Abstract
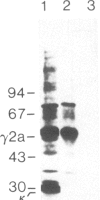
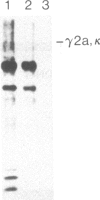
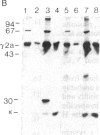
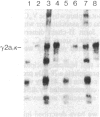
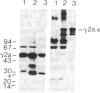
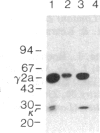
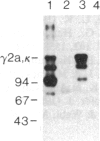
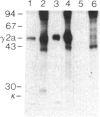
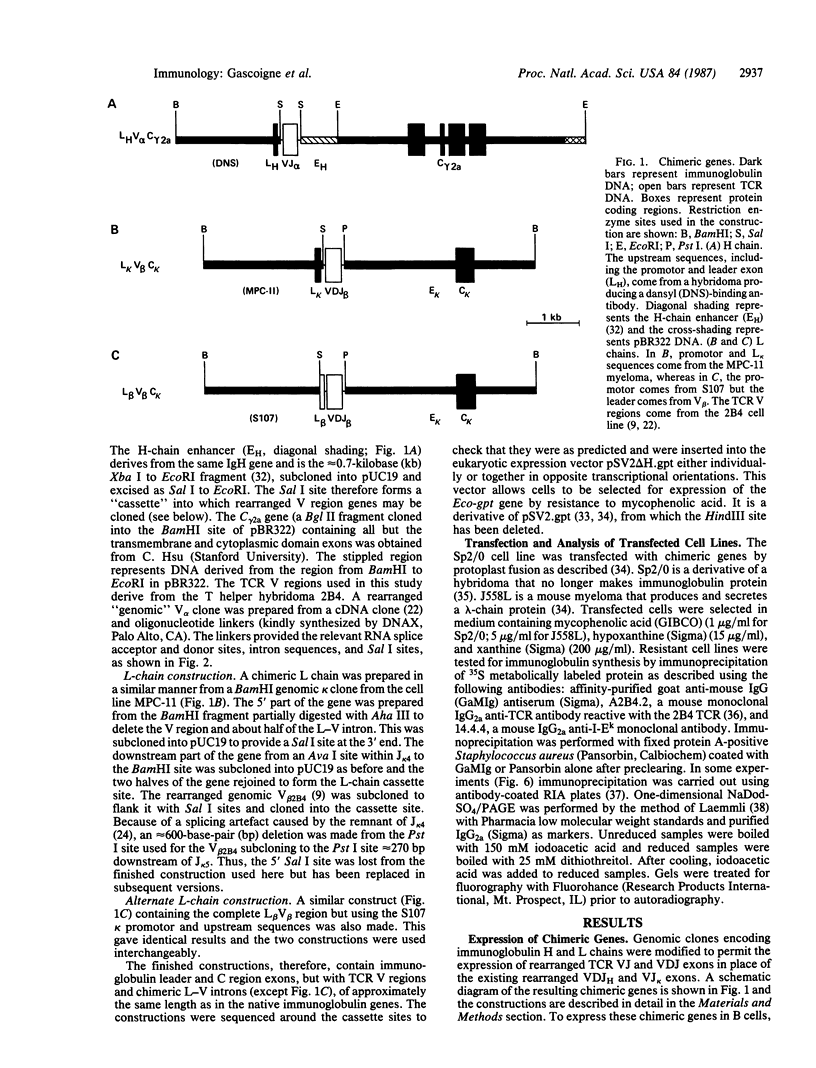
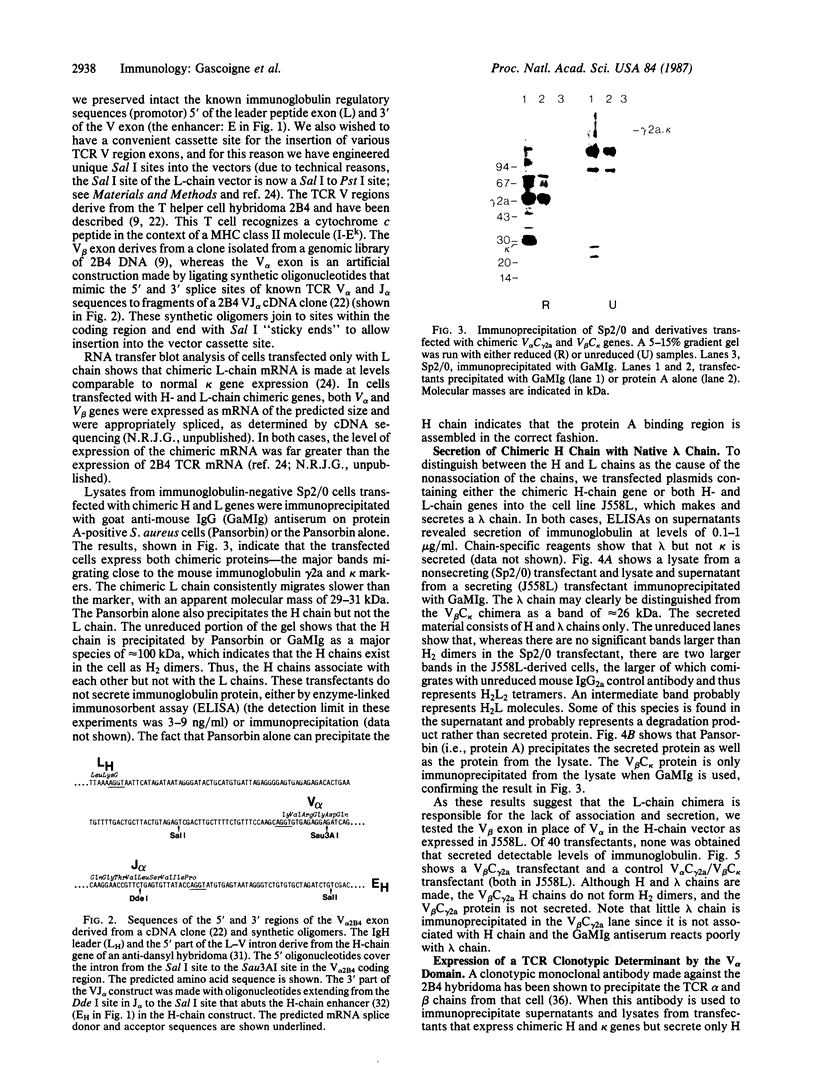
To produce sufficient quantities of soluble T-cell receptor protein for detailed biochemical and biophysical analyses we have explored the use of immunoglobulin--T-cell receptor gene fusions. In this report we describe a chimeric gene construct containing a T-cell receptor alpha-chain variable (V) domain and the constant (C) region coding sequences of an immunoglobulin gamma 2a molecule. Cells transfected with the chimeric gene synthesize a stable protein product that expresses immunoglobulin and T-cell receptor antigenic determinants as well as protein A binding sites. We show that the determinant recognized by the anticlonotypic antibody A2B4.2 resides on the V alpha domain of the T-cell receptor. The chimeric protein associates with a normal lambda light chain to form an apparently normal tetrameric (H2L2, where H = heavy and L = light) immunoglobulin molecule that is secreted. Also of potential significance is the fact that a T-cell receptor V beta gene in the same construct is neither assembled nor secreted with the lambda light chain, and when expressed with a C kappa region it does not assemble with the chimeric V alpha C gamma 2a protein mentioned above. This indicates that not all T-cell receptor V regions are similar enough to immunoglobulin V regions for them to be completely interchangeable.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Pattern P., Chien Y., Yokota T., Eshhar Z., Giedlin M., Gascoigne N. R., Goodnow C., Wolf R., Arai K. Variability and repertoire size of T-cell receptor V alpha gene segments. Nature. 1985 Oct 3;317(6036):430–434. doi: 10.1038/317430a0. [DOI] [PubMed] [Google Scholar]
- Behlke M. A., Spinella D. G., Chou H. S., Sha W., Hartl D. L., Loh D. Y. T-cell receptor beta-chain expression: dependence on relatively few variable region genes. Science. 1985 Aug 9;229(4713):566–570. doi: 10.1126/science.3875151. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulianne G. L., Hozumi N., Shulman M. J. Production of functional chimaeric mouse/human antibody. Nature. 1984 Dec 13;312(5995):643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Concannon P., Pickering L. A., Kung P., Hood L. Diversity and structure of human T-cell receptor beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6598–6602. doi: 10.1073/pnas.83.17.