Abstract
Self/nonself perception governs mate selection in most eukaryotic species. It relies on a number of natural barriers that act before, during and after copulation. These hurdles prevent a costly investment into an embryo with potentially suboptimal genetic and immunological properties and aim at discouraging fertilization when male and female gametes exhibit extensive sharing of alleles. Due to the fact that several genes belonging to the extended major histocompatibility complex (xMHC) carry out crucial immune functions and are the most polymorphic within vertebrate genomes, it is likely that securing heterozygosity and the selection of rare alleles within this gene complex contributes to endowing the offspring with an advantage in fighting infections. Apart from MHC class I and II antigens, the products of several other genes within the xMHC are candidates for participating in mate choice, especially since the respective loci are subject to long-range linkage disequilibrium which may aid to preserve functionally connected alleles within a given haplotype. Among these loci are polymorphic odorant receptor genes that are expressed not only in the olfactory epithelium, but also within male reproductive tissues. They may thus not only be of importance in olfaction-influenced mate choice, by recognizing MHC-dependent individual-specific olfactory signals, but could also guide spermatozoa along chemical gradients to their target, the oocyte. By focusing on the human HLA complex and genes within its vicinity, we show here that the products of several xMHC-specified molecules might be involved in self/nonself perception during reproduction. Although the molecular details are often unknown, the existence of highly diverse, yet intertwined pre- and post-copulatory barriers suggests that xMHC-encoded proteins may be important for various stages of mate choice, germ cell development, as well as embryonic and foetal life in mammals and other vertebrates. Many of these genes should thus be regarded as crucial not only within the immune system, but also in reproduction.
Key words: cryptic female choice, extended major histocompatibility complex, gene polymorphism, human leukocyte antigen complex, mate choice, odorant receptor, reproduction, self/nonself discrimination
Introduction
Charles Darwin's statement that “the females...select the more agreeable partners”1 has greatly influenced our concepts about how two potential mating partners evaluate their capability to create viable offspring. Although religious, social, dynastic and pecuniary considerations have had an influence on human mate choice for thousands of years, up to the present,2 visual, auditory and chemical (e.g., olfactory) cues play a role in humans as well3–11 and govern mate choice in other vertebrates,12–20 invertebrates,21–25 plants26 and even in fungi.27
Birds provide particularly instructive examples: the elaborate tail feathers of male peacocks signal to hens that the male is the possessor of ‘good genes’ that allowed him to survive despite the obvious disadvantage of having to carry his fancy tail around. Similarly, the sophisticated songs of the males in some bird species and the way in which these songs are displayed appear to allow choosy females to enhance their breeding success by selecting males according to their ‘personalities.’28 And even in a flying mammal such as the sac-winged bat (Saccopteryx bilineata), males use their wings in a fan-like fashion to direct mixtures of individual-specific odors from glands below their wings towards females in order to catch their attention.29 In mice, chemical signals received via olfaction play a decisive role in pre-copulatory mate choice: in a seminal publication, Yamazaki and colleagues demonstrated that these animals can sense the type of genes belonging to the major histocompatibility complex (MHC) of another mouse through MHC-dependent odors.30 Mice exploit this ‘knowledge’ for choosing an MHC-dissimilar mate, and comparable results have been obtained in several further vertebrate species, from fish to man.31–36 Therefore, apart from its well-established, crucial role in innate and adaptive immunity,37–39 the MHC appears also important in mate choice in the vast majority of vertebrates which have to date been investigated regarding this aspect. The exceptional degree of polymorphism exhibited by several MHC loci is thus not only advantageous in Self/Nonself recognition processes during T cell development40 and presentation of foreign antigens by MHC molecules to receptors on effector cells,41–43 but also in reproduction, where a number of barriers help in securing the creation of genetically and immunologically optimal offspring.
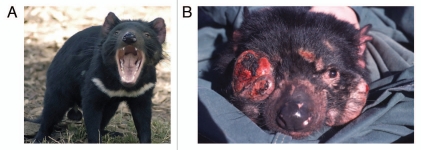
Not all vertebrate species, however, appear to adhere to the idea that it is desirable to combine dissimilar MHC alleles in the offspring to fight infections effectively. Instead, they may prefer to endow their young with an ‘optimal’ number of alleles within the MHC,44,45 or rely on other criteria.46,47 These results indicate that although individuals with identical or similar MHC types are usually not attracted by each other, additional factors can modulate these aversions and preferences or replace them altogether.48 On the other hand, possessing an MHC with a low degree of polymorphism15 can be associated with very severe problems, as demonstrated by the Tasmanian devil (Sarcophilus harrisii) (Fig. 1), whose population is threatened by a clonal tumor (devil facial tumor disease)49 exhibiting exceptional properties: since there are only marginal differences between the MHC antigens on the malignant cells and those on normal cells of this marsupial,50 the tumor cells cannot be recognized as ‘nonself’ by the immune system of infected animals. Therefore, the tumor is not rejected, in consequence of which more than half of the Tasmanian devil population has already succumbed to the disease.51
Figure 1.
Limited MHC diversity in the Tasmanian devil facilitates the spread of a contagious tumor. (A) Tasmanian devil. (Source: wikipedia.org, Photo: Wayne McLean). (B) Tasmanian devil with devil facial tumor disease. Molecular genetic and karyotypic evidence indicates that this unusual infective cancer originated in a single individual about 15 years ago, and can thus be regarded as ‘a clonally reproducing mammal that is an obligate parasite’.49 (Source: ref. 224. Photo: Menna Jones).
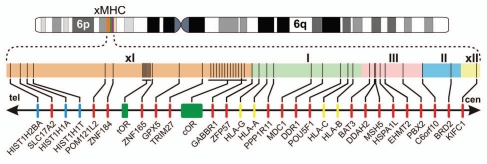
In the following sections, we will initially discuss which barriers have to be surmounted in mate choice, followed by a description of the products of those MHC genes that are likely to participate in the various stages of reproduction, focusing mainly on the situation in man. We will then examine to what extent pre- and post-copulatory mate selection relies on MHC genes. In particular, we will explore possible molecular mechanisms which might help females to evaluate the suitability of potential mates. However, we will not restrict ourselves just to the MHC, but extend the analysis to its immediate vicinity as well, both telomeric and centromeric. The several hundred genes present within this chromosomal segment, in man 7,800 kilobasepairs (kb), have collectively been termed the ‘extended MHC’ (xMHC; Fig. 2)52 due to the exceptional level of linkage disequilibrium (LD) observed over the entire region in selected haplotypes.53 The presence of polymorphic odorant receptor (OR) genes in close linkage to the MHC54–60 and the possible existence of a functional relationship between OR and MHC loci have even led to the suggestion to designate the xMHC an ‘immuno-olfactory supercomplex’.61
Figure 2.
