Abstract
Aim:
This study was designed to retrospectively review our experience with the multimodality management of hepatoblastomas (HB).
Materials and Methods:
Thirteen patients were treated for HB between 2000 and 2007. The clinical presentations, chemotherapy tolerance and response, surgical procedure undertaken, and complications were analysed.
Results:
Median age of the population was 12 months (3-60 months), with a male-to-female ratio of 3.3:1. Nine patients were treated with neoadjuvant chemotherapy incorporating cisplatin and adriamycin. Primary surgery was done in four patients. Extent of hepatic resection in the operated patients varied. Mixed type was the predominant histopathological diagnosis. Adjuvant chemotherapy was well tolerated with no morbidity or mortality. Five-year event-free survival (EFS) and overall survival (OS) of all the 13 patients is 76.9%. All the nine patients who could complete multimodality treatment are alive with no evidence of disease or complications with median follow-up of 63 months (46-122 months).
Conclusions:
Treatment of HB with multidisciplinary approach was well tolerated. OS and EFS of patients were comparable with published studies.
Keywords: Chemotherapy, hepatic resections, hepatoblastoma, multimodality treatment, neoadjuvant treatment
INTRODUCTION
Hepatoblastomas (HB) are rare pediatric neoplasms, with incidence of 1.5 per million, and comprising 1% of pediatric malignancies.[1] Till 1970s, surgery was the primary modality of treatment of HB. Unfortunately, up to 60% of the patients present in an unresectable stage.[1,2] Later, the chemo-responsiveness of the tumor was demonstrated which led to the incorporation of adjuvant chemotherapy with cisplatinum and doxorubicin in the treatment of HB.[3–5] International Society of Pediatric Oncology (SIOP) pioneered the concept of neoadjuvant chemotherapy in the management of HB.[6,7] Surgical resection of the tumors were made easier by reducing the size and vascularity of the tumor, and the chances for obtaining negative margins of resection were more.[7,8] A partial response (PR) status could be achieved in 82% of the cases in SIOPEL-1 study. Surgical resection after neoadjuvant chemotherapy could be done in 87% of the cases whereas historically only 30% of the cases were operable upfront. Surgical morbidity was also less if resection was performed after neoadjuvant chemotherapy. Extended surgical resections of HB have been safely performed in the pediatric age group.[8] Orthotopic Liver transplant (OLT) is an effective treatment for unresectable HB with survival rates of 82% if done as a primary treatment.[1] Combined modality treatment is now the standard of care in HB. Management of HB at a tertiary care cancer centre over 8 years is reviewed in this article.
MATERIALS AND METHODS
This is a retrospective analysis of patients diagnosed to have HB and treated in our institution between January 2000 and December 2007. Patient demographics, mode of presentation, method of diagnosis, extent of tumor at diagnosis, surgical procedures performed, complications of treatment and outcome were compiled by reviewing medical histories, radiology, pathology, and surgery reports. Based on imaging using a computerized tomography (CT) scan or magnetic resonance imaging (MRI), all patients were assigned a PRETEXT (Pre-treatment extent of disease) stage and four groups of patients were identified as PRETEXT I-IV.[2]
Up to 2001, patients were treated in our institution by primary surgery followed by adjuvant chemotherapy. Later when neoadjuvant chemotherapy followed by surgical resection became the standard of care, cisplatinum and adriamycin as in the PLADO (PLA, Platinum; DO, Doxorubicin) regimen was administered to all patients every 21 days. PLADO chemotherapy consisted of PLA on day 1 at a dose of 80 mg/m2, administered in a continuous 24-h infusion and DO at a dose of 30 mg/m2 per day, administered as a continuous 24-h intravenous (IV) infusion on days 2 and 3. Both drugs were administered via a central venous catheter.
Patients were routinely reassessed after three cycles for surgical resection. If the tumor was found to be inoperable, patient was given one more cycle of chemotherapy. The decision regarding timing of surgery was taken by the surgical oncology team. Postoperatively, two to three cycles of chemotherapy were given to a total of six cycles. The patients were followed up after treatment according to the institution's Protocol with serial AFP levels and imaging. Follow-up was complete for all treated patients, and patient details were updated as of August 2010. Statistical analyses were performed using SPSS (version 14.0). The Kaplan-Meier method was used to estimate survival curves.
