Abstract
Temperature is suggested to determine the upper limit of tree life. Therefore, future climate warming may be of importance for tree distribution within the European Alps, where low temperatures limit carbon metabolism.
We focused on the effects of air and soil temperature on net photosynthesis (Pn) of Pinus cembra an evergreen climax species of the timberline ecotone of the Central Austrian Alps. Light response and temperature response curves were estimated along an altitudinal gradient ranging from the forest limit up to the krummholz limit in both summer and fall.
In general, Pn was significantly lower in fall as compared to summer. Nevertheless, independent from season mean Pn values tended to increase with elevation and were positively correlated with root zone temperatures. The specific leaf area by contrast declined with increasing elevation. Furthermore, the temperature optimum of net photosynthesis declined with increasing elevation and was positively correlated with the mean maximum air temperature of the 10 days prior the date of measurement.
Thus, our findings appear to reflect a long-term adaptation of the photosynthetic apparatus of Pinus cembra to the general temperature conditions with respect to elevation combined with a short term acclimation to the prevailing temperature regime.
Keywords: net photosynthesis, temperature, cembran pine, timberline ecotone, global warming
1. INTRODUCTION
The alpine timberline is one of the most conspicuous ecological boundaries (Körner, 2003). Rather than being an abrupt boundary the upper timberline usually forms an ecotone which stretches from the forest limit or the upper limit of the continuous closed forest canopy to the treeless alpine zone above (Tranquillini, 1979; Wieser and Tausz, 2007). Pathway up through the timberline ecotone there are two other boundaries; the tree limit and the krummholz limit. The tree limit is the upper limit of trees; higher than 2 (Wieser and Tausz, 2007) to 3 m (Körner, 2007). Such a minimum tree height ensures that the tree`s crown is well coupled to the atmosphere and protrudes above the snow cover. Above the tree limit tree species are finally deformed to krummholz, and at their highest ascent the krummholz limit they are only low growing stunted forms which either hug the ground or attain at most the height of the snow cover (Tranquillini, 1976) and thus experience microclimates similar to dwarf plants (Grace et al., 2002).
Interest currently increased in timberline-associated forest trees and ecosystems because they are expected to undergo significant alterations due to climate warming (Grace et al., 2002; Holtmeier and Broll, 2007; Walther et al., 2005; Wieser and Tausz, 2007) which is supposed to cause both an upward advancement of trees beyond the present tree limit (Walther, 2003) and denser forests at the forest limit (cf. also Grace et al., 2002). Evidence exists concerning climate warming by about 0.6 °C during the last century (Jones et al., 1998) and global change models predict even further temperature increase by 1.4 to 5.8 °C during the upcoming decades (IPCC, 2007). Such changes seem to be most pronounced in the European Alps (Beniston et al., 1997; Diaz and Bradley, 1997). Data from the timberline ecotone in the Central Austrian Alps show that during the last decade mean annual air temperature was, on average, 1 °C higher as compared to the period before and changes appear to be most pronounced in spring and summer (Tab. I).
Table I.
Annual mean, winter (December–February), spring (March–May), summer (June–August), and fall (September–November) air temperatures (°C) at timberline on Mt. Patscherkofel, Austria 1950 m a.s.l. (Klimahaus Research Station) during the period 1963–1997 and 1998–2007.
| 1963–1997 | 1998–2007 | Difference | |
|---|---|---|---|
| Annual mean | 2.2 ± 0.9 | 3.2 ± 0.5 | +1.0 |
| Winter | −4.0 ± 1.5 | −3.8 ± 1.4 | +0.2 |
| Spring | 0.4 ± 1.3 | 2.0 ± 1.0 | +1.6 |
| Summer | 9.0 ± 1.2 | 10.6 ± 1.2 | +1.6 |
| Fall | 3.5 ± 1.3 | 3.8 ± 1.5 | +0.3 |
Furthermore, within the timberline ecotone air temperature, growing season length and soil depth decrease with altitude (Wieser and Tausz, 2007), while soil temperatures found under the closed canopy at the forest limit are generally lower than those at the tree limit (Körner and Paulsen, 2004; Oberhuber, 2007). Such small scale patterns of environmental conditions along altitudinal gradients provide an ideal opportunity for comparative ecophysiological research for plant adaptation to environmental changes (cf. also Körner, 2003; Larcher, 1967) with a minimum of confounding biogeographic influences and a maximum of interpretability (Ledig and Korbobo, 1983). On the other hand, it is well established that by evolutionary adaptation the ecotype-specific temperature optimum of net photosynthesis tends to decreases with increasing altitude (Benecke and Havranek, 1980; Pisek et al., 1969; 1973; Slatyer, 1978). To our knowledge however, there is little information on the temperature adaptation of net photosynthesis of conifers within the timberline ecotone of the Central European Alps. Therefore, our specific goals were to quantify the effect of temperature along an altitudinal transect from the forest limit up to the krummholz limit on net photosynthesis of Pinus cembra L., an evergreen climax tree species of the timberline ecotone of the Central European Alps (Ellenberg, 1996; Tranquillini, 1979).