The human extended MHC. Human chromosome 6 is shown with the short (6p) and the long arm (6q). A schematic map of the xMHC is depicted below, with the extended class I (xI, ∼3,900 kb), class I (I∼1,900 kb), class III (III∼700 kb), class II (II∼900 kb) and the extended class II (xII, ∼200 kb) regions indicated by different colors. Nearly all genes mentioned in the main text are shown, with their approximate locations within a given subregion indicated by vertical lines. Histones are shown in blue, the two odorant clusters with a total of 34 genes in green, selected HLA class I genes in yellow and all other genes in red. The directions towards telomere (tel) and centromere (cen) are also given. In the mouse, all genes telomeric of (and including) TRIM27 in the xclass I region are not linked to the MHC, but form a syntenic group of loci on chromosome 13.
Reproductive Barriers in Vertebrates
Barriers that influence mate choice exist before, during and after copulation (Table 1). As mentioned above, pre-copulatory barriers in vertebrates may be of a visual, an auditory or an olfactory nature. Whereas an involvement of the xMHC in auditory contexts is as yet unlikely, despite the presence of the COL11A2 gene within the extended class II subregion (xclass II) (Fig. 2) which is associated also with dominant autosomal deafness,52 there is evidence that visual and, in particular, olfactory cues are employed in mate selection, and that the latter are dependent on polymorphic proteins specified by the MHC. This is in contrast to the products of OR genes within the xMHC, for which a role in pre-copulatory mate choice has so far not been demonstrated. As there are several excellent reviews on pre-copulatory mate choice,10,11,15,16,62–66 we will discuss these issues only briefly in one of the following sections.
Table 1.
Reproductive barriers in vertebrates
| (A) | Before copulation |
| visual | |
| auditory | |
| olfactory | |
| (B) | During copulation |
| optimal complementarity between male and female sexual organs | |
| (C) | After copulation |
| before oocyte fertilization | |
| during sperm—oocyte interaction | |
| after oocyte fertilization, but before zygote formation | |
| after zygote formation |
During copulation, optimal complementarity between male and female sexual organs is helping to secure reproductive success, as shown in closely related species of spiders.67 However, there is evidence for comparable mechanisms also in vertebrates. For example, in species of birds without forced copulations, such as the harlequin duck (Histrionicus histrionicus) or the African goose (Anser cygnoides), the males have short phalli and the females simple vaginas. On the other hand, genital co-variation has led to long phalli and very elaborate vaginas in species with high levels of forced copulations like the long-tailed duck (Clangula hyemalis) and the mallard (Anas platyrhynchos).68 In primates, differences in the anatomy of sexual organs can also be linked to particular sexual behaviors, e.g., to promiscuity.69 To our knowledge, a relationship to genes within the xMHC has not been described for any of these characteristics and we will not consider them further.
Finally, post-copulatory barriers are part of ‘cryptic female choice’ mechanisms that involve hidden female effects that impact on the success of males in fertilizing ova. They can be grouped in mechanisms that are effective before oocyte fertilization, during the fertilization process itself or after sperm—oocyte interaction in the interval preceding the formation of the zygote. Furthermore, these barriers may also affect the pre- or post-implantation embryo and the foetus. Cryptic female choice includes seemingly exotic phenomena such as sperm dumping,70 but also hurdles that must be surmounted by spermatozoa that traverse towards an oocyte within the female reproductive tract. In the latter process, there may well be functions for MHC class I antigens and other xMHC-encoded proteins, particularly OR.
However, a recent study71 demonstrates that it may sometimes be difficult to separate pre- and post-copulatory aspects from each other. Gillingham and colleagues found that, when presented with two MHC-distinct females, male red junglefowl (Gallus gallus) allocated more sperm to that mate with the most pronounced MHC dissimilarity. This behavior is likely to influence fertilization success, since it has been shown that the relative number of sperm inseminated by competing males into a female chicken reliably permits to predict fertilization success.72 How a male bird perceives that its MHC type is dissimilar from that of a female is, however, currently enigmatic. Chickens do have numerous OR genes, but next to nothing is known of their genomic organization and ligands73 as well as the avian sense of smell in general, and it appears equally plausible that these birds rely on other sensory modalities, including post-copulatory mechanisms, for MHC-influenced mate choice. Mate choice is thus not only dependent on decisions made by the female, but, at least in selected cases, on male assessments as well.
In the next section, we will point out a number of xMHC loci whose products have either been shown to play a role or are likely to be involved in mate selection, as well as, more generally, in reproduction.
Participation of xMHC Genes in Reproduction
It may not be surprising that some proteins encoded by the xMHC participate in reproduction, but it seems striking how many of them execute functions that are specifically devoted to pre-copulatory mate choice, germ cell differentiation and function, as well as to embryogenesis (Table 2). However, it must be pointed out that the genes which are part of the human xMHC are not always part of this chromosomal segment also in other species. For example, the human TRIM27 locus (xclass I region) and genes telomeric of it are part of a syntenic assembly on chromosome 13 of the mouse, while the MHC and genes centromeric to TRIM27 are located on chromosome 17 in this species.60 It seems therefore unlikely that possible functional relationships between the MHC and, for example, histone genes are necessarily dependent on a genetic linkage. The fact that 157 tRNA genes, also requiring intense transcription, are intermingled with more than fifty histone loci in the vicinity of the human MHC, the human leukocyte antigen (HLA) complex, points to another explanation: it might be that co-clustering of these genes serves to maximize their transcriptional levels.52
Table 2.
