.
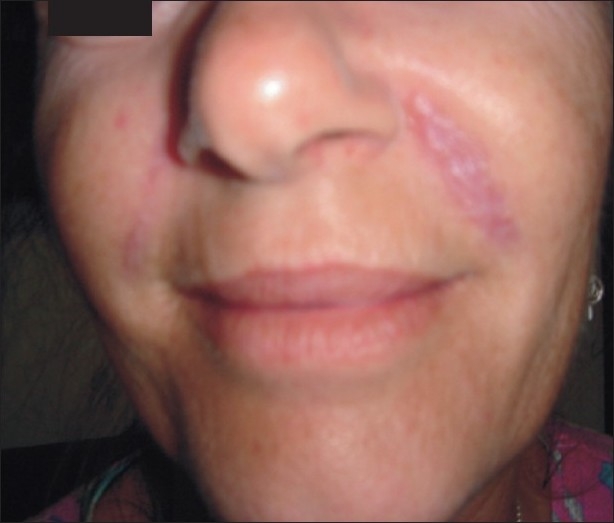
Nasolabial swellings appearing 2 years following injection of cosmetic filler Dermalive
Figure 2.
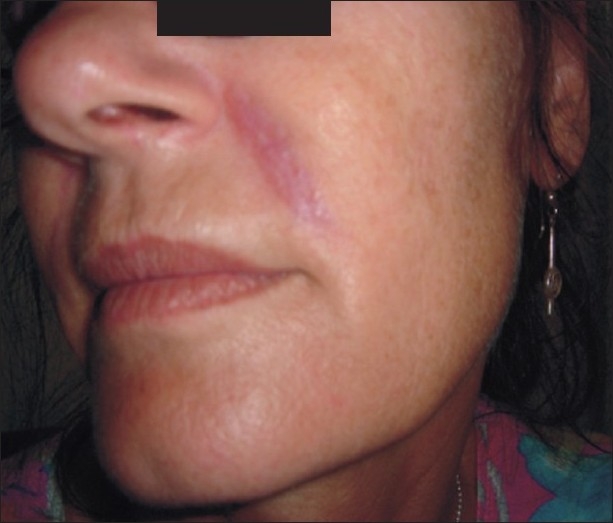
Firm erythematous longitudinal swellings along the line of injection of fillers
Figure 3.
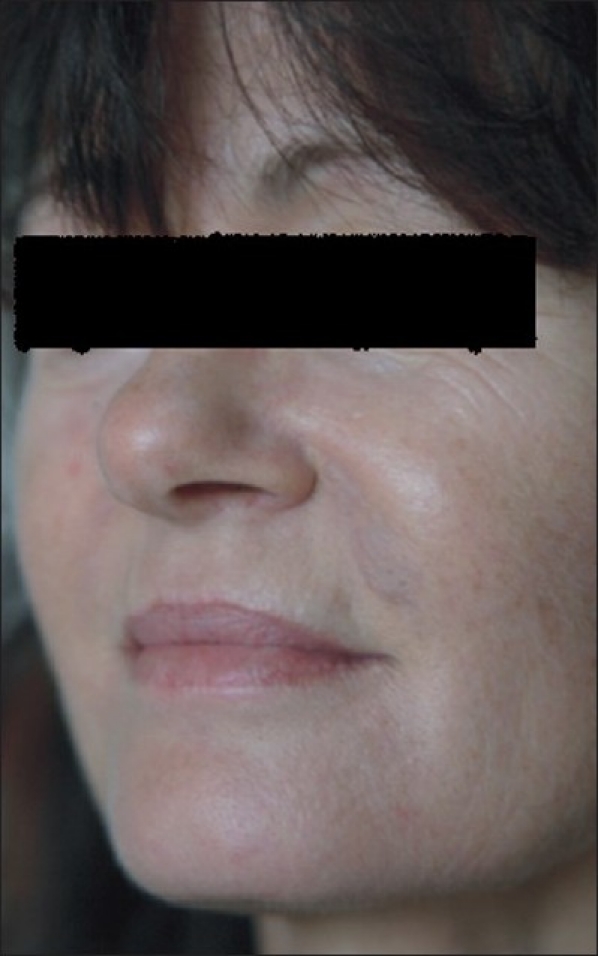
With camouflage make-up
Figure 4.
