Abstract
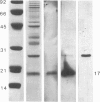
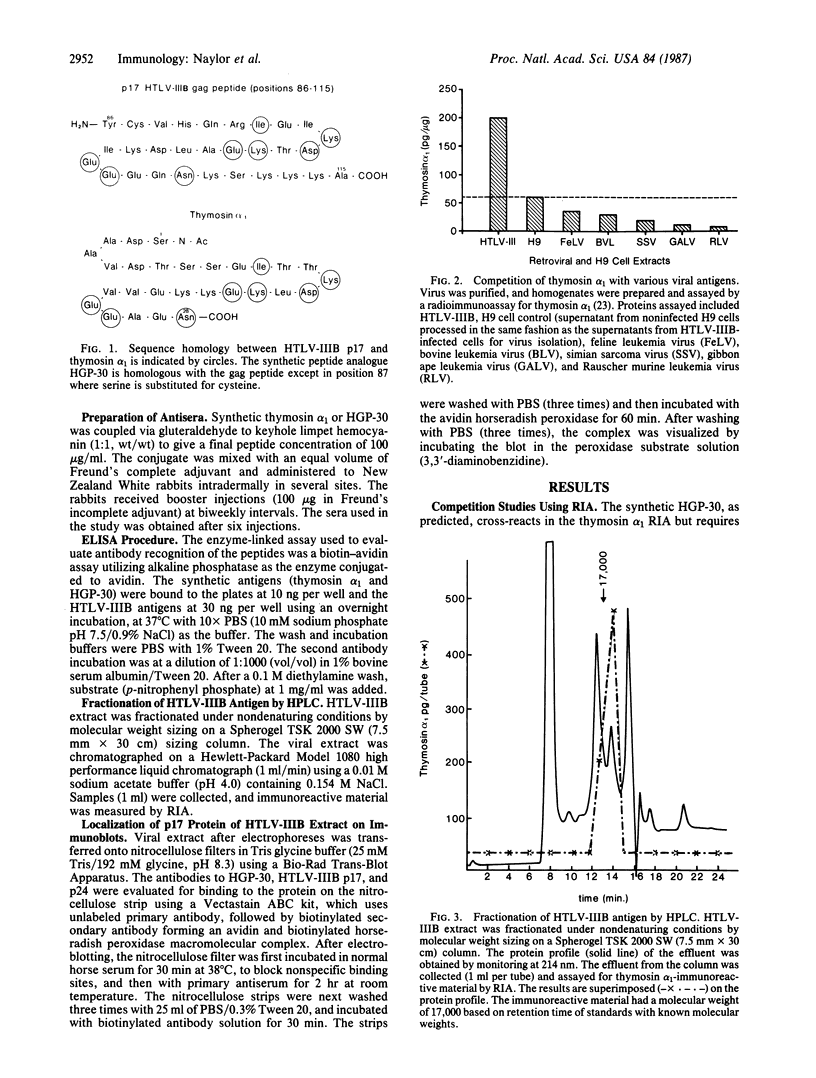
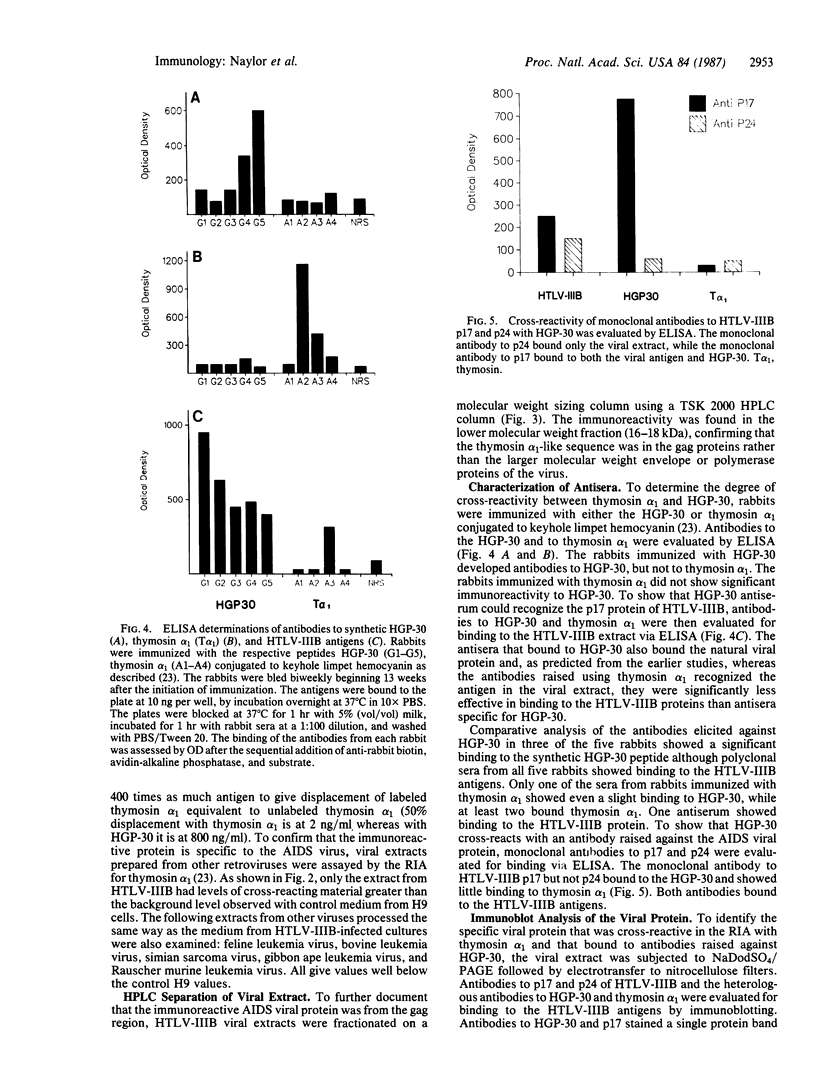
We have reported that an antiserum prepared against thymosin alpha 1 [which shares a region of homology with the p17 protein of the acquired immunodeficiency syndrome (AIDS)-associated human immunodeficiency virus] effectively neutralized the AIDS virus and prevented its replication in H9 cells. Using HPLC and immunoblot analysis, we have identified from a clone B, type III human T-lymphotropic virus (HTLV-IIIB) extract a protein with a molecular weight of 17,000 that is immunoreactive with thymosin alpha 1. In contrast, no immunoreactivity was found in retroviral extracts from a number of nonhuman species including feline, bovine, simian, gibbon, and murine retroviruses. Heterologous antiserum prepared against a 30-amino acid synthetic peptide analogue (HGP-30) does not cross-react with thymosin alpha 1 but does react specifically with the p17 protein of the AIDS virus in a manner identical to that seen with an HTLV-IIIB p17-specific monoclonal antibody. The demonstration that this synthetic analogue is immunogenic and that antibodies to HGP-30 cross-react not only with the synthetic peptide but also with the HTLV-IIIB p17 viral protein provides an additional, and potentially more specific, candidate for development of a synthetic peptide vaccine for AIDS. In addition, the p17 synthetic peptide (HGP-30) may prove to be useful in a diagnostic assay for the detection of AIDS virus infection in seronegative individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann A. J. The acquired immunodeficiency syndrome in infants and children. Ann Intern Med. 1985 Nov;103(5):734–737. doi: 10.7326/0003-4819-103-5-734. [DOI] [PubMed] [Google Scholar]
- Biggar R. J., Taylor P. H., Goldstein A. L., Melbye M., Ebbesen P., Mann D. L., Strong D. M. Thymosin alpha 1 levels and helper:suppressor ratios in homosexual men. N Engl J Med. 1983 Jul 7;309(1):49–49. doi: 10.1056/NEJM198307073090113. [DOI] [PubMed] [Google Scholar]
- Ciobanu N., Welte K., Kruger G., Venuta S., Gold J., Feldman S. P., Wang C. Y., Koziner B., Moore M. A., Safai B. Defective T-cell response to PHA and mitogenic monoclonal antibodies in male homosexuals with acquired immunodeficiency syndrome and its in vitro correction by interleukin 2. J Clin Immunol. 1983 Oct;3(4):332–340. doi: 10.1007/BF00915794. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Bach J. F., Safai B. Low serum thymic hormone levels in patients with acquired immunodeficiency syndrome. N Engl J Med. 1983 Jul 7;309(1):48–49. doi: 10.1056/NEJM198307073090112. [DOI] [PubMed] [Google Scholar]