Genes within the human xMHC with a role in reproduction
| Region, gene designation | Function |
| xclass I region | |
| HIST1H2BA and others | Testis-specific histones; destabilization of nucleosome |
| SLC17A2 | Solute carrier protein |
| POM121L2 | Nucleoporin; nuclear envelope formation |
| ZNF184, ZNF165 | Testis-specific zinc-finger proteins; unknown function |
| GPX5 | Glutathione peroxidase; protects against peroxide damage |
| TRIM27 | Member of tripartite motif gene family of zinc-finger proteins; transcriptional regulation of sperm differentiation (?) |
| tOR and cOR | Telomeric and centromeric OR clusters; involvement in mate selection |
| GABBR1 | Subunit of the receptor for γ-amino butyric acid; regulation of ion channels |
| class I region | |
| ZFP57 | KRAB zinc-finger protein; imprint maintenance |
| HLA-G | Expressed on extravillous cytotrophoblast; interaction with maternal NK cells |
| HLA-A | Involvement in sperm receptor selection (?) |
| PPP1R11 | Complex formation with protein phosphatase γ1, actin and PPP1R7; involved in germ cell morphogenesis; absence causes male infertility |
| MDC1 | Involved in repair of DNA double strand breaks |
| DDR1 | Tyrosine kinase; essential developmental functions |
| POU5F1 | Master regulatory transcription factor; maintenance of germ cell lineage |
| HLA-C | Expressed on extravillous cytotrophoblast; interaction with maternal NK cells |
| HLA-B | Involvement in sperm receptor selection (?) |
| class III region | |
| BAT3 | Deficiency leads to male infertility through HSPA1L degradation |
| DDAH2 | Regulates levels of nitric oxide synthase inhibitors; maintains myometrial quiescence during gestation |
| MSH5 | Involvement in meiotic recombination |
| HSPA1L | Testis-specific variant of heat shock protein 70; absence leads to male infertility |
| EHMT2 | Repressor of imprinted genes in trophoblast |
| PBX2 | TALE homeodomain protein; non-essential function (?) |
| class II region | |
| C6orf10 | Testis-specific basic protein with unknown function |
| BRD2 | Bromodomain-containing transcription factor with a possible role in spermatogenesis |
| xclass II region | |
| KIFC1 | Motor protein involved in acrosome biogenesis; expressed also in the syncytiotrophoblast of the placenta |
The description will begin at the telomeric end of the human xMHC (Fig. 2). We will omit, however, the majority of genes that are not specifically expressed in reproductive tissues.
Histone loci.
The cluster of histone genes within the xMHC is the largest within the human genome.52 Histones are required in enormous quantities not only in mitotic cells, but also in cells undergoing meiosis. In male germ cells, they contribute to large-scale genome compaction which is necessary to enclose the haploid DNA within the small spermatozoal head.74 The major role of several xMHC-encoded testis-specific histone variants appears to be the creation of less stable nucleosomes, thus preparing the DNA for the interaction with very basic non-histone proteins and eventually protamines. It has been estimated that only 4% of the DNA within a spermatozoon retains nucleosomes.75 At least three histone genes, HIST1H2BA, HIST1H1A and HIST1H1T within the xMHC are testis-specific and participate in nucleosome destabilization.
Solute carrier proteins.
There are five genes specifying these ion transporters within the human xMHC,52 but only SLC17A2 is known to be testis-expressed. It co-transports sodium and phosphate. A specific reproduction-related function has not been determined for this locus.
POM121L2.
This polymorphic gene encodes a protein that is essential for the formation of the nuclear pore.76 It is abundantly expressed in the testis, particularly in pachytene spermatocytes, but its precise function in male germ cells is unknown.
Zinc-finger proteins.
There are 36 genes within the xMHC encoding polypeptides belonging to this large family. ZNF184 and ZNF165,77 are testis-specific, but their functions have not been determined. In case of TRIM27, a member of the tripartite motif family of zinc-finger proteins, evidence indicates that it may be involved in the transcriptional regulation of sperm differentiation.78
GPX5.
Located between ZNF165 and TRIM27 in the human xMHC, this locus specifies a polypeptide belonging to the glutathione peroxidase family, but it can be distinguished from other family members because its mRNA does not contain a selenocystein codon. It is strongly expressed in the epididymis within the testis, but also in spermatozoa where it is thought to protect membrane lipids against peroxide damage.
Members of OR clusters.
Since the work of Parmentier and colleagues, who were the first to demonstrate that transcripts of OR genes cannot only be found within the main olfactory epithelium (MOE) within the nose, but also in testicular tissue,79 a role for these chemoreceptors in reproduction had to be taken into account. In man, there are two clusters of OR genes within the xMHC. The centromeric of these is also MHC-linked in most other mammals so far examined,225 while the telomeric cluster may be found on other chromosomes. Therefore, a species-independent, functional relationship between MHC loci and MHC-linked OR genes relying on LD is likely to encompass primarily members of the centromeric OR cluster. A prerequisite for the participation of these genes in Self/Nonself recognition is the presence of polymorphisms that affect their function. With the possible exception of a few pseudogenes, all HLA-linked OR loci have been found to be polymorphic, with so far up to eight alleles (OR12D2, in the center of the centromeric OR gene cluster), 55,58,59,80 and it is known that mouse MHC-linked OR loci exhibit variability as well.60 In man, several HLA-linked OR genes are expressed in the testis,81 in addition to those described previously,79 although a role in human reproduction has not yet been firmly established for any of them. When reflecting on pre- and post-copulatory mate selection, we will return again to OR genes and consider how they might interact with MHC class I molecules.
GABBR1.
This polymorphic82 gene belongs to a family of G-protein-coupled receptors (GPCR) involved in neurotransmission. Together with the GABABR2 subunit, GABABR1 constitutes a heterodimeric protein complex in which only the GABABR1 subunit binds γ-amino butyric acid with high affinity, while GABABR2 interacts with G proteins.83 The receptor mediates the coupling to adenyl cyclase, voltage-gated Ca2+ channels and inwardly rectifying K+ channels84 and is expressed not only in neurons, but also in testicular tissue and spermatozoa.85,86 An interesting observation concerning GABBR1 is its apparently obligatory linkage to OR genes.225 This is the case in all mammals so far examined, irrespective of linkage between OR loci and the MHC, as well as in amphibia (Xenopus tropicalis) and in fish (Danio rerio). Since the linkage (between GABA-B-R1, Or33a, Or33b, Or33c and Or35a) extends even to the fruit fly (Drosophila melanogaster), despite the fact that OR of this species are evolutionarily unrelated to those of vertebrates,87 the GABBR1-OR linkage is suggestive of an important functional relationship between these genes which requires them to be in close physical proximity that has been retained for at least 990 million years.88
ZFP57.
