Abstract
Meningiomas are slow-growing intracranial/intraspinal tumours, with a wide range of histopathological variants. The more aggressive atypical and malignant types can disseminate via the venous system, lymphatics or cerebrospinal fluid, with the lungs and pleura being the most common site of extracranial metastasis. We look at a 68-year-old woman presenting with abdominal pain, who had previously been treated for an intracranial meningioma with a ventriculo-peritoneal shunt in situ. Investigation revealed a lesion in segment 4 of the liver with the shunt tip being in close proximity. Biopsy was consistent with metastatic meningioma. A liver resection was subsequently performed. We postulate that this is the first reported case of dissemination of an intracranial meningioma via cerebrospinal fluid by means of a ventriculo-peritoneal shunt.
Key Words: Hepatic metastasis, Meningioma, Ventriculo-peritoneal shunt
Case Report
A 61-year-old lady presented with headaches and was subsequently diagnosed with a cerebral atypical meningioma on cross-sectional imaging. She underwent a craniotomy and resection, and further resections for recurrence 2 and 4 years later. During this last operation a ventriculo-peritoneal (VP) shunt was inserted at the time of resection. Three years later during routine follow-up, a further small recurrence was detected, located around the region of the shunt, which was treated with external beam radiotherapy. Following therapy she was living independently with no neurological functional deficit. Follow-up cross-sectional imaging revealed no residual disease. She had hypothyroidism but was otherwise fit and well. At the age of 68 years she presented to a local hospital with a history of right-sided abdominal pain and nausea. She also described loss of appetite, mild weight loss, and a short history of altered bowel habit which swiftly resolved. Clinical examination revealed a right upper quadrant mass.
At the time of presentation her serum liver function tests and coagulation studies were normal. Hepatitis serology and autoantibody screening were negative. An ultrasound scan confirmed the presence of a large irregular mass in the liver. A staging computed tomography scan was then performed which found an ill-defined and heterogeneous mass in segment 4b of the liver with multiple incidental cysts. A magnetic resonance imaging scan demonstrated a 7.6 × 8.3 × 6.6 cm lobulated lesion inferiorly in segment 4 abutting but not invading the gallbladder and extending to at least the border of segment 5. This demonstrated a heterogeneous signal, slightly increased in T2. Interestingly post-gandolium there was no marked hypervascularity demonstrated. This lesion was slightly distorting the liver capsule. The spleen was of normal size and no ascites was seen. The VP shunt was also noted to be in very close proximity to the lesion. The liver was otherwise normal. These cross-sectional imaging findings of a solitary large hypovascular liver lesion excluded a cyst, haemangioma or focal nodular hyperplasia. They did not represent typical appearances of a liver primary cancer and hence the lesion was deemed indeterminate.
A percutaneous liver biopsy revealed a partly necrotic tumour composed of small groups and strands of medium-sized cells set in a collagenous stroma. The tumour cells showed oval, monotonous nuclei with homogenous chromatin. Occasional intranuclear inclusions were seen but no mitoses were recognised in the very small amount of viable tumour present. Immunohistochemically the tumour showed strong, diffuse expression for vimentin and strong staining for epithelial membrane antigen in a proportion of cells, an immunophenotype typical for meningioma. There was only very focal positivity for cytokeratin and the tumour cells were negative for GFAP, S100 and leucocyte common antigen excluding carcinoma, melanoma, neural tumours and lymphoma.
The appearances of the tumour in the diagnostic liver core biopsy were compared with those of the previously resected recurrent WHO grade 2 meningioma and the features in both specimens were similar. In particular, the latest recurrence displayed a similar patternless growth with focal necrosis. It also showed a high mitotic rate of 17 per 10 high power fields just falling short of a grade 3 anaplastic meningioma. The liver biopsy was therefore consistent with metastatic meningioma.