- Elie R., Laroche A. C., Arnoux E., Guérin J. M., Pierre G., Malebranche R., Seemayer T. A., Dupuy J. M., Russo P., Lapp W. S. Thymic dysplasia in acquired immunodeficiency syndrome. N Engl J Med. 1983 Apr 7;308(14):841–842. doi: 10.1056/NEJM198304073081412. [DOI] [PubMed] [Google Scholar]
- Gallo R. C. The AIDS virus. Sci Am. 1987 Jan;256(1):46–56. [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature. 1986 May 15;321(6067):244–246. doi: 10.1038/321244a0. [DOI] [PubMed] [Google Scholar]
- Grody W. W., Fligiel S., Naeim F. Thymus involution in the acquired immunodeficiency syndrome. Am J Clin Pathol. 1985 Jul;84(1):85–95. doi: 10.1093/ajcp/84.1.85. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Reuben J. M., Rios A., Mansell P. W., Newell G. R., McClure J. E., Goldstein A. L. Elevated serum thymosin alpha 1 levels associated with evidence of immune dysregulation in male homosexuals with a history of infectious diseases or Kaposi's sarcoma. N Engl J Med. 1983 Jan 6;308(1):45–46. doi: 10.1056/nejm198301063080113. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Narayan O. Neutralizing antibodies to visna lentivirus: mechanism of action and possible role in virus persistence. J Virol. 1986 Jul;59(1):37–44. doi: 10.1128/jvi.59.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler C. M., Schulof R. S., Alabaster O., Goldstein A. L., Naylor P. H., Phillips T. M., Luban N. L., Kelleher J. F., Reaman G. H. Inverse correlation between age related abnormalities of T-cell immunity and circulating thymosin alpha 1 levels in haemophilia A. Br J Haematol. 1984 Oct;58(2):325–336. doi: 10.1111/j.1365-2141.1984.tb06091.x. [DOI] [PubMed] [Google Scholar]
- Kreiss J. K., Lawrence D. N., Kasper C. K., Goldstein A. L., Naylor P. H., McLane M. F., Lee T. H., Essex M. Antibody to human T-cell leukemia virus membrane antigens, beta 2-microglobulin levels, and thymosin alpha 1 levels in hemophiliacs and their spouses. Ann Intern Med. 1984 Feb;100(2):178–182. doi: 10.7326/0003-4819-100-2-178. [DOI] [PubMed] [Google Scholar]
- Lange J. M., Coutinho R. A., Krone W. J., Verdonck L. F., Danner S. A., van der Noordaa J., Goudsmit J. Distinct IgG recognition patterns during progression of subclinical and clinical infection with lymphadenopathy associated virus/human T lymphotropic virus. Br Med J (Clin Res Ed) 1986 Jan 25;292(6515):228–230. doi: 10.1136/bmj.292.6515.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. M., Paul D. A., Huisman H. G., de Wolf F., van den Berg H., Coutinho R. A., Danner S. A., van der Noordaa J., Goudsmit J. Persistent HIV antigenaemia and decline of HIV core antibodies associated with transition to AIDS. Br Med J (Clin Res Ed) 1986 Dec 6;293(6560):1459–1462. doi: 10.1136/bmj.293.6560.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- McClure J. E., Lameris N., Wara D. W., Goldstein A. L. Immunochemical studies on thymosin: radioimmunoassay of thymosin alpha 1. J Immunol. 1982 Jan;128(1):368–375. [PubMed] [Google Scholar]