This gene belongs to the growing list of xMHC loci with an ‘exciting’ role in reproduction and development. It was recently detected that it encodes a KRAB zinc-finger protein that is highly expressed in developing oocytes and preimplantation embryos and which is involved in maintaining DNA methylation at specific loci. Its absence leads to a severe phenotype causing early lethality in newborn mice.89 In man, homozygous mutations affecting ZFP57 function can cause transient neonatal diabetes.90
HLA-G.
This HLA class I gene exhibits only a low degree of polymorphism. Its product appears to play a role in materno—foetal interactions, where it is expressed on cells of the placental extravillous cytotrophoblast, together with HLA-C antigens.91,92 HLA-G interacts with receptors on natural killer (NK) cells and has long been suspected to be involved in recurrent spontaneous abortions. However, only a recent analysis demonstrated that promoter variants of this gene are involved in this common pregnancy complication.93
HLA-A.
This is one of the most polymorphic genes in the human genome. Despite the absence of β2-microglobulin (β2m), and the concomitant lack of intact class I molecules, it is expressed in certain spermatogenic cells, but not spermatozoa. Since seminiferous tubules are an immunologically privileged tissue to which immune cells have no access, this site of HLA-A class I heavy chain (HC) synthesis points to a non-immunological function for this molecule. As previously suggested by us,36,94 certain polymorphic HLA class I HC, including the product of the HLA-A locus, might be involved in the selection of OR for expression on spermatozoa that lack reactivity with self-molecules.
PPP1R11.
Defects in the protein phosphatase 1 (PP1) γ1 and γ2 isoforms of mice are associated with defective germ cell morphogenesis and apoptosis, but do not cause abnormalities in other tissues. It has recently been detected that the basis for this puzzling observation lies in the association of PP1γ2, actin, and the regulatory subunits (PPP1R7 and PPP1R11) of PP1γ2. Complex formation between these four proteins leads to retention of high levels of PPP1R11 and prevention both of its proteolysis and germ cell apoptosis.95 This indicates that PPP1R11 fulfils an important function within the male reproductive system, and it is known that defects of this gene lead to male infertility in mice.96 The expression of different PPP1R11 isoforms begins at the pachytene stage of spermatogenesis, but the protein(s) play(s) an important role also in the context of sperm motility.97,98 By using in silico analyses, we have detected that this locus is always linked to GABBR1, and even the opposing transcriptional orientation of the two genes is invariably retained, both in mammals and two species of fish (zebrafish, Danio rerio and medaka, Oryzias latipes).
MDC1.
The product of this polymorphic52 gene fulfils an essential function in the repair of DNA double strand breaks. It is not expressed in spermatogonia and preleptotene spermatocytes in mice, possibly explaining the radiosensitivity of these early germ cells, but the MDC1 protein is detectable at later stages of spermatogenesis. In mice with a defect of this gene, all spermatocytes enter apoptosis in epithelial stage IV, pointing to a crucial role of MDC1 also in the production of male gametes.99
DDR1.
This gene specifies a receptor tyrosine kinase that exerts multiple functions in mice. Major sites of its expression are the skeletal bones, skin and the urogenital tract, but also in the uterus during implantation. Mice defective for DDR1 gave birth to offspring that were smaller in size than their normal littermates. Furthermore, the majority of mutant females could not give rise to offspring due to a lack of appropriate blastocyst implantation into the uterine wall, and they exhibited also defects of mammary gland architecture.100
POU5F1.
Although this gene does not seem to play a role in the immune system, it is clearly one of the most crucial in the MHC. It is a highly polymorphic101 master regulatory transcription factor that is present in the genomes of all mammals and plays a critical role in the maintenance of pluripotency in various cell lineages of the embryo as well as in germ cells.102–105 A striking finding is its conservation within the mammalian xMHC, where POU5F1 is an obligatory neighbor of another transcription factor, TCF19.52 The POU5F1-TCF19 linkage is retained also in a lizard, the Caroline anole (Anolis carolinensis), together with the opposing transcriptional orientation of these genes.106 However, a functional interaction of the products of these loci has so far not been detected. Being right in the middle of the human MHC, POU5F1 alleles are part of a region that is subject to marked LD,80 linking them to distinct HLA-B alleles in most populations and in some (e.g., Caucasians) also to HLA-A alleles. Obviously, POU5F1 alleles are also part of ‘ancient’ HLA haplotypes, such as A1-B8-DR3 which are characterized by very strong LD. Although there is no reason to assume that these considerations play any role during spermatogenesis, they could become important after fertilization and during embryonic development, and we will later return to them.
HLA-C.
This moderately polymorphic class I gene is expressed on cells of the extravillous cytotrophoblast of the placenta, where it can interact with suitable receptors on maternal NK cells.91,92
HLA-B.
With more than 1,000 alleles, this is the most polymorphic gene in the human genome and, like HLA-A, it is expressed at late stages of spermatogenesis, but not on sperm. The deliberations made with respect to HLA-A apply also to this locus.
BAT3.
This is a polymorphic52 gene whose targeted inactivation leads to complete male infertility due to apoptosis of meiotic male germ cells. BAT3 deficiency is accompanied by degradation of the protein product of the HSPA1L gene, also resident within the xMHC, a testis-specific chaperone, through polyubiquitinylation and subsequent degradation.107 Therefore, BAT3 must be regarded as a crucial regulator of HSPA1L activity.
DDAH2.
This locus encodes a dimethylarginine dimethylaminohydrolase, an enzyme which hydrolyzes inhibitors of endogenous nitric oxide synthase and modulates their intracellular concentrations. It is involved in regulation of myometrial contractivity during pregnancy in the rat, thereby controlling delivery at term.108
MSH5.
This polymorphic gene encodes a repair protein that is involved in meiotic recombination. Its expression begins at the early primary spermatocyte stage and ends when elongated spermatids form. The C85T polymorphism is associated with male infertility, specifically azoospermia or severe oligozoospermia, due to an anomalous chromosome synapsis and meiotic failure.109,110
HSPA1L.