2. MATERIAL AND METHODS
2.1. Study site and climatic conditions
The study was conducted in the timberline ecotone at Mt. Patscherkofel (47° 12′ 37″ N, 11° 27′ 07″ E; Tuxer Alps as part of the Central Tyrolean Alps) south of Innsbruck/Austria, where Pinus cembra is the dominating tree species. 45-y of meteorological data recorded from the weather station at the forest limit (Klimahaus Research Station, 1950 m a.s.l.) show a mean annual air temperature of 2.5 °C, a continuous snow cover from October through May, and the possibility of frost during each summer month. The mean annual precipitation is 995 mm, with the majority falling during the growing season May through October. Over the last 15 y mean of annual soil temperature at 5 cm soil depth ranged between 2.6 and 5.7 °C with summer maxima up to 15 °C and winter minima down to −6.5 °C. The geology of the Mt. Patscherkofel region is dominated by gneisses and schist. According to the World Base for Soil Resources (FAO, 1998), a Haplic Podzol, a soil type typical for the Central Austrian Alps (Neuwinger, 1970; 1980), prevailed at the study site.
Our three study sites along a south west exposed transect were at 1 950 (forest limit), 2 100 (tree line; isolated trees ≥ 3 m height), and at 2 180 m a.s.l. (krummholz limit with outposts of short-stature individuals). At the krummholz limit only individuals with no damage due to extreme environmental conditions, such as winter desiccation, were selected. At each site air temperature, relative humidity (HMP45C, Campbell Scientific, Shepshed; UK), solar radiation (SP-Lite, Campbell Scientific), wind velocity (A100R, Campbell Scientific), precipitation (ARG100, Campbell Scientific), soil temperature (107 Temperature Probe, Campbell Scientific, Shepshed; UK) and soil water potential (EQ3 Equitensiometer, Liu, Dachau, Germany) were monitored and recorded with a CR10X data logger (Campbell Scientific) programmed to record 30-min averages of measurements taken every minute throughout the growing seasons of 2006 and 2007.
2.2. Gas exchange measurements
At each study site three representative Pinus cembra trees were selected for gas exchange measurements of the current flush of sun exposed twigs from the upper canopy. Measurements were made in fall 2006 (day 282–day 294) and in summer 2007 (day 226–day 246).
The light response of net photosynthesis (Pn) was measured in situ with a portable gas exchange system (CIRAS 1, PP Systems, Hitchin, Hertfordshire, UK) equipped with a climate-controlled PLC6 leaf chamber under standardized conditions of 15 °C air temperature, 70% relative humidity, and 360 μmol mol−1 CO2. An LED light unit was used to maintain a photosynthetic active radiation (PAR) of up to 2 000 μmol m−2 s−1 at the needle surface. After estimating Pn under non limiting PAR irradiance was decreased in 10 steps and needles were allowed to reach a stable net CO2 exchange rate before a reading was taken.
Subsequent to the determination of the light response curve the twigs were detached (commonly between 11:00–13:00) for determining the temperature response of Pn at a temperature range from 5 to 30 °C under laboratory conditions by means of a temperature controlled chamber (Walz, Effeltrich, Germany) connected to a LI-Cor (LI-6262; LI-Cor, Lincoln, NE, USA) gas analyzer operating in the differential mode. In order to avoid any embolisms twigs were re-cut under water and kept watered throughout the entire measurements (cf. also Wang et al., 2008). The gas exchange chamber was supplied with 2 L of humidified ambient air per minute to maintain the relative humidity within the chamber close to 70 to 80%. As a result leaf to air vapour pressure difference was always below 5 PakPa−1 and thus unlikely to have significantly affected stomatal conductance for our specific temperature range (Wieser, 2002).
The temperature response of Pn was measured under a PAR of 1200 μmol m−2 s−1 (artificial light source Osram HQI-T 400 W DH) and estimated as follows: the chamber temperature was raised in 2.5 to 5° steps starting at 15 °C and finishing at 30 °C. After that the temperature was decreased in similar steps down to 5 °C and finally raised again to 15 °C. The twigs were allowed to equilibrate at each measurement temperature before each reading was taken. In order to ensure that no effects of enclosure duration as well as any hysteresis in the temperature response of Pn were influencing our measurements, Pn at 15 °C was measured three times: once at the beginning of the measurement cycle, once during the middle under falling temperature and finally at the end of the cycle under decreasing temperature (cf. also Battagila et al., 1996). Furthermore, prior tests of CO2 gas exchange rates estimated in situ versus those values estimated on detached P. cembra twigs showed no significant differences (author's unpublished data).
Pn values were calculated according to von Caemmerer and Farquhar (1981) and related to total leaf surface area. The latter was calculated by multiplying the projected leaf area estimated by means of a leaf area image analyser (Skye Instruments, Landrindod, UK) with 2.6 (H. Kronfuss pers. communication). The specific leaf area (SLA) was defined as projected needle area divided by needle dry mass. The latter was determined by oven drying at 75 °C for 48 h.