The patient was referred to the regional Hepato-Pancreatico-Biliary centre where the cross-sectional imaging and histology were reviewed and discussed in the multi-disciplinary team. Following patient consultation and satisfactory cardiopulmonary exercise testing, a segment 4b and 5 liver resection was performed. Intraoperative ultrasound examination was used to demarcate the margins of the tumour which were well separated from the right and left vascular inflow. A cholecystectomy was performed along with division of omental adhesions. Blood loss was measured as 450 ml.
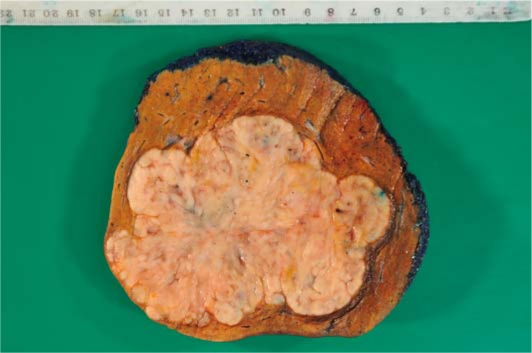
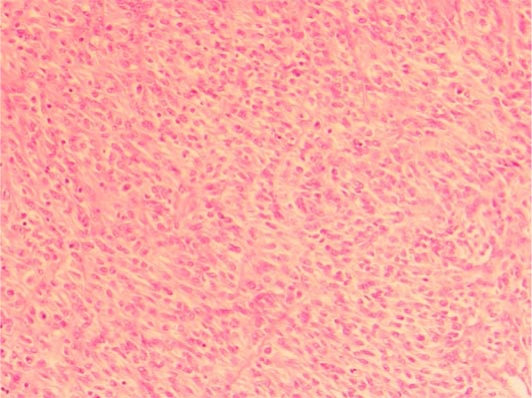
The liver resection specimen weighed 800 g and contained an nonencapsulated, multinodular, cream-coloured, rubbery tumour measuring 123 mm in greatest dimension, occupying segment 4b and 5 almost entirely (fig. 1). Histology confirmed a highly cellular tumour with a patternless appearance composed of round to elongated cells with ovoid nuclei with focal nuclear vacuoles (fig. 2). The tumour showed large areas of necrosis and up to 21 mitoses per 10 high power fields. In view of the mitotic count of >20/10 high power fields the appearances were classified as metastatic anaplastic/malignant meningioma, likely transferred through the VP shunt. The tumour margin was >10 mm.
Fig. 1.
Macroscopic specimen of 123 mm tumour occupying liver segments 4b and 5 almost entirely.
Fig. 2.
Microscopic specimen of highly cellular tumour with a patternless appearance.
Postoperatively a bile leak was detected. This was managed initially with continued HDU care, antibiotics, and a percutaneous abdominal drain. Subsequently two ERCP procedures were needed to achieve adequate biliary drainage by placement of a plastic biliary stent placement into the left hepatic duct. The patient made a good recovery and was discharged home 14 days postoperatively.
Discussion
Meningiomas are slow-growing neoplasms that make up 14-19% of all primary intracranial and intraspinal tumours [1]. The WHO have classified 15 histopathological variants of meningioma [2]. Of these subtypes meningiothelial, fibrous and transitional are the most common (table 1).
Table 1.
WHO 2000 classification of meningiomas [2]
| WHO grade 1 | WHO grade 2 | WHO grade 3 |
|---|---|---|
| Benign, 90% | Atypical, 7% | Malignant, 2% |
|
|
|
Most meningiomas are benign, however WHO grade 2 and 3 meningiomas have less favourable outcomes. Malignant progression is a continuum of increasing atypia. WHO grade 2 and 3 are determined by either a particular histological subtype or by a combination of morphological features. Chordoid and clear cell meningioma are automatically classified as WHO grade 2 whereas papillary and rhabdoid meningioma correspond to WHO grade 3. According to the WHO classification morphological criteria for an atypical meningioma are either more than 3 mitoses/10 high power fields [3] or at least three of the following features: increased cellularity, small cells with a high nuclear:cytoplasmic ratio, prominent nucleoli, uninterrupted patternless or sheet-like growth and foci of necrosis. Anaplastic/malignant meningioma shows obviously malignant cytology and/or >20 mitoses/10 high power fields [4]. The anaplastic type is the most aggressive, with mean overall survival for anaplastic meningiomas being 3.3 years as opposed to 11.9 years for atypical meningiomas [5].