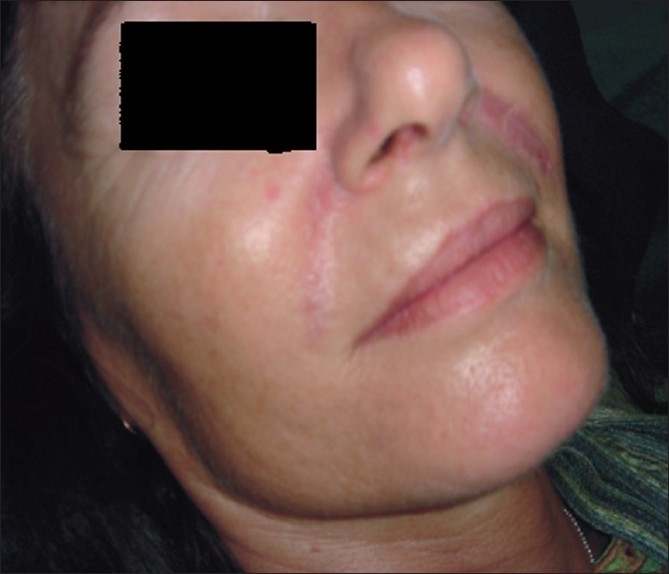
After 6 injections of intralesional Triamcinolone
As the patient was willing to accept cosmetic surgery, a surgical excision was carried out by the plastic surgeons and the tissue was sent for a histopathological evaluation. Operative findings indicated well encapsulated, grayish white, multilobulated dermal nodules. The wound was thoroughly cleaned and sutured in layers after achieving complete haemostasis. The subcutaneous tissues were approximated with 5-0 Monocryl interrupted sutures (Ethicon, Inc). The skin closure was done with 8-0 Ethilon (Ethicon, Inc) [Figure 5]. Operation site healed well and suture removal was done on the 10th day.
Figure 5.
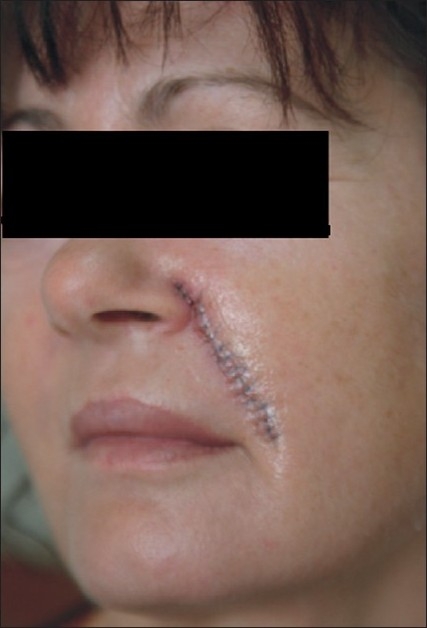
The sutures
The excision biopsy showed a dermal and subcutaneous adipose tissue infiltrate of foreign body giant cells and numerous irregular crystalline structures [Figures 6 and 7]. The surgical site showed good healing and our patient was satisfied with the result [Figure 8].
Figure 6.
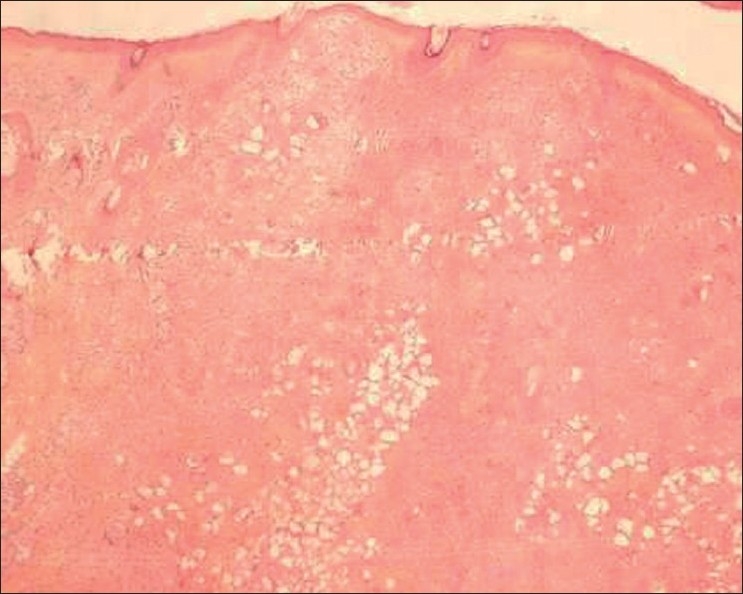
Dermal and subcutaneous tissue infiltrate with crystalline structures
Figure 7.