The temperature response of Pn was calculated according to the parabolic function (Battagilia et al., 1998; Sall and Pettersen, 1994):
| (1) |
where Pn is the measured net photosynthesis at the measurement temperature T , Pnopt is the CO2 uptake rate at the temperature optimum (Topt), and the parameter a describes the spread of the parabola.
3. RESULTS
Environmental conditions obtained at the three study sites during fall 2006 and summer 2007, respectively are summarized in Table II. Daily mean air temperature decreased by 0.8 K per 100 m in altitude in both during fall 2006 (r2 = 0.95) and during summer 2007 (r2 = 0.98). Root zone temperature in 10 cm soil depth by contrast increased by 0.6 K per 100 m in altitude in fall 2006 (r2 = 0.86) and per 1.3 K per 100 m in altitude during summer 2007 (r2 = 0.95), respectively. Irradiance also increased with altitude, which however, was more pronounced in fall as compared to summer (Tab. II). Independent of elevation, ample precipitation (Tab. II) resulted in soil water potentials higher than −0.08 MPa during fall 2006 and higher than −0.04 MPa in summer 2007, indicating that the trees at the study sites did not experience soil water stress before both measurement periods.
Table II.
Climatic parameters for the 30 days prior to the measurements. All values are based on 30 day means, except precipitation which is based a 30-day sum.
| Site | Period | Air temperature (°C) |
Soil temperature (°C) |
Irradiance (W m−2) |
Precipitation (mm) |
|---|---|---|---|---|---|
| Forest limit | Fall 2006 Summer 2007 |
8.6 11.1 |
6.7 8.1 |
277 433 |
82 125 |
| Tree limit | Fall 2006 Summer 2007 |
7.7 10.0 |
7.2 10.5 |
344 442 |
45 135 |
| Krummholz limit | Fall 2006 Summer 2007 |
6.7 9.1 |
8.2 10.8 |
360 453 |
70 148 |
Independent from altitude the maximum rate of Pn followed seasonal trends in environmental conditions. As a consequent of shorter days, lower irradiance, lower air, and soil temperatures (Tab. II) the maximum rate of Pn was significantly lower (p = 0.021) in fall as compared to summer values (Figs. 1 and 2).
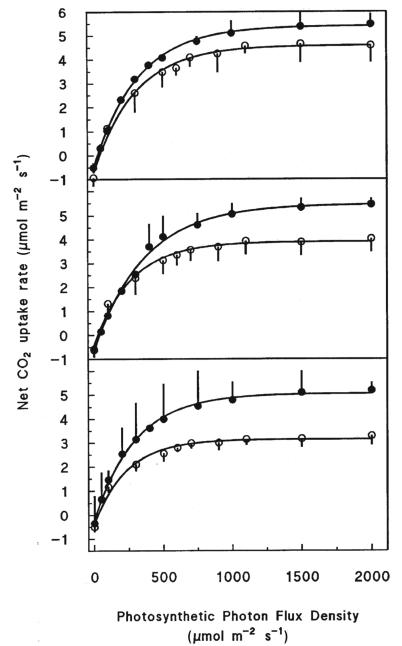
Figure 1.
Light response curves of fully current-year Pinus cembra needles at 2 180 m a.s.l. (krummholz belt; top), 2 100 m a.s.l (tree line; middle), and 1 950 m a.s.l. (forest limit; bottom) assessed in fall (open symbols) and in summer (closed symbols). Measurements were made in situ at ambient CO2, 15 °C, and 70% of relative humidity.
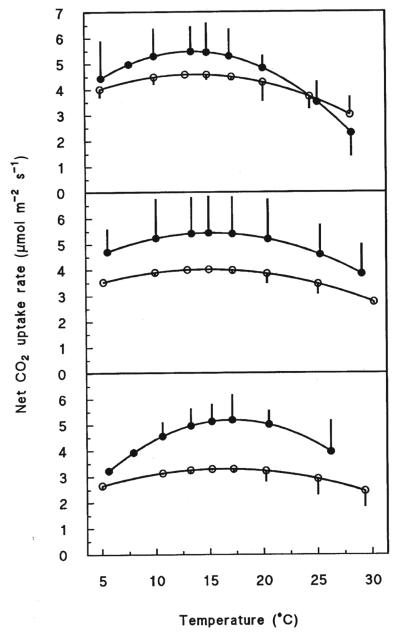
Figure 2.
The temperature responses of net photosynthesis of Pinus cembra needles at 2 180 m a.s.l. (krummholz belt; top), 2 100 m a.s.l (tree line; middle), and 1 950 m a.s.l. (forest limit; bottom) assessed in fall (open symbols) and summer (closed symbols). Measurements were made under laboratory conditions at ambient CO2, 1 200 μmol m−2 s−1 PPFD, and 70% of relative humidity.
The photosynthetic light response curves of fully developed Pinus cembra needles conducted in situ at 15 °C and ambient CO2 showed a light saturation point of close to 500 μmol m−2 s−1 (Fig. 1). Moreover, the initial slopes of the light response curves, indicating the apparent quantum efficiency, did not differ significantly with respect to altitude and averaged 0.02 ± 0.002 and 002 ± 0.001 mol mol−1 in fall 2006 and in summer 2007, respectively.