There are three heat shock protein 70 genes, and all map to the class III region of the xMHC. HSPA1L is the most basic of the three and represents a testis-specific variant.111 As described above in the context of BAT3, its absence leads to male infertility. The pleiotropic functions of the HSPA1L protein offer a plausible explanation for this finding. It is interesting to note that this particular chaperone has also been found in the MOE of mouse and man, and there is evidence that it helps the expression of OR in heterologous cells.112 Whether HSPA1L fulfils this function also in spermatogenic cells, remains to be determined. Nothing is known regarding the consequences of its polymorphism. HSPA1A and HSPA1B (not shown in Fig. 2, immediately centromeric of HSPA1L) are two distinct genes, but encode proteins with identical sequence.113 HSPA1A expression is activated by oxidative stress and hyperosmotic shock; it may be the first gene that is physiologically activated and tightly regulated after fertilization in mammals in consequence of the osmotic stress experienced by very immature embryos.114
EHMT2.
This is a gene within the xMHC that is, like ZFP57, involved in imprinting. EHMT2 however, acts by carrying out the majority of dimethylation of histone H3 at lysine 9. In the mouse, placenta-specific imprinting appears to be dependent on this gene to repress developmentally regulated genes during embryonic stem cell differentiation.115
PBX2.
This is one of four mammalian genes that encode TALE homeodomain proteins. They serve as DNA binding partners for a subset of Hox transcription factors. While other members of the PBX family fulfil essential roles in mammalian development, the PBX2 protein may be dispensable, although it is strongly expressed during embryonic development as well.116 The authors conclude that it is likely that other PBX members might compensate for the loss of PBX2 in the mutant animals.
C6orf10.
This polymorphic locus encodes a testis-specific basic protein with no known function in spermatogenesis.
BRD2.
This gene specifies a double bromodomain protein which is part of a family of transcription factors that associate preferentially with hyperacetylated chromatin of transcribed genes. In addition, BRD2 has intrinsic histone chaperone activity and is a transcriptional co-activator/co-repressor.117,118 The gene is ubiquitously expressed,119 is polymorphic120 and may play a role also during spermatogenesis in the context of chromatin modifications, in addition to a related gene (BRDT) that encodes a testis-specific double bromodomain protein.121
KIFC1.
Members of the kinesin superfamily are motor proteins that perform diverse functions. Depending on the cell type, these may include the transport of various cellular organelles, vesicles, chromosomes, protein complexes, entire nuclei and the regulation of microtubule dynamics. KIFC1 belongs to the class of C-terminal kinesins in which the motor activity is directed towards the microtubule minus end. In mammals, this gene is vital for normal spermatogenesis122 and concerned with acrosome biogenesis, by maintaining the structure of the manchette close to the nuclear membrane.123,124 The protein first associates with vesicles produced by the Golgi apparatus, then with the growing acrosome of the spermatid and with the caudal end of the nucleus in elongated spermatids. The role of KIFC1 in spermatogenesis has also been investigated in an invertebrate, the Chinese mitten crab (Eriocheir sinensis), resulting in similar findings.125 This points to an extreme conservation of the function of this molecule, and the retention of its chromosomal location in the xclass II region within the xMHC of mammals points into the same direction (Fig. 1).126
Facial Attractiveness, Individual-Specific Odors and the Extended MHC
There can be no doubt that the choice of a sexual partner is crucially affected by facial attractiveness.3,4,10 It has been suspected that the MHC might be involved in other sensory modalities apart from olfaction,127 but it came as a surprise that the MHC appears to influence also facial preferences. In a typical study, females had to assess the attractiveness of men from photographs, followed by a comparison of the female and male HLA types. Other than in odor assessment studies such as that carried out by Wedekind and colleagues,32 where preferences were more pronounced when women and men carried dissimilar HLA types, Roberts and co-workers observed a tendency that similar HLA types correlated with facial attractiveness.128 Likewise, the level of heterozygosity at the HLA complex was found to be associated positively with this characteristic.129 It must be mentioned, however, that comparable findings were not reported by all investigators, most likely due to differences in methodological design (reviewed in ref. 10). Nevertheless, the majority of these studies indicate that genes of the HLA complex do not only contribute to facial features, but suggest also that the preference for a particular facial characteristic correlates with a similar HLA constitution.
The few analyses carried out so far do not permit to pinpoint a particular gene within a subregion of the human xMHC as being responsible for the observed effects. However, the possibility that properties of the skin might be influenced by polymorphic genes within the HLA complex should be taken into consideration. There are loci between DDR1 and POU5F1 as well as just centromeric of the latter, within the HLA class I region, that are associated with disorders of this organ. Among them are corneodesmosin (CDSN; hypotrichosis simplex of the scalp) and several genes associated with psoriasis (PSORS1C1, PSORS1C2 and PSORS1C3). At least CDSN and PSORS1C1 are known to be polymorphic, and might thus contribute to distinct skin phenotypes in individuals with different HLA haplotypes. Polymorphisms have also been established for COL11A2 (xclass II region) which, apart from being associated with a hearing disorder,52 is a candidate locus for shaping facial characteristics.10,130 However, additional studies will have to aim at identifying the xMHC gene(s) responsible for facial attractiveness. As pointed out before, the extensive LD within the human xMHC is a severe hurdle for such detailed studies.52
Much more, however, is known about MHC-influenced odor preferences.10,11,15,16,30–36,131–138 These studies concentrated initially on rodents, but have, since the pioneering work of Wedekind and his colleagues,32 also included humans. A number of facts appear to be relatively well established, across species: (1) genes that are part of the MHC play an important role in determining individual differences in body smell; (2) based on olfaction alone, males and females mostly prefer mates possessing dissimilar MHC types; (3) there may be a female preference for males exhibiting MHC heterozygosity. In species such as mouse and man, several additional results have been obtained. For example, β2m-deficient mice possess an individual-specific odor (odor-type) that is distinct from that of wild type mice carrying the same MHC, and is apparently connected to the greatly reduced number of molecules (among them MHC class I antigens) that normally associate with β2m on the surface of cells and within body fluids.139 Furthermore, the natural MHC mutant mouse strains bm1 and bm3, whose H-2K molecules are distinguished by only five amino acid exchanges, are characterized also by distinct odortypes that can be smelled by untrained mice.134
This suggests that peptides (or other ligands) bound to these proteins might play a role in olfactory recognition of an MHC type. In support of this contention, it has been found that peptides bound to MHC class I molecules exert effects on the MOE in which OR genes are expressed.135 These effects are only observed when the peptides retain their anchor residues, i.e., those amino acids with which they are firmly bound to a given MHC class I antigen. For example, in case of the human class I molecule HLA-B*27:05, arginine at peptide position 2 is an obligatory anchor, while Leu, Val, Phe, Tyr, Arg and Lys have been established as secondary anchors at the C-terminus.140–142 The exchange of such anchors precludes high-affinity binding not only to MHC class I molecules,143 but also to receptors on neurons of the MOE135 and of the vomeronasal organ (VNO) in rodents (this organ is present only in vestigial, non-functional form within the human nose). Although the precise identity of nearly all of these receptors remains to be determined, they are commonly thought to be typical OR within the MOE,144–146 while the VNO expresses a variety of distinct GPCR, at least in mice (reviewed in ref. 145,147–149), and may thus be more complex.