RESULTS
Thirteen patients were diagnosed and treated for HB in our institution. All the patients had elevated AFP levels and pathological confirmation was done in all feasible cases. There were ten males and three were females in the group (M:F, 3.3:1). The median age was 12 months (range, 3-60 months). Only one patient was preterm by birth. One child had congenital anomaly with dysmorphic facies and mental retardation. None of the patients had any cardiac anomalies. The commonest presenting feature was that of an abdominal mass (in 12 out of 13 patients). One patient was diagnosed while being evaluated for fever. None of the patients had jaundice or was seropositive for Hepatitis B or C. All the patients had palpable hepatomegaly. AFP was elevated in all the cases with a median level of 30,000 ng/ml (range, 702-1,40,000 ng/ml). CT scan was done for 12 patients and MRI for one. PRETEXT staging distribution was as follows; PRETEXT Stage I 7.7% (n = 1), Stage II 46.1% (n = 6), Stage III 38.4% (n = 5), and Stage IV 7.7% (n = 1). Patients who underwent straight surgery did not have preoperative biopsy. Eight of the remaining nine patients had pretreatment fine needle aspiration (FNA) or Trucut biopsy.
Treatment
Neoadjuvant chemotherapy was given to 9 of 13 patients. The remaining four had initial surgery [Table 1]. A 3-month-old baby with PRETEXT 3 disease died of pneumonia after second cycle of neoadjuvant chemotherapy. Another 1-year-old baby had sudden aspiration and expired after the second cycle. Of the remaining seven children, three patients completed four cycles and another four completed three cycles of chemotherapy before surgery. A 5-year-old boy with PRETEXT stage 4 disease was taken up for laparotomy after neoadjuvant chemotherapy and was found to have extensive infiltrations to colon and mesentery and hence was declared inoperable. The remaining six patients received adjuvant chemotherapy also for a total of six cycles. Among the four patients who had primary surgery, three received adjuvant chemotherapy also. An 18-month-old patient with raised AFP levels, who had undergone marginal excision of the liver lesion outside had no malignancy on extended resection. The pathological diagnosis of HB was confirmed on review of the excision biopsy specimen. He was not given chemotherapy and is a long-term survivor.
Table 1.
Patient characteristics and results of treatment with multimodality management
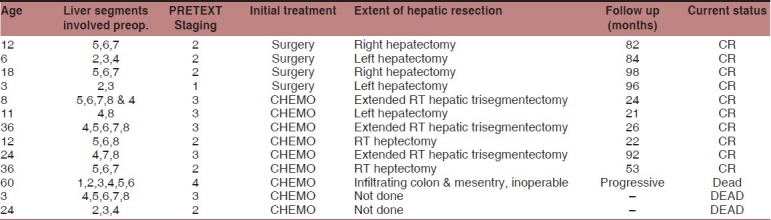
Administration of chemotherapy for the infant population required close monitoring for complications. Dose reductions were done as required at the discretion of the treating pediatric oncologist, avoiding undue toxicities. Neutropenia requiring growth factor support, even after dose reduction as mentioned in SIOPEL 1, was documented in four patients. There were two mortalities (22%) after neoadjuvant chemotherapy. None of the patients had cardiac failure after anthracycline administration. Long-term toxicities were not reported in any patient.
Surgical procedures performed included right hepatectomy in four patients, extended right hepatectomy in three patients, and left hepatectomy in three. The median duration of surgery was 190 min (range, 110-350 min). The median blood loss was 300 ml (100-500 ml). The median length of postoperative hospital stay was 9.5 days (range, 7-21 days). The only postoperative complication was intestinal obstruction in one patient which was managed conservatively. There was no postoperative mortality.
Histopathology and margins
The histological types encountered were as follows: embryonal and mixed embryonal/foetal subtype in four patients and mixed epithelial and mesenchymal in six. Pathological complete remission was noted in one patient after neoadjuvant chemotherapy, others having residual tumor. Among the ten patients who underwent hepatectomy, six had tumor-free margin of at least 5 mm, three had close margins (<5 mm), and one had microscopic positive margins.
Outcome
Out of 13 patients, two patients expired after two cycles of neoadjuvant chemotherapy. The patient who had inoperable disease after neoadjuvant chemotherapy was offered only palliative care. He expired after 9 months. The patient who had no residual tumor after re-resection was not given adjuvant chemotherapy and is long-term disease free. All the remaining nine patients who could complete the planned multimodality protocol treatment are alive and disease free after a median follow-up period of 63 months (46-122 months). The 5-year overall survival (OS) and event-free survival (EFS) of the total patient population is 76.9%. The 5-year OS according to the PRETEXT stage is as follows: PRETEXT I, 100%; PRETEXT II, 100%; PRETEXT III, 66.7%; and PRETEXT IV, 0%. The patient who had positive margin has completed 70 months of follow-up. The median survival for patients with close margins is 114 months.