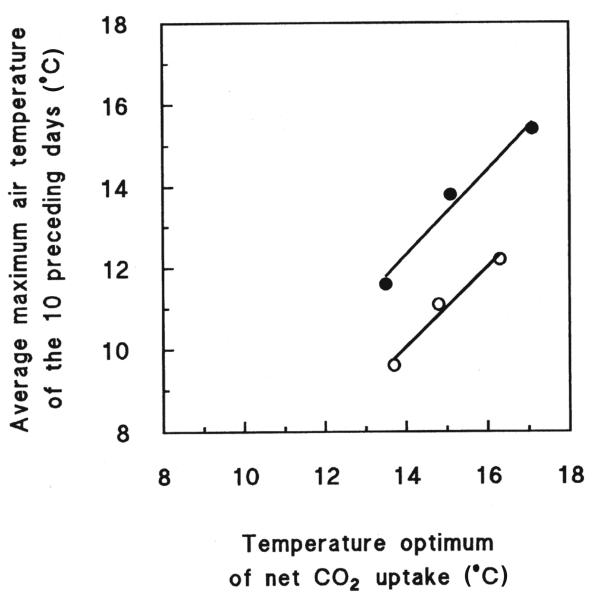
The photosynthetic temperature response curves and the parameterization of equation 1, describing the relationship between Pn and T are shown in Figure 2 and Table III, respectively. Although the temperature response curves of Pn were similar in form with respect to altitude and season, P. cembra needles showed a more marked temperature response in summer than in fall (Fig. 2). In summer the Topt of Pn declined from 17.1 °C at the forests limit, to 15.1 °C at the tree limit, and to 13.5 °C at the krummholz limit. The corresponding values for fall were 16.3, 14.8, and 13.7 °C, respectively (Tab. II). Thus the observed decline in Topt was 1.6 K per 100 m in altitude in summer (r2 = 0.98) and 1.15 K per 100 m in altitude in fall (r2 = 0.98), respectively. Furthermore, the Topt of Pn was linearly correlated to the mean maximum air temperature of the 10 days preceding the measurements obtained (Fig. 3).
Table III.
Characteristics of the photosynthetic temperature response as defined by equation (1) for fall 2006 and summer 2007. Pnopt is the maximum net CO2 uptake rate at the temperature optimum Topt, and the factor a describes the spread of the parabola.
| Site | Year | Pnopt (μmol m−2 s−1) | Topt (°C) | a |
|---|---|---|---|---|
| Forest limit | Fall 2006 | 3.30 ± 0.21 | 16.3 | 0.0050 |
| Summer 2007 | 5.19 ± 0.72 | 17.1 | 0.0148 | |
| Tree limit | Fall 2006 | 4.02 ± 0.46 | 14.8 | 0.0052 |
| Summer 2007 | 5.45 ± 1.41 | 15.1 | 0.0082 | |
| Krummholz limit | Fall 2006 | 4.59 ± 0.21 | 13.7 | 0.0076 |
| Summer 2007 | 5.48 ± 0.66 | 13.5 | 0.0147 |
Figure 3.
The relationship between the temperature optimum at which maximum net CO2 uptake occurred and the average maximum air temperature of the 10 days preceding the measurements obtained in fall (open symbols) and summer (closed symbols). Points were fit by linear regression: fall: y = 0.98x − 3.7, r2 = 0.97; summer: y = 1.05x − 2.3, r2 = 0.98.
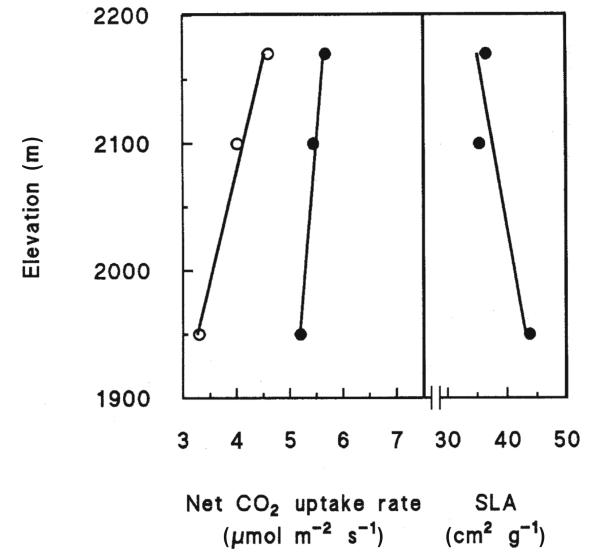
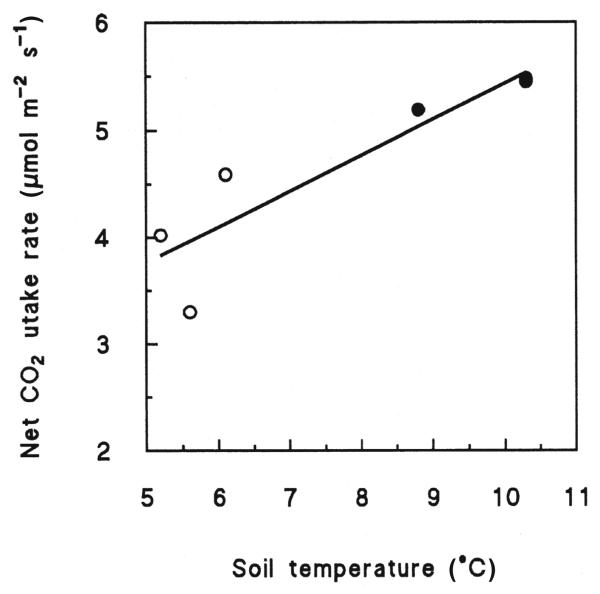
Average Pnopt increased with increasing altitude, while the opposite trend was observed for SLA (Fig. 4). Furthermore, independent of season and altitude of all environmental parameters examined Pnopt was best correlated with the daily mean soil temperature of the 10 preceding days (Fig. 5).