- Montagnier L. Lymphadenopathy-associated virus: from molecular biology to pathogenicity. Ann Intern Med. 1985 Nov;103(5):689–693. doi: 10.7326/0003-4819-103-5-689. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Naylor P. H., Friedman-Kien A., Hersh E., Erdos M., Goldstein A. L. Thymosin alpha 1 and thymosin beta 4 in serum: comparison of normal, cord, homosexual and AIDS serum. Int J Immunopharmacol. 1986;8(7):667–676. doi: 10.1016/0192-0561(86)90001-9. [DOI] [PubMed] [Google Scholar]
- Naylor P. H., Schulof R. S., Sztein M. B., Spira T. J., McCurdy P. R., Darr F., Kessler C. M., Simon G. L., Goldstein A. L. Thymosin in the early diagnosis and treatment of high risk homosexuals and hemophiliacs with AIDS-like immune dysfunction. Ann N Y Acad Sci. 1984;437:88–99. doi: 10.1111/j.1749-6632.1984.tb37125.x. [DOI] [PubMed] [Google Scholar]
- Oleske J., Minnefor A., Cooper R., Jr, Thomas K., dela Cruz A., Ahdieh H., Guerrero I., Joshi V. V., Desposito F. Immune deficiency syndrome in children. JAMA. 1983 May 6;249(17):2345–2349. [PubMed] [Google Scholar]
- Reichert C. M., O'Leary T. J., Levens D. L., Simrell C. R., Macher A. M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983 Sep;112(3):357–382. [PMC free article] [PubMed] [Google Scholar]
- Rubinstein A., Novick B. E., Sicklick M. J., Bernstein L. J., Incefy G. S., Naylor P. H., Goldstein A. L. Circulating thymulin and thymosin-alpha 1 activity in pediatric acquired immune deficiency syndrome: in vivo and in vitro studies. J Pediatr. 1986 Sep;109(3):422–427. doi: 10.1016/s0022-3476(86)80111-1. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Gallo R. C. Human T-lymphotropic retroviruses in adult T-cell leukemia-lymphoma and acquired immune deficiency syndrome. J Clin Immunol. 1984 Nov;4(6):415–423. doi: 10.1007/BF00916570. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Sun D. K., Thornton A. H., Naylor P. H., Goldstein A. L. Neutralization of HTLV-III/LAV replication by antiserum to thymosin alpha 1. Science. 1986 May 30;232(4754):1135–1137. doi: 10.1126/science.3010464. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977 Jul;88(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Stahl R. E., Friedman-Kien A., Dubin R., Marmor M., Zolla-Pazner S. Immunologic abnormalities in homosexual men. Relationship to Kaposi's sarcoma. Am J Med. 1982 Aug;73(2):171–178. doi: 10.1016/0002-9343(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Weber J. N., Clapham P. R., Weiss R. A., Parker D., Roberts C., Duncan J., Weller I., Carne C., Tedder R. S., Pinching A. J. Human immunodeficiency virus infection in two cohorts of homosexual men: neutralising sera and association of anti-gag antibody with prognosis. Lancet. 1987 Jan 17;1(8525):119–122. doi: 10.1016/s0140-6736(87)91964-7. [DOI] [PubMed] [Google Scholar]