DISCUSSION
Hepatoblastomas are the most common pediatric primary liver malignancy accounting for more than 90% of the liver tumors in less than 5 years of age.[1] Most of the cases are asymptomatic and present in an advanced stage. The exact etiology is unknown. The association with preterm births and genetic conditions like Beckwith-Wiedemann syndrome is well documented in the literature.[9,10] Surgical resection is the cornerstone for successful management of HB. Management of HB has evolved from extensive surgical resections through the incorporation of adjuvant chemotherapy to the current standard of care of neoadjuvant chemotherapy followed by surgery. The survival in 1970s with surgical resection alone was a meagre 10-20%.[11] However with the arrival of chemotherapeutic regimens such as PLADO, the surgical outcomes improved tremendously. Neoadjuvant chemotherapy pioneered by SIOP has reduced the surgical complications, facilitated more complete resections and improved the cure rates.[7] Presently, survival is between 75% and 90%.[12–14] Multi-institutional trials have confirmed the feasibility of this approach in limited resource settings also.[15,16] An initial surgical approach may be acceptable for resectable disease, but a neoadjuvant approach may be preferable in advanced stages.[13]
Maibach et al. reviewed SIOPEL experience with the low AFP level at presentation of HB. This group had extensive disease, poor histology, poor response to chemotherapy, and consequently poor outcome.[17] Survival is influenced by the pathological subtype with foetal subtype having the best outcome and anaplastic subtype being resistant to treatment.[18] PRETEXT staging had a significant impact on survival with 100% survival in stage I dropping to only 57% in stage IV.[7,19,20] We observed a similar pattern in our series as well. The long-term survival of patients with positive or close margins in our series could be attributed to the administration of chemotherapy.
The patients in our study had a lower median age of presentation (12 months) and male predominance compared to some of the previous reports. PRETEXT stage II predominated in our series (46.1%). Around 80% patients in our series had a mixed type of HB comprising foetal, embryonal, and epithelial elements. Chemotherapy-related long-term toxicities were absent in our study. Most importantly all the nine patients who completed multimodality treatment are long-term survivors. Relapse of the disease was not observed in any of the survivors. Of the three deaths seen in our study, only one patient died due to chemotherapy-induced neutropenic sepsis. None of the deaths were related to surgery. In the SIOPEL 1 study, 38 deaths were reported, of which three were attributable to chemotherapy.[7]
Excellent results have been reported from tertiary care centres in HB, thus reaffirming the role of expertise in the management of this rare neoplasm. Bajpai et al. reported excellent outcome in 10 patients with HB. Three patients recurred in their study. Fetal subtype was the most common histology.[16] Ang et al. analysed outcome of 30 patients with HB treated at Royal Children's Hospital Australia, from 1984 to 2004. The 5-year EFS was 75.7%.[14]
SIOPEL 2 and 3 were trials aimed at improvement of results for advanced stages.[21,22] SIOPEL-3 HR trial utilising a dose dense regime has shown a promising 69% survival rate for high-risk patients. Orthotopic liver transplant is an option for unresectable cases of HB.[23] Irionotecan maintenance has shown promising clinical activity in a small study.[24] Malogolowkin et al. reported the feasibility of hepatic artery chemoembolisation in 11 cases of refractory pediatric liver tumors and had three survivors.[25]
From a malignancy with dismal outcome, HB has come a long way through mainly because of the combined modality treatment with improved chemotherapeutic regimes and surgical techniques. HB is one cancer where the coordinated effort of multiple specialities has given a leap in the cure rates.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mueller Bu, Terrada DL, Finegold MJ. Principles and practice of pediatric oncology. In: Pizzo PA, Poplack DG, editors. Hepatoblastoma. 5th ed. Houston: Lippincott-Williams Wilkins Publishers; 2006. p. 887. [Google Scholar]
- 2.Exelby PR, Filler RM, Grosfeld JL. Liver tumours in children in particular reference to hepatoblastoma and hepatocellular carcinoma: American Academy of Pediatrics Surgical Section Survey - 1974. J Pediatr Surg. 1975;10:329–37. doi: 10.1016/0022-3468(75)90095-0. [DOI] [PubMed] [Google Scholar]
- 3.Quinn JJ, Altman AJ, Robinson T. Adriamycin and cisplatin for hepatoblastoma. Cancer. 1985;56:1926–9. doi: 10.1002/1097-0142(19851015)56:8<1926::aid-cncr2820560805>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Ortega JA, Krailo MD, Haas JH. Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: A report from the Childrens Cancer Study Group. J Clin Oncol. 1991;9:2167–76. doi: 10.1200/JCO.1991.9.12.2167. [DOI] [PubMed] [Google Scholar]