Figure 4.
Mean maximum net CO2 uptake rate obtained in fall (open symbols) and summer (closed symbols) and specific leaf area (SLA) with respect to elevation in the timberline ecotone at Mt Patscherkofel between 1 950 and 2 175 m a.s.l. Points were fit by linear regression: mean maximum net CO2 uptake rate fall: y = 0.0057x − 7.9, r2 = 0.98; mean maximum net CO2 uptake rate summer: y = 0.0021x + 1.1, r2 = 0.97; SLA: y = −0.0037x + 115, r2 = 0.82.
Figure 5.
The relationship between the mean maximum net CO2 uptake rate obtained in fall (open symbols) and summer (closed symbols) and the corresponding daily mean soil temperature of the 10 preceding days. Points were fit by linear regression: y = 0.33x + 2.1, r2 = 0.81.
4. DISCUSSION
Our Pnopt values for current-year Pinus cembra needles obtained during the summer ranged from 5.19 to 5.48 μmol m−2 s−1, which is within the reported range of the maximum photosynthetic capacity at ambient CO2 (sensu Larcher, 2001) obtained in situ for conifers in the timberline ecotone of the European Alps (2.9−7.5 μmol m−2 s−1; Wieser and Tausz, 2007). At the alpine timberline variations in Pn of cembran pine tracked seasonal changes in climate. Several studies (Cartellieri, 1935; Häsler. 1994; Pisek and Winkler, 1958; Wieser, 2004) have shown that apparent Pn of Pinus cembra needles in fall is about 30 to 50% lower as compared to maximum CO2 uptake rates obtained in mid-summer. In comparison, in this study we observed Pnopt to be reduced by 26 to 36 in fall as compared to summer. In general, variations in air temperature within an entire growing season do not affect CO2 uptake of Pinus cembra to a large extent, because the temperature response curve of Pn is relatively wide. In a growing season round study Pn of cembran pine at the forest limit has been shown to operate at more than 90% of its maximum over a range of 8 to 10 K (Wieser, 2004). As a matter of fact, thermal limitation of Pn is primarily restricted to situations during which carbon uptake is restricted by low irradiance and an accompanied lower air and needle temperatures. Thus, due to the effect of temperature on enzyme activity (Sternberg et al., 1995) the temperature optimum of Pn shifts with irradiance towards lower values when photon flux density is low and towards higher values when photon flux density is high (Pisek et al., 1969; Pisek and Winkler, 1958; Wieser, 1997).
In our study we also observed a paramount influence of soil temperature on Pnopt of Pinus cembra, emphasizing the role root zone temperature on foliar gas exchange (Day et al., 1989; DeLucia, 1986) and growth (Gruber et al., 2009a) within the timberline ecotone. Our findings on the effect of soil temperature on Pn with respect to season and altitude are corroborated by observations of Havranek (1972) who found a linear correlation between of daily carbon gain of Pinus cembra seedlings at timberline near Obergurgl, Austria (2070 m a.s.l.) and daily mean soil temperature at 10 cm soil depth between 0 and 7 °C, while higher root zone temperatures lose their role as a limiting factor for carbon gain. Moreover, growing season round artificial soil warming at our study plot at the krummholz limit triggered a raise in stem diameter increment of stunted Pinus cembra trees (Gruber et al., 2009b).
Environmental conditions change with increasing altitude. Our study plots were selected along an altitudinal gradient within the timberline ecotone ranging from the forest limit (1950 m a.s.l.) up to the krummholz limit (2180 m a.s.l) and hence included the upper distribution limit of Pinus cembra. Along this altitudinal gradient air temperature and irradiance decreased with increasing altitude while root zone temperature in 10 cm soil depth increased with increasing altitude. The observed lower soil temperatures at the forest limit can mainly be attributed to the closed canopy which preventing soil heat flux and radiative warming of the rooting zone (Aulitzky, 1961; Gruber et al., 2009a; Körner and Paulsen 2004; Oberhuber 2007) when compared to the open stands at the tree and the krummholz limit.