The occurrence of distant metastasis from a benign meningioma is reported to be rare, being estimated at around 0.1% [6]. This occurrence however is significantly higher in atypical and malignant cases, being 5 and 30% respectively [7, 8]. The proposed method of dissemination is via the venous system, lymphatics or cerebrospinal fluid (CSF). Spread to the lymphatic system is postulated to occur as the tumour spreads in the skull and scalp, or to the lymphatics around the cranial nerves, for instance in the cavernous sinus [9]. Surgical resection allows seeded cells to access the blood and lymphatic system [9], however the more common reported route of metastasis is via invasion of the dural venous sinuses and cranial veins, with resultant spread into the pulmonary circulation, azygous and hemiazygous systems and vertebral venous plexus [10, 11]. This would account for the majority of sites for extracranial metastasis, most commonly being the lung and pleura (60%), and in order of decreasing frequency: liver, long bones, vertebrae, ribs, pleura, mediastinum, and lymph nodes [12, 13].
Meningiomas arise from the arachnoid cap cells and thus are invariably in contact with CSF. Seeding through CSF pathways however is reported to be rare [14]. What makes our case particularly unique is the fact that there is no previously reported case in the literature regarding spread in CSF via a VP shunt, into the abdominal cavity. Furthermore there are very few reported cases of metastasis to the liver. Figueroa et al. reported on a case of metastasising transitional meningioma, spreading initially to the lungs and then to the liver [15].
Interestingly, and certainly worth considering following surgical resection, is the risk of iatrogenic metastasis. Skin metastasis localised around the scar of the resection incision has been reported in five cases: one in the temporal muscle [16], one in the abdominal wall [17], and three in the scalp [18, 19, 20]. The first case was a transitional meningioma, whilst the latter three were atypical or anaplastic meningiomas. In view of this a thorough operative field washout and frequent changing of surgical tools has been recommended [19].
Due to the slow-growing nature of metastases and their good prognosis after resection, surgery is the recommended treatment for meningiomas. Radiotherapy is ineffective as a primary treatment modality; however as an adjuvant it decreases local recurrence and time to recurrence after complete or subtotal resection [21].
Conclusion
We believe this to be the first reported case of dissemination of an intracranial meningioma via CSF by means of a VP shunt. Whilst spread by the more commonly postulated venous route could not be entirely excluded, in view of the reported recurrence of the intracranial meningioma at the shunt site and the close proximity of the shunt to the liver lesion, it appears very likely that the spread occurred via the VP shunt. This case also demonstrates the importance of considering the possibility of metastasis in a patient with a liver lesion and past history of meningioma. We recommend early referral to the local hepato-pancreatico-biliary centre, and furthermore, given the reported risks of cutaneous seeding [16, 17, 18, 19, 20], we would strongly advise against percutaneous liver biopsy in a patient with a potentially resectable tumour.