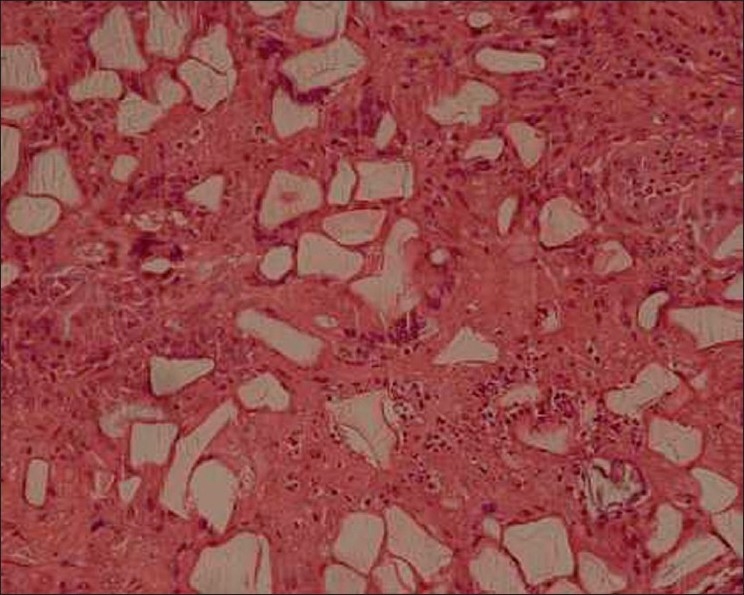
Translucent foreign body containing crystalline structures, surrounded by macrophages and giant cells H and E, 10×
Figure 8.
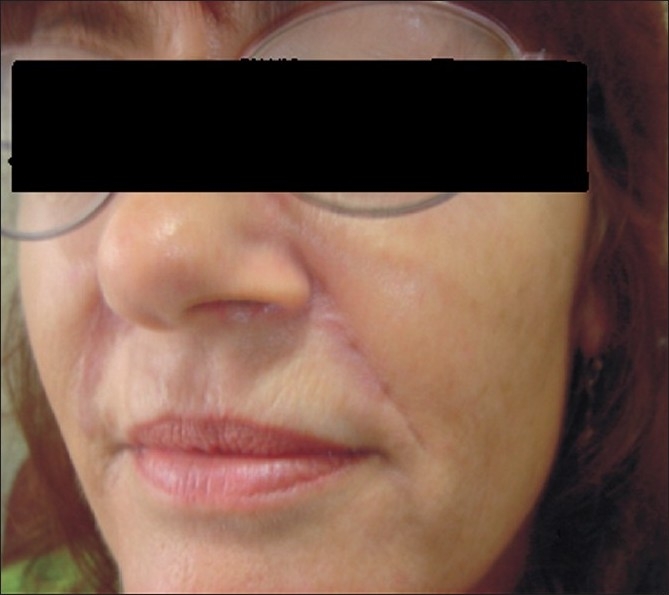
Post-surgery healing with secondary intention
The further treatment plan is to inject autologous fat as a filler to enhance the appearance following surgical excision. Autologous fat transplant is preferred both because of the hesitance on the part of the patient to accept another filler and also to prevent formation of new granulomas and foreign body reactions, which are known to occur even with temporary fillers like hyaluronic acid.