As a general rule, Pn of Pinus cembra needles increased with increasing altitude in either summer and fall as also observed for Pnopt of Espeletia schulztii (Rada et al., 1998)and E. neriifolia (Cavieres et al., 2000) along an elevational transect in the timberline ecotone of the Venezuelan Andes. Such findings suggest a higher efficiency of carbon uptake per unit leaf area, which might be attributed to altitude related difference in needle morphology. In our study we found that the SLA of Piuns cembra needles declines with increasing elevation. Similar altitudinal reductions in the SLA of conifers have also been observed by others (Benecke et al., 1981; Hurtin and Marshall, 2000; Richardson et al., 2001). On the other hand, dry weight related foliar nitrogen concentrations of trees at high elevation stands tend to be similar or even higher when compared to low elevation sites (Richardson et al., 2001; Sparks and Ehleringer, 1997). Thus, the combination of thicker needles (≈lower SLA; considered as a morphological feature of sun type needles) with high nitrogen contents may cause the amount of photosynthetic enzymes to increase with increasing elevation and hence provides a high cost-effective system for plants in marginal habitats where assimilation is restricted to a short growing season (cf. also Ledig and Korbobo, 1983).
Moreover, the temperature optimum of Pn shows a clear decline with increasing elevation. Leaves of trees at timberline in Europe (Pisek et al., 1969, 1973), Australia (Slatyer, 1978), and New Zealand (Benecke and Havranek, 1980) display a significantly lower ecotype-specific Topt of Pn than leaves from trees at lower elevation sites. As shown in this study for Pinus cembra even within the timberline ecotone the Topt of Pn decreased with altitude and was linearly correlated to the mean maximum air temperature of the 10 d prior the date of measurement. Similar correlations between Topt for Pn and the prevailing air temperature at the study site were also obtained for Eucalyptus pauciflora (Slatyer, 1977), Eucalyptus pauciflora (Battagila et al., 1996; Cunningham and Read, 2002), Espeletia schultzii (Rada et al., 1998) and Espeletia neriifolia (Cavieres et al., 2000).
In conclusion, our findings appear to reflect a long-term adaptation of the photosynthetic apparatus of Pinus cembra to the general temperature conditions at each site combined with a short term acclimation to the prevailing temperature regime. Taking into account that growth and regeneration of trees within the timberline ecotone is mainly controlled by temperature (Körner, 2003), trees within the timberline ecotone may benefit from future global warming as temperature-driven respiratory carbon losses are likely to be over-compensated by the concurrently increasing gross primary production (Wieser and Stöhr, 2005). Future climate warming is also supposed to cause both an upward advancement of trees beyond the present tree limit (Walther, 2003) and denser forests below the forest limit (Grace et al., 2002). However, one has to be aware that episodic extreme events by e.g. drought, severe frost during the growing season or biotic stress (by pathogens/herbivores) rather than the gradual temperature increase will control tree population dynamics within the timberline ecotone of the Central European Alps in the future environment (Wieser et al., 2009).
Acknowledgements
This work was supported by the Austrian Science Fund (Project No. FWF P18819-B03 “Temperature dependence of Pinus cembra (L.) stem growth and respiration along an altitudinal transect”).
REFERENCES
- Aulitzky H. Die Bodentemperaturverhältnisse in der Kampfzone oberhalb der Waldgrenze und im subalpinen Zirben-Lärchenwald. Mitt. Forstl. Bundesvers. Mariabrunn. 1961;59:153–208. [Google Scholar]
- Battagila M, Beadle C, Loughead S. Photosynthetic temperature response of Eucalyptus globulus and Eucalyptus nitens. Tree Physiol. 1996;16:81–99. doi: 10.1093/treephys/16.1-2.81. [DOI] [PubMed] [Google Scholar]
- Benecke U, Havranek WM. Gas exchange of trees at altitudes up to timberline, Craigieburn Range, New Zealand. In: Benecke U, Davies MD, editors. Mountain environments and subalpine tree growth. 1980. pp. 195–212. Technical report 70, New Zealand Forest Service. [Google Scholar]
- Benecke U, Schulze E-D, Matyssek R, Havranek WM. Environmental control of CO2-assimilation and leaf conductance in Larix decidua Mill. I. A comparison of contrasting natural environments. Oecologia. 1981;50:54–61. doi: 10.1007/BF00378793. [DOI] [PubMed] [Google Scholar]
- Beniston M, Diaz HF, Bradley RS. Climate change at high elevation sites: an overview. Clim. Change. 1997;36:233–251. [Google Scholar]