Since MHC-bound peptides provide, by way of their anchor residues, fairly accurate mirror images of the pockets that serve to anchor them within an MHC class I molecule's binding groove, they inform the recipient indirectly about the peptide donor's MHC type and could thus provide the basis for self/nonself perception outside of the immune system.36,146,150,151 In assessing the ability of cells carrying a particular chemoreceptor (V2r1b) to bind individual peptides within the VNO, the conclusion was reached that receptors within this organ are likely to distinguish peptides derived from MHC class I and II antigens.151 As there is evidence in some species that also MHC class II alleles influence the outcome of pre-copulatory mate choice (reviewed in ref. 16), these findings in mice provide a general conceptual framework for the involvement of peptides derived from both types of antigen presenting molecules within the MHC. Although the interactions between peptides and any of the chemoreceptors within the MOE or VNO are clearly not yet understood at a molecular level, the very existence of these interactions must be regarded as supporting a role for peptide-presenting MHC molecules in pre-copulatory mate choice.
In this context, could there be a role for OR genes within the xclass I region in these processes? Following the discovery of MHC-linked clusters of OR loci telomeric of the HLA complex (xclass I region),54,55,58,59 MHC-linked OR clusters have been found in nearly all mammals investigated.225 Remarkably, it was observed that many of the HLA-linked OR are polymorphic,55,58,80 although the degree of polymorphism exhibited by individual OR genes was not comparable to that observed for HLA-A, -B or -C loci. The diversity of entire OR haplotypes, however, comprising 34 OR loci in case of the human xMHC, was found to match or even exceed that of HLA haplotypes.58,80 It is still unclear to what extent these two groups of haplotypes are associated with each other through LD, since the analyses are so far limited. Nonetheless, very strong LD is found in the case of the ‘ancient’ HLA haplotypes A1-B8-DR3 and A3-B7-DR15,152,153 and our in silico analyses of eleven ethnically diverse human populations with more than 1,000 individuals (http://hapmap.ncbi.nlm.nih.gov/) demonstrate also the existence of LD between alleles within the OR clusters and loci within the HLA complex in all analyzed populations.80 Despite the recent publication of a study with an inbred Caucasian population which arrived at the opposite conclusion,154 these observations indicate that a functional connection between HLA class I molecules and the products of HLA-linked OR genes is a reality.61
Although still unproven, we suggest that HLA-linked OR will have been selected such that their interaction with HLA molecules that are part of the same xMHC (expressing ‘self-HLA’ molecules) will be minimized, while interaction with nonself-HLA molecules would be favored. Such interactions might be accomplished in molecular terms through peptides or their fragments with affinity for both types of proteins.135,146 As previously pointed out by us,36,94 this scenario is strikingly similar to mechanisms employed by certain fungi to prevent self-fertilization. For example, in the ink cap (Coprinopsis cinerea, formerly Coprinus cinereus), the products of genetically linked, polymorphic loci for pheromones and pheromone receptors are only able to interact with each other when they are specified by unrelated haplotypes.27 This sophisticated system contributes to the existence of a large number (∼20,000) of genetically distinct ‘mating types’ in this mushroom, thus resembling the vast number of HLA haplotypes in human beings. From a structural genetics perspective, it appears plausible that an interdependence of xMHC gene products, e.g., in reproduction, is one of the prerequisites that favors an exceptional, long-range LD such as that observed in the human xMHC.
The relatively high molecular weight of peptides that are presented by MHC class I or II molecules (∼1,000 Dalton or more) poses the problem of how these almost certainly non-volatile substances traverse from one individual to another. Other than in aquatic animals, this is not immediately obvious in case of terrestrial vertebrates. It seems that close bodily contact between two potential mates is necessary to ensure an efficient exchange of peptides that signal the MHC type to OR and other GPCR. However, it is very likely that other ligands are part of these pre-copulatory signals as well. For example, since dogs can distinguish individual-specific scents of humans even when they are not in close physical contact,155,156 volatile substances that permit the animal's chemoreceptors to respond to the scent of individual human beings must exist. Following the identification of 373 candidate substances, Penn and co-workers found evidence for both individual and gender-specific axillary compounds.138 These included alcohols, phenols, aldehydes, ketones, esters, hydrocarbons and other organic substances of low molecular weight. Although the origin of the observed inter-individual differences is currently unclear and could include genetic as well as environmental factors, such as commensal bacteria which colonize an individual in an HLA type-dependent manner, it appears reasonable to assume that the MHC type of an individual might influence the composition of axillary sweat (in man) or other body fluids (vertebrates in general), thereby influencing individual-specific odors.