References
- 1.Wara WM, Sheline GE, Newman H, Townsend JJ, Boldrey EB. Radiation therapy of meningiomas. Am J Roentgenol Radium Ther Nucl Med. 1975;123:453–458. doi: 10.2214/ajr.123.3.453. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Scheithauer BW, Budka H, Von Deimling A, Kepes JJ. Meningiomas. In: Kleihues P, Cavenee WK, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000. pp. 176–184. [Google Scholar]
- 3.Miller DC, Ojemann RG, Proppe KH, McGinnis BD, Grillo HC. Benign metastasizing meningioma: case report. J Neurosurg. 1985;62:763–766. doi: 10.3171/jns.1985.62.5.0763. [DOI] [PubMed] [Google Scholar]
- 4.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 5.Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79:574–580. doi: 10.1136/jnnp.2007.121582. [DOI] [PubMed] [Google Scholar]
- 6.Strang RR, Tovi D, Nordenstam H. Meningioma with intracerebral and visceral metastases. J Neurosurg. 1964;21:1098–1102. doi: 10.3171/jns.1964.21.12.1098. [DOI] [PubMed] [Google Scholar]
- 7.Enam SA, Abdulrauf S, Mehta B, Malik GM, Mahmood A. Metastasis in meningioma. Acta Neurochir (Wien) 1996;138:1172–1177. doi: 10.1007/BF01809747. discussion 1177-1178. [DOI] [PubMed] [Google Scholar]
- 8.Gezen F, Kahraman S, Canakci Z, Bedük A. Review of 36 cases of spinal cord meningioma. Spine. 2000;15:727–731. doi: 10.1097/00007632-200003150-00013. [DOI] [PubMed] [Google Scholar]
- 9.Shuangshoti S, Hongsaprabhas C, Nersky MG. Metastasizing meninigioma. Cancer. 1970;26:832–841. doi: 10.1002/1097-0142(197010)26:4<832::aid-cncr2820260416>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Ueyama Y, Marita K, Ochiai C, Ohsawa N, Hata J, Tamaoki N. Xenotransplantation of a human meningioma and its lung metastasis in nude mice. Br J Cancer. 1978;37:644–647. doi: 10.1038/bjc.1978.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kepes JJ. Biology, Pathology and Differential Diagnosis. New York: Masson Publishing; 1982. Meningiomas; pp. 190–200. [Google Scholar]
- 12.Karasick JL, Mullan SF. A survey of metastatic meningiomas. J Neurosurg. 1974;40:209–212. doi: 10.3171/jns.1974.40.2.0206. [DOI] [PubMed] [Google Scholar]
- 13.Allen IV, McClure J, McCormick D, Gleadhill CA. LDH isoenzyme pattern in a meningioma with pulmonary metastases. J Pathol. 1977;123:187–191. doi: 10.1002/path.1711230309. [DOI] [PubMed] [Google Scholar]
- 14.Russell DS, Rubinstein LJ. Pathology of Tumours of the Nervous System. Baltimore: Williams and Wilkins; 1977. pp. 68–74. 89-91. [Google Scholar]
- 15.Figueroa BE, Quint DJ, McKeever PE, Chandler WF. Extracranial metastatic meningioma. Br J Radiol. 1999;72:513–516. doi: 10.1259/bjr.72.857.10505022. [DOI] [PubMed] [Google Scholar]
- 16.Singh RVP, Yeh JS, Campbell DA. Implantation meningioma in temporalis muscle – case report. Br J Neurosurg. 1994;8:93–95. doi: 10.3109/02688699409002400. [DOI] [PubMed] [Google Scholar]
- 17.Sadahira Y, Sugihara K, Manabe T. Iatrogenic implantation of malignant meningioma to the abdominal wall. Virchows Arch. 2001;438:316–318. doi: 10.1007/s004280000347. [DOI] [PubMed] [Google Scholar]
- 18.Ludemann WO, Obler R, Tatagiba M, Samii M. Seeding of malignant meningioma along a surgical trajectory on the scalp. J Neurosurg. 2002;97:683–686. doi: 10.3171/jns.2002.97.3.0683. [DOI] [PubMed] [Google Scholar]
- 19.Mahore A, Chagla A, Goel A. Seeding metastases of a benign intraventricular meningioma along the surgical track. J Clin Neurosci. 2010;17:253–255. doi: 10.1016/j.jocn.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Akai T, Shiraga S, Iizuka H, Kishibe S, Ueda Y. Recurrent meningioma with metastasis to the skin incision – case report. Neurol Med Chir (Tokyo) 2004;44:600–602. doi: 10.2176/nmc.44.600. [DOI] [PubMed] [Google Scholar]
- 21.Pramesh CS, Saklani AP, Pantvaidya GH, Heroor AA, Naresh KN, Sharma S, Deshpande RK. Benign metastasizing meningioma. Jpn J Clin Oncol. 2003;33:86–88. doi: 10.1093/jjco/hyg022. [DOI] [PubMed] [Google Scholar]