- Cartellieri E. Jahresgang von osmotischem Wert, Transpiration und Assimilation einiger Ericaceen der alpinen Zwergstrauchheide und von Pinus cembra. Jahrb. Wiss. Bot. 1935;82:460–506. [Google Scholar]
- Cavieres LA, Rada F, Azocar A, Garcia-Nunez C, Cabera HM. Gas exchange and low temperature resistance in two tropical high mountain tree species in the Venezuelan Andes. Acta Oecol. 2000;21:203–211. [Google Scholar]
- Cunningham SC, Read J. Comparison of temperature and tropical rainforest tree species: photosynthetic response to temperature. Oecologia. 2002;133:112–119. doi: 10.1007/s00442-002-1034-1. [DOI] [PubMed] [Google Scholar]
- Day TA, DeLucia EH, Smith WK. Influence of cold soil and snow cover on photosynthesis and leaf conductance in two Rocky Mountain conifers. Oecologia. 1989;80:546–552. doi: 10.1007/BF00380080. [DOI] [PubMed] [Google Scholar]
- DeLucia EH. Effect of low root temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engelmann spruce (Picea engelmanii Parry ex Engelm.) seedlings. Tree Physiol. 1986;2:143–154. doi: 10.1093/treephys/2.1-2-3.143. [DOI] [PubMed] [Google Scholar]
- Diaz HF, Bradley RS. Temperature variations during the last century at high elevation sites. Clim. Change. 1997;36:253–279. [Google Scholar]
- Ellenberg H. Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. Vol. 5. Auflage; Ulmer, Stuttgart: 1996. p. 1095. [Google Scholar]
- FAO, ISRIC, and ISSS . World reference for soil resources. FAO; Rome: 1998. p. 109. [Google Scholar]
- Grace J, Berninger F, Nagy L. Impact of climate change on the treeline. Ann. Bot. 2002;90:537–544. doi: 10.1093/aob/mcf222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Zimmermann J, Wieser G, Oberhuber W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine timberline ecotone. Ann. For. Sci. 2009a;66:503. doi: 10.1051/forest/2009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Wieser G, Oberhuber W. Effects of simulated soil temperature on stem diameter increment of Pinus cembra at the alpine timberline: a new approach based on root zone roofing. Eur. J. For. Res. 2009b doi: 10.1007/s10342-009-0305-3. Doi: 10.1007/s10342-009-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsler R. Ecophysiological investigations on cembran pine at timberline in the Alps, an overview. In: Schmidt WC, Holtmeier F-K, editors. Proceedings of an International workshop on Subalpine stone pines and their environment: the status of knowledge; St. Moritz, Switzerland. Sept. 5-11; 1994. pp. 61–66. Tech. Rep. INT-GTR-309, US Department of Agriculture, Forest Service, Intermountain Research Station, Ogden, UT. [Google Scholar]
- Havranek WM. Über die Bedeutung der Bodentemperatur für die Photosynthese und die Transpiration junger Forstpflanzen und für die Stoffproduktion an der Waldgrenze. Angew. Bot. 1972;46:101–116. [Google Scholar]
- Holtmeier F-K, Broll G. Treeline advance - driving processes and adverse factors. Landscape Online. 2007;1:1–33. [Google Scholar]
- Hurtin KR, Marshall JD. Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia. 2000;123:32–40. doi: 10.1007/s004420050986. [DOI] [PubMed] [Google Scholar]
- IPCC . Climate change. Cambridge University Press; Cambridge: 2007. 2007. [Google Scholar]
- Jones PD, Wigley TML, Folland CK, Parker DE, Angelli JK, Jebedeff S, Hansen JE. Evidence of global warming in the last decade. Nature. 1988;332:791. [Google Scholar]
- Körner Ch. Alpine plant life: functional plant ecology of high mountain ecosystems. 2nd ed. Springer; Berlin: 2003. p. 344. [Google Scholar]
- Körner C. Climatic treelines: conventions, global patterns, causes. Erdkunde. 2007;61:316–324. [Google Scholar]
- Körner C, Paulsen L. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004;31:713–732. [Google Scholar]
- Larcher W. Die Berge einzigartiges Versuchsfeld der Natur. Jahrb. Ver. Schutze Alpenpflanzen Tiere. 1967;32:1–7. [Google Scholar]
- Larcher W. Ökophysiologie der Pflanzen: Leben, Leistung und Stressbewältigung der Pflanzen in ihrer Umwelt, Ulmer, Stuttgart. 2001:408. [Google Scholar]
- Ledig FT, Korbobo DR. Adaptation of sugar maple populations along altitudinal gradients: photosynthesis, respiration, and specific leaf weight. Am. J. Bot. 1983;70:256–265. [Google Scholar]
- Neuwinger I. Böden der subalpinen und alpinen Stufe in den Tiroler Alpen. Mitt. Ostalpin-Dinarischen Ges. 1970;11:135–150. [Google Scholar]
- Neuwinger I. Erwärmung, Wasserrückhalt und Erosionsbereitschaft subalpiner Böden. Mitt. Forstl. Bundesvers. Wien. 1980;129:113–144. [Google Scholar]