One of the most interesting aspects of pre-copulatory mate preferences is the emerging consensus that facial preferences tend to be assortative (favoring similarity), as opposed to odor-based studies, most of which indicate disassortative preferences (favoring dissimilarity). Although these seemingly contradictory associations were initially considered puzzling, they could indeed make sense, as pointed out and discussed in more depth by Havlicek and Roberts.10 A plausible hypothesis128 assumes that potential mates achieve, by relying on two very different assessment systems, an intermediate level of genetic dissimilarity among themselves. This is reminiscent of theoretical considerations44 as well as experimental studies with three-spined sticklebacks (Gasterosteus aculeatus) which have shown that females prefer males that complement their own MHC class II alleles optimally.33,45
Pre-copulatory mate choice appears thus as a multi-facetted, highly complex subject in which not only MHC-encoded molecules but also OR are involved. The proven interactions between MHC antigen-derived peptides and OR (or other GPCR within the VNO) continue to be a fascinating research topic. Further work in this area should aim at identifying the OR responsible for binding a given peptide, thereby opening the opportunity to conduct X-ray crystallographic studies of OR involved in mate choice. Furthermore, if additional experiments will reinforce the concept that particular haplotypes of the MHC and those of OR clusters near the MHC are connected by LD, as suggested by the existence of ancient HLA haplotypes53,152 and our recent analyses,80,153 this would have far-reaching functional implications and provide an explanation for the nearly obligatory proximity of these two chromosomal regions which is found at least in mammals.225
Enabling Spermatozoa to Function in Self/Nonself Perception
Most xMHC genes which we have alluded to in the foregoing sections, play roles in human spermatogenesis. This is a very complex process whose details are still being unravelled. It entails the proliferation of diploid spermatogonia, their differentiation and division into haploid spermatids, and finally the formation of spermatozoa, perhaps the most specialized cells in the body. Following spermiogenesis, the last stages of spermatogenesis, mammalian spermatozoa have to undergo additional steps of maturation during their storage in the epididymis until ejaculation. If self/nonself discrimination and female choice were to find their continuation within the mammalian female reproductive tract, and if such processes would rely also on the interactions of polymorphic proteins, as in pre-copulatory mate selection, spermatozoa might have to signal their ‘self’ in some way to the female. Being part of the most polymorphic region within the genome, certain genes within the xMHC could indeed be involved. Consequently, a participation of MHC antigens has already been suggested,157 and the authors reflected on the possibility that the presence of these molecules on the surface of male gametes might provide the female with an indication of the MHC haplotype carried by an individual spermatozoon. There is, however, a problem with this theory: it has been established that neither spermatozoa nor oocytes, at least in man, carry MHC class I and II antigens on their surfaces.158–160
Although cryptic female choice may appear as a logical complement to pre-copulatory mate selection, how could the former be accomplished in the absence from male and female gametes of the potentially most useful proteins that enable self/nonself discrimination? Prompted by the finding that certain HLA class I genes are abundantly expressed during spermatogenesis, though in seemingly non-functional form due to the lack of β2m,94 and the presence of OR transcripts in human testis,79,81 we have previously reasoned that polymorphic OR on the surface of spermatozoa might be the principal molecules that allow to signal the male's MHC type. We proposed the term ‘Sperm Receptor Selection’ (SRS) hypothesis for a process that suggests a plausible molecular mechanism for the selection of a genetically ‘optimal’ spermatozoon which is deemed fit by a given female to fertilize an oocyte.36,94 We postulate that there are a number of prerequisites which would have to be fulfilled in order to accomplish this task:
Before expression on the spermatozoal surface, chemoreceptors (e.g., OR) would have to undergo a testicular selection step during which they are assessed for potential self-reactivity with polymorphic molecules (for simplicity, we will assume that these are specified by MHC haplotypes ‘W’ and ‘Z’, although non-MHC-encoded molecules could in principal be involved as well).
Receptors with self-reactivity (anti-W or -Z) would have to be eliminated; in this way, only receptors lacking self-reactivity will obtain the chance to participate in Self/Nonself discrimination processes during cryptic female choice.
Some of the selected receptors (and, by inference, the spermatozoa on which they reside) might then be able to interact with nonself-molecules (molecules that are specified by haplotypes that are not W or Z).
Females with at least one haplotype other than W or Z should thus be able to attract spermatozoa using molecules within their reproductive tract.
The SRS hypothesis succeeds in providing a framework for Self/Nonself discrimination that bears some resemblance to the negative selection of T cells within the thymic medulla (Table 3).40,143 In both cases, those receptors that do not interact beyond a certain affinity threshold with self-molecules that are provided in the context of a selection process ‘survive’, and they offer the cells on which they reside, the opportunity to interact with nonself-molecules (MHC-presented ‘foreign’ peptides in case of T cells, ligands within the female's reproductive tract in case of spermatozoa). Therefore, comparable to the interaction of MHC-derived peptides with OR within the MOE or chemoreceptors within the VNO,135,146,151 such nonself-reactive OR would indirectly indicate the polymorphic ‘self’ of the male and thereby provide the basic prerequisites for participating in Self/Nonself discrimination.
Table 3.
Outcome of selections within thymus and testis
| Feature | Type of selection | |
| T cell selection in the thymus | Sperm receptor selection in the testis | |
| Positive selection on MHC molecules | T cells with low affinity for self-MHC survive | Probably not necessary |
| Negative selection with self-molecules on/in cells with ‘promiscuous’ transcription | T cells lacking high affinity to self-MHC:peptide complexes survive | Spermatozoa are fitted with receptors that lack self-reactivity |
| Consequence | T cells recognize nonself-MHC:peptide complexes | Spermatozoa recognize nonself-ligands |
Logical as all this may possibly be, is there also experimental support for the SRS hypothesis? Clearly, most of the evidence is so far circumstantial, but there are also some important facts that must be taken into account:
Transcription within the testis has been termed ‘promiscuous’,161 and, to a considerable extent, resembles that observed within epithelial cells of the thymic medulla.162 These cells are crucial for establishing peripheral T cell tolerance during negative T cell selection and rely on the AIRE protein for the transcription of many genes that are otherwise only transcribed within peripheral organs.163 Remarkably, the AIRE gene is expressed also in the testis, where its prime function appears to assure normal apoptosis during spermatogenesis.164 As in medullary thymic epithelial cells, promiscuous transcription within sperm precursor cells might serve to enlarge the repertoire of self-proteins for optimal negative selection purposes.
At least the two most highly polymorphic HLA class I loci, HLA-A and HLA-B, are abundantly expressed in spermatocytes I and II (ref. 94 and Hutter et al. unpublished results). It is very unlikely that these molecules fulfil their established antigen presentation function, since β2m is not expressed in intratubular cells within the testis. From an immunological point of view, HLA class I antigens serve no meaningful purpose in spermatogenic cells, since there are no effector cells (T cells, natural killer cells) in seminiferous tubules. Although it appears possible that these molecules are only ‘accidentally’ produced and fulfil no useful function at all, the SRS hypothesis suggests a plausible way to explain their presence within an immunologically privileged tissue.
OR genes start to be expressed in spermatocytes II, are very strongly expressed in spermatids and more weakly on spermatozoa. In human testicular cells, transcripts from approximately fifty OR genes, about one third encoded by the xMHC, are present.79,81 Similar findings have been described in mice,165–167 rats,166,168 and dogs.169 A given OR gene is expressed in a variable percentage of mammalian sperm precursors (∼30–90%) and spermatozoa (∼5–40%).165,170–172 These percentages imply that a single spermatozoon will express more than one type of OR, in marked contrast to the situation within the MOE, where a given olfactory neuron expresses only a single OR species.145 Consequently, individual spermatozoa should be able to respond to a larger spectrum of ligands than olfactory neurons.