DISCUSSION
Dermalive consists of 40% acrylic hydrogel particles, a copolymer of hydroxy-ethyl-methacrylate (HEMA) and ethyl-methacrylate (EMA), and 60% cross-linked hyaluronic acid.[1] HEMA and EMA are microspheres with a variable diameter, which are non-biodegradable, and therefore, ensure a long-term nature of the product. Hyaluronic acid is merely a carrier substance that prevents the microspheres from agglomerating during tissue ingrowth. The carrier gel is removed within weeks and is definitely replaced by neocollagenesis response in the surrounding host tissue. The acrylic hydrogel particles produce longer lasting results compared to temporary fillers and are associated with a reportedly low incidence of adverse reactions.[1]
Many physicians claim no complications with semi-permanent fillers. In 2001, the global complication rate of Dermadeep/Dermalive was estimated at 1.2 per thousand patients, occurring on an average of 6 months after injection, in which 60% of cases had disregarded basic recommendations for use.[1] Although most of these complications were felt to be due to improper technique or use in the presence of contraindications, it must be remembered that adverse reactions have been reported with all injectable fillers, and with higher frequency when permanent or semi-permanent fillers are involved. The long-term side effects of Dermalive fillers include skin indurations including nodules and granulomas with or without fistulation. Systemic complications include fever, arthralgia, arthritis and rash.[2,3]
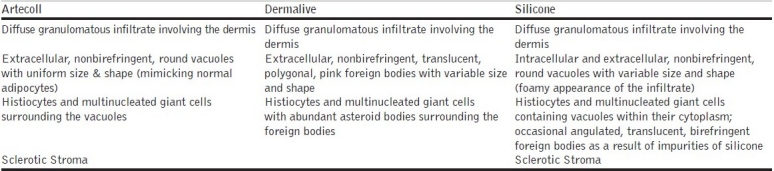
Dermalive fillers are of non-animal origin; so pre-treatment skin testing has not been recommended. Dermalive is considered a semi-permanent implant and its effects should last a minimum of 12 months. Dermalive should be implanted in the deep dermis, which is dependent on operator skill, and a maximum of 2 or 3 injections at a minimum of 3-monthly intervals may be necessary to achieve optimal results.[4] However, treatment is contraindicated in areas previously injected with other dermal fillers, in patients with a tendency to develop hypertrophic scars, in those with a history of autoimmune or inflammatory disease or receiving immunotherapy, and in patients with multiple allergies or those allergic to sodium hyaluronate.[4] Factors which may influence granuloma development include properties of the filler, volume injected, and previous infections or trauma. The shape of the methacrylate microspheres, which are comparatively irregular and sharp edged structures in Dermalive, maybe an important factor in inducing a more severe granulomatous reaction.[5] Our patient's biopsy revealed numerous irregular crystalline structures of variable size and shape with uniform transdermal distribution [Figures 6,7]. The crystalline bodies enclosed sharply circumscribed, translucent, non-birefringent foreign bodies with variable diameter. A sparse lymphocytic infiltrate intermingled with several multinucleated foreign body giant cells was also noted. This is the typical histopathological picture described in Dermalive granulomas by Steenkiste et al. in 2005.[6] The particular shape of the foreign body reflects the shape of the injected implant particles and allows the correct identification of the implanted material.[7,8] Table 1 above outlines the histopathological differences between Artecoll, Dermalive and Silicone granulomas.[8]
Table 1.
Histopathology in granulomas caused by different fillers
Treatment options include intralesional steroids, 5-fluorouracil (5-FU), anti-inflammatory and immunomodulatory drugs like minocycline, rifampicin or hydroxychloroquine.[9,10] Anecdotal reports also suggest some relief with the use of nonsteroidal anti-inflammatory drugs (NSAIDs), antihistamines and Tacrolimus.[11] In case of widespread lesions or repeated failure of conservative therapies, surgical excision is the treatment of choice.[12] Surgical extirpation can also allow a dermatologist to prevent the cutaneous side effects of intradermal steroid or 5-FU injection.[13] A histopathological confirmation is recommended before undertaking a surgical excision of the granuloma.