- 5.Douglass EC, Reynolds M, Finegold M, Cantor AB, Glicksman A. Cisplatin, vincristine and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1993;11:96–9. doi: 10.1200/JCO.1993.11.1.96. [DOI] [PubMed] [Google Scholar]
- 6.von Schweinitz D, Byrd DJ, Hecker M. Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma. Eur J Cancer. 1997;33:1243–9. doi: 10.1016/s0959-8049(97)00095-6. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks-Mireaux C, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–28. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 8.Schnater JM, Aronson DC, Plaschkes J, Perilongo G, Brown J, Otte JB, et al. Surgical View of the Treatment of Patients with hepatoblastoma: : results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer. 2002;94:1111–20. [PubMed] [Google Scholar]
- 9.Ansell P, Mitchell CD, Roman E, Simpson J, Birch JM, Eden TO. Relationships between perinatal and maternal characteristics and hepatoblastoma: a report from the UKCCS. Eur J Cancer. 2005;41:741–8. doi: 10.1016/j.ejca.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan WG, Sanders DW, Grosfeld JL, Plumley DA, Rescorla FJ, Scherer LR, et al. Favorable outcome in children with Beckwith-Wiedemann syndrome and intraabdominal malignant tumors. J Pediatr Surg. 1995;30:1042–4. doi: 10.1016/0022-3468(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 11.Hermann RE, Lonsdale D. Chemotherapy, radiotherapy and hepatic lobectomy for hepatoblastoma in an infant: report of a survival. Surgery. 1970;68:383–8. [PubMed] [Google Scholar]
- 12.Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, et al. Randomized comparison of cisplatin/ vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665–75. doi: 10.1200/JCO.2000.18.14.2665. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu-Mares W, Douglass EC, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol. 2002;20:2789–97. doi: 10.1200/JCO.2002.06.155. [DOI] [PubMed] [Google Scholar]
- 14.Ang JP, Heath JA, Khurana S, Auldist A. Treatment outcomes for hepatoblastoma: an institution's experience over two decades. Pediatr Surg Int. 2007;23:103–9. doi: 10.1007/s00383-006-1834-1. [DOI] [PubMed] [Google Scholar]
- 15.Udupa KV, Navadgi SM, Mullerpatan P, Chhabra D, Shah RC, Jagannath P. Neoadjuvant chemotherapy before surgery of Hepatoblatoma. Indian J Paediatr. 2006;73:735–7. doi: 10.1007/BF02898456. [DOI] [PubMed] [Google Scholar]
- 16.Bajpai M, Pal K, Agarwala S, Seth T, Gupta AK. Midterm results with hepatectomy after preoperative chemotherapy in hepatoblastoma. Paediatr Surg Int. 2005;21:364–8. doi: 10.1007/s00383-005-1381-1. [DOI] [PubMed] [Google Scholar]
- 17.De Ioris M, Brugieres L, Zimmermann A, Keeling J, Brock P, Maibach R, et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer. 2008;44:545–50. doi: 10.1016/j.ejca.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Brown J, Dicks-Mireaux C, Philips A, Vos A, Plaschkes J, Brock P, et al. Pretreatment prognostic factors for children with hepatoblastoma-results from the International Society of Pediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418–25. doi: 10.1016/s0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 19.Haas JE, Muezynski KA, Krailo M, Ablin A, Land V, Vietti IJ, et al. Histopathology and prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer. 1989;64:1082–95. doi: 10.1002/1097-0142(19890901)64:5<1082::aid-cncr2820640520>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Aronson DC, Schnater JM, Staalman CR, Weverling GJ, Plaschkes J, Perilongo G, et al. predictive value of the pretreatment extent of disease system in hepatoblastoma: Results From the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 Study. J Clin Oncol. 2005;23:1245–52. doi: 10.1200/JCO.2005.07.145. [DOI] [PubMed] [Google Scholar]
- 21.Perilongo G, Shafford E, Maibach R, Aronson D, Brugieres L, Brock P, et al. Risk-adapted treatment for childhood hepatoblastoma.final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer. 2004;40:411–21. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Zsiros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, et al. Successful Treatment of Childhood High-Risk Hepatoblastoma With Dose-Intensive Multiagent Chemotherapy and Surgery: Final Results of the SIOPEL-3HR Study. J Clin Oncol. 2010;28:2584–90. doi: 10.1200/JCO.2009.22.4857. [DOI] [PubMed] [Google Scholar]
- 23.Beaunoyer M, Vanatta JM, Ogihara M, Strichartz D, Dahl G, Berquist WE, et al. Outcomes of transplantation in children with primary hepatic malignancy. Pediatr Transplant. 2007;11:655–60. doi: 10.1111/j.1399-3046.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 24.Qayed M, Powell C, Morgan ER, Haugen M, Katzensteinet HM. Irinotecan as maintenance therapy in high-risk hepatoblastoma. Pediatr Blood Cancer. 2010;54:761–3. doi: 10.1002/pbc.22408. [DOI] [PubMed] [Google Scholar]
- 25.Malogolowkin MH, Stanley P, Steele DA, Ortega JA. Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol. 2000;18:1279–84. doi: 10.1200/JCO.2000.18.6.1279. [DOI] [PubMed] [Google Scholar]