The combination of promiscuous gene expression, in particular the synthesis of polymorphic HLA class I antigens and a variety of OR, during spermatogenesis, supports the SRS hypothesis, but it would be crucial to demonstrate a direct and functionally relevant interaction between HLA-A or HLA-B molecules and OR within testicular tissue to prove the correctness of its basic assumptions.
Cryptic Female Choice Before Fertilization
These deliberations suggest that spermatozoa can express chemoreceptors that do not exhibit high affinity to any self-molecule due to negative selection within the testis. They would thus potentially be receptive to foreign ligands supplied by the female. These ligands should also be polymorphic to provide the genetic signature of the female. Again, ideal candidates seem to be MHC class I antigens or the peptides bound to them.
Human oocytes do not express these molecules, but granulosa cells that surround them exhibit strong reactivity with antibodies directed at intact HLA class I and II complexes.159 These HLA:peptide complexes possess only a limited lifespan (hours to days),143 and will sooner or later disintegrate. Their extracellular domains will also be shed into the follicular fluid. The repertoire of chemoattractants within this fluid173–175 should thus also contain polymorphic polypeptides or peptides that were once bound to them. Even in case of OR for which an interaction with a specific ligand has been demonstrated,170 several problems remain.175 Probably the most important of these is the genuine nature of the ligand for a sperm-expressed OR. For example, more than 90% of sperm respond to the low-molecular weight compound bourgeonal, although only about 10% of the spermatozoa are capacitated.170,176 The relevance of bourgeonal as a physiological ligand has therefore been questioned,175,176 and chemotactic experiments with sperm are complicated by the varying functional states of these cells.175 Nevertheless, in order to prove the basic assumptions of the SRS hypothesis, MHC-derived peptides, MHC class I molecules or their fragments would have to be shown to exert chemotactic effects on sperm from MHC-typed donors.
Additionally, the SRS hypothesis suggests a molecular basis for sperm competition. This term refers to the competition between spermatozoa from two or more males to fertilize a given set of oocytes.177 In promiscuous species, sperm competition is common and may influence the mobility or shape of spermatozoa.178 Since soluble MHC class I antigens are present in seminal fluid, at least in man,179 these self-molecules should not be able to interact with chemoreceptors on sperm from the same male. In contrast, there is a high chance that they will be able to bind to sperm from males that carry other haplotypes. An interaction could possibly prevent a directed movement of these competing sperm towards the oocyte and soluble MHC molecules might therefore be the ‘decoy’ compounds envisaged by Branscomb and colleagues.166 Although currently largely speculative, this plausible scenario should at least be taken into consideration, in the absence of another molecular explanation, to clarify sperm competition.
A compelling case for this process can even be made for spermatozoa within one ejaculate, as previously pointed out by us.36 It involves male mice that are heterozygous for wild type and t-complex haplotypes. The latter comprise long variant segments of chromosome 17 that exist as natural polymorphisms.180 Spermatozoa harboring the wild type allele exhibit a greatly reduced chance to fertilize an egg (down to 1%) in comparison to those carrying a t-complex haplotype. It has been found that several t-complex distorter genes act additively to enhance the transmission rate of the respective haplotypes by increasing the motility of the t-complex bearing spermatozoa, leading to the speculation that not only the t-complex distorter genes, but also others involved in male gametogenesis may have the potential to evolve functionally different alleles, causing the phenotypic inequality of male gametes.181
Furthermore, the female can influence the outcome of sperm competition not only by providing gradients of chemical attractants to which sperm from different males might respond distinctly, but also by ‘adjusting’ the length of her oviducts: in promiscuous primates (e.g., the chimpanzee, Pan troglodytes), oviducts are longer than in non-promiscuous species (e.g., the gorilla, Gorilla gorilla), suggesting that more time can be spent on selecting the ‘best’ spermatozoon among those available.69 If also the combination of different OR expressed on a single spermatozoon should be random, as suggested in the previous section,170,172 a simple calculation indicates that the vast majority of cells in an ejaculate will express a unique ‘signature’ of chemoreceptors, leading to distinct responses upon chemical stimulation. As there is evidence that cryptic female choice involving the MHC does indeed take place in vertebrates, even in primates,69,182 we must think of molecular mechanisms that could provide a framework for post-copulatory Self/Nonself discrimination.
Female Choice during Fertilization and in the Prezygotic State
The analysis of several different taxa of invertebrates has shown that these animals have evolved very elaborate systems to ensure that fertilization occurs mainly between unrelated members of a species, thus preventing self-fertilization and inbreeding. In many taxa, in particular in free-spawning animals, the absence of pre-copulatory mate selection and cryptic female choice before fertilization made it necessary to develop other means of selecting compatible gametes. Gamete recognition proteins play decisive roles whose molecular basis is already well understood in sea urchins (in particular, Echinometra species) and a marine mollusc, the abalone (Haliotis sp.). In many species belonging to these taxa, only conspecific spermatozoa are permitted to enter the egg (reviewed in ref. 24). However, gamete interactions may partially fail, as in reef building corals,183 or may work only one-way: A-species sperm cannot fertilize B-species eggs, but B-species sperm is able to fertilize also A-species eggs.184 Positive selection of repeated domains in sperm-binding polypeptides, such as the product of the VERL gene on the surface of abalone eggs, results in rapid evolution of these proteins.185
Furthermore, a sophisticated system of self-incompatibility exists in an ascidian species. In Ciona intestinalis, it involves four proteins, two on the sperm surface (s-Themis-A and s-Themis-B) and two that are part of the egg's vitelline coat (v-Themis-A and v-Themis-B). Each of the v-Themis genes are located on different chromosomes and within the first intron of the respective s-Themis gene, but in opposite transcriptional orientation. All four genes are extremely polymorphic and the regions exhibiting polymorphism in both s- and both v-Themis loci are near each other. It has been suggested that the interaction of the products of both sperm-expressed Themis genes with the respective proteins on the egg surface leads to fertilization failure.23 The basic genetics of this interesting system had already been discovered more than sixty years ago by Thomas Hunt Morgan, who also proposed a ‘Haploid Sperm Hypothesis