The production of Dermalive was stopped in 2007 following multiple reports of severe adverse events, with granuloma formation being the most dreaded long-term complication.[14] Dermalive fillers had never received FDA approval for cosmetic use in the United States. Dermalive fillers were never available in India. Products available in India include hyaluronic acid fillers, calcium hydroxylapatite, polylactic acid fillers, collagen-based fillers, silicone and polyacrylamide-based fillers. All of the aforementioned fillers can potentially induce nodules, lumps and granulomas; however, hyaluronic acid fillers are safer due to their temporary nature and response to hyaluronidase injections.
CONCLUSION
The purpose of this case report is to highlight the awareness on the risk of complications in semi-permanent fillers, and to reinstate the importance of proper techniques in minimizing complications. The long-term complications can be particularly devastating, and thus, it is important to have a long term follow-up of patients injected with permanent and semi-permanent fillers. Informed consent on potential inflammatory sequelae when working with these injectables is mandatory. Official documentation of all adverse effects with dermal filler(s) implantation must be made mandatory in order for cosmetic physicians and surgeons to make an informed choice when using injectable fillers.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bergeret- Galley C, Latouche X, Illouz YG. The value of a new filler material in corrective and cosmetic surgery: Dermalive and Dermadeep. J Aesth Plas Surg. 2001;25:249–55. doi: 10.1007/s002660010131. [DOI] [PubMed] [Google Scholar]
- 2.Alijotas-Reig J, Garcia-Gimenes V. Delayed immune- mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22:150–61. doi: 10.1111/j.1468-3083.2007.02354.x. [DOI] [PubMed] [Google Scholar]
- 3.Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31:1616–25. [PubMed] [Google Scholar]
- 4.Angus JE, Affleck AG, Leach IH, Millard LG. Two cases of delayed granulomatous reactions to the cosmetic filler Dermalive, a hyaluronic acid and acrylic hydrogel. Br J Dermatol. 2006;154:1077–9. doi: 10.1111/j.1365-2133.2006.07482.x. [DOI] [PubMed] [Google Scholar]
- 5.Vargas-Machuca I, González-Guerra E, Angulo J, del Carmen Fariña M, Martín L, Requena L. Facial granulomas secondary to Dermalive microimplants.Report of a case with histopathologic differential diagnosis among the granulomas secondary to different injectable permanent filler material. Am J Dermatopathol. 2006;28:173–7. doi: 10.1097/01.dad.0000181108.46909.8e. [DOI] [PubMed] [Google Scholar]
- 6.Steenkiste E, Marien K, Van den Oord J. Dermalive granuloma: A lesion with distinctive histological features. Internet J Dermatol. 2005;3:1531–3018. [Google Scholar]
- 7.Sidwell RU, Dhillon AP, Butler PE, Rustin MH. Localized granulomatous reaction to a semi- permanent hyaluronic acid and acrylic hydrogel cosmetic filler. Clin Exp Dermatol. 2004;29:630–2. doi: 10.1111/j.1365-2230.2004.01625.x. [DOI] [PubMed] [Google Scholar]
- 8.Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001;23:197–202. doi: 10.1097/00000372-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 9.De Boulle K. Management of complications after implantation of fillers. J Cosmet Dermatol. 2004;3:2–15. doi: 10.1111/j.1473-2130.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- 10.Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893–97. doi: 10.1097/00042728-200508000-00001. [DOI] [PubMed] [Google Scholar]
- 11.De Boulle K. Management of complications after implantation of fillers. J of Cosmetic Dermatol. 2004;3:2–15. doi: 10.1111/j.1473-2130.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- 12.Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. J Plast Reconstr Surg. 2006;118:92S–107S. doi: 10.1097/01.prs.0000234672.69287.77. [DOI] [PubMed] [Google Scholar]
- 13.Wolfram D, Tzankov A, Piza-Katzer H. Surgery for foreign body reactions due to injectable fillers. Dermatology. 2006;213:300–4. doi: 10.1159/000096193. [DOI] [PubMed] [Google Scholar]
- 14.Rossner M, Rossner F, Bachmann F, Wiest L, Rzany B. Risk of severe adverse reactions to an injectable filler based on a fixed combination of Hydroxyethylmethacrylate and ethylmethacrylate with Hyaluronic acid. Dermatol Surg. 2009;35:367–74. doi: 10.1111/j.1524-4725.2008.01062.x. [DOI] [PubMed] [Google Scholar]