- Oberhuber W. Limitation by growth processes. In: Wieser G, Tausz M, editors. Trees at their upper limit. Treelife limitation, at the alpine timberline, Plant Ecophysiology. Vol. 5. Springer; Dorthrecht, The Netherlands: 2007. pp. 131–143. [Google Scholar]
- Pisek A, Larcher W, Moser W, Pack I. Kardinale Temperaturbereiche der Photosynthese und Grenztemperaturen des Lebens der Blätter verschiedener Spermatophyten. III. Temperaturabhängigkeit und optimaler Temberaturbereich der Netto-Photosynthese. Flora Abt. B. 1969;158:608–630. [Google Scholar]
- Pisek A, Larcher W, Vegis A, Napp-Zinn K, Precht H. The normal temperature range. In: Christophersen J, Hensel H, Larcher W, editors. Temperature and life. Springer; Berlin, Heidelberg, New York: 1973. pp. 102–194. [Google Scholar]
- Pisek A, Winkler E. Assimilationsvermögen und Respiration der Fichte (Picea excelsa LINK) in verschiedenen Höhenlagen und der Zirbe (Picea abies L.) an der alpinen Waldgrenze. Planta. 1958;51:518–543. [Google Scholar]
- Rada F, Azocar A, Gonzales J, Briceno B. Leaf gas exchange in Espeletia schultzii Wedd, a giant caulescent rosette species, along an altitudinal gradient in the Venezuelan Andes. Acta Oecol. 1998;19:73–79. [Google Scholar]
- Richardson AD, Berlyn GP, Gregorie TG. Spectral reflectance of Picea rubens (Pinaceae) and Abies balsamifera (Pinaceae) needles along an elevational gradient, Mt. Moosilauke, New Hampshire, USA. Am. J. Bot. 2001;88:667–676. [PubMed] [Google Scholar]
- Sall T, Pettersen P. A model of photosynthetic acclimation as a special case of reaction norms. Theor. Biol. 1994;166:1–8. [Google Scholar]
- Slatyer RO. Altitudinal variation in the photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. ex Sreng. I. Seasonal changes under field conditions in the Snowy Mountains area of South-east australy. Aust. J. Bot. 1977;25:1–20. [Google Scholar]
- Slatyer RO. Altitudinal variation in the photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. Ex Sreng. VII. Relationships between gradients of field temperature and photosynthetic temperature optima in the Snowy Mountains area. Aust. J. Bot. 1978;26:111–121. [Google Scholar]
- Sternberg P, De Lucia EH, Schoettle AW, Smolander H. Photosynthetic light capture and processing from cell to canopy. In: Smith WK, Hinckley TM, editors. Ecophysiology of coniferous forests. Academic Press; San Diego: 1995. pp. 3–38. [Google Scholar]
- Tranquillini W, Lange OL. Water relations at timberline. In: Kappen L, Schulze E-D, editors. Water relations and plant life. Problems and modern approaches, Ecological Studies. Vol. 19. Springer; Berlin, Heidelberg, New York: 1976. pp. 473–491. [Google Scholar]
- Tranquillini W. Ecol. Stud. Vol. 31. Springer Verlag; Berlin: 1979. Physiological ecology of the alpine timberline; p. 137. [Google Scholar]
- Von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Walther G-R. Plants in a warmer world. Perspect. Plant. Ecol. Evol. Syst. 2003;6:169–185. [Google Scholar]
- Walther G-R, Beißner S, Pott R. Climate change and high mountain vegetation shifts. In: Broll G, Keplin B, editors. Mountain ecosystems, Studies in Treeline Ecology. Springer; Berlin, Heidelberg: 2005. pp. 77–95. [Google Scholar]
- Wang Q, Ilo A, Tenhunen J, Kalkubari Y. Annual and seasonal variations in photosynthetic capacity of Fagus crenata along an elevation gradient in the Naeba Mountains, Japan. Tree Physiol. 2008;28:277–285. doi: 10.1093/treephys/28.2.277. [DOI] [PubMed] [Google Scholar]
- Wieser G. Carbon dioxide gas exchange of cembran pine (Pinus cembra) at the alpine timberline during winter. Tree Physiol. 1997;17:473–477. doi: 10.1093/treephys/17.7.473. [DOI] [PubMed] [Google Scholar]
- Wieser G. Exchange of trace gases at the tree - atmosphere interface: ozone. In: Gasche R, Papen H, Rennenberg H, editors. Trace gas exchange in forest ecosystems, Tree Physiology. Vol. 3. Kluwer Academic Publishers; Dordrecht, Boston, London: 2002. pp. 211–226. [Google Scholar]
- Wieser G. Environmental control of carbon dioxide gas exchange in needles of a mature Pinus cembra tree at the alpine timberline during the growing season. Phyton. 2004;44:145–153. [Google Scholar]
- Wieser G, Matyssek R, Luzian R, Zwerger P, Pindur P, Oberhuber W, Gruber A. Effects of atmospheric and climate change at the timberline of the Central European Alps. Ann. For. Sci. 2009;66:402. doi: 10.1051/forest/2009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser G, Stöhr D. Net ecosystem carbon dioxide exchange dynamics in a Pinus cembra forest at the upper timberline in the central Austrian Alps. Phyton. 2005;45:233–242. [Google Scholar]
- Wieser G, Tausz M. Trees at their upper limit: Treelife limitation at the alpine timberline, Plant Ecophysiology. Vol. 5. Springer; 2007. p. 232. [Google Scholar]