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Raison R. L. Structural analysis of the myeloma-associated membrane antigen KMA. J Immunol. 1985 Aug;135(2):1276–1280. [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. II. Failure of physiologic cooperative interactions between T and B lymphocytes from allogeneic donor strains in humoral response to hapten-protein conjugates. J Exp Med. 1973 Jun 1;137(6):1405–1418. doi: 10.1084/jem.137.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred B., Shreffler D. C. H-2 dependence of co-operation between T and B cells in vivo. J Immunol. 1972 Nov;109(5):940–943. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Zamoyska R. A beginner's guide to major histocompatibility complex function. Nature. 1982 Jun 24;297(5868):628–628. doi: 10.1038/297628a0. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Johnson M. J., Herzenberg L. A., Oi V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L., Oi V. T. Transfer and expression of immunoglobulin genes. Annu Rev Immunol. 1984;2:239–256. doi: 10.1146/annurev.iy.02.040184.001323. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Vuong T. M., Hardy R., Reidler J., Dangle J., Herzenberg L. A., Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984 Jan 12;307(5947):136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- Patten P., Yokota T., Rothbard J., Chien Y., Arai K., Davis M. M. Structure, expression and divergence of T-cell receptor beta-chain variable regions. Nature. 1984 Nov 1;312(5989):40–46. doi: 10.1038/312040a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Germain R. N., Schwartz R. H. Monoclonal antibodies against the antigen receptor on a cloned T-cell hybrid. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6972–6976. doi: 10.1073/pnas.80.22.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Gefter M. L., Manser T., Morrison S. L., Oi V. T., Ptashne M. Expression of a VHC kappa chimaeric protein in mouse myeloma cells. Nature. 1984 May 24;309(5966):364–367. doi: 10.1038/309364a0. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Augustin A. A. V beta gene polymorphism and a major polyclonal T cell receptor idiotype. Cell. 1985 Aug;42(1):89–92. doi: 10.1016/s0092-8674(85)80104-5. [DOI] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Dolder B., Karjalainen K. A novel approach for preparing anti-T cell receptor constant region antibodies. Eur J Immunol. 1986 Jul;16(7):851–854. doi: 10.1002/eji.1830160722. [DOI] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Yagüe J., White J., Coleclough C., Kappler J., Palmer E., Marrack P. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985 Aug;42(1):81–87. doi: 10.1016/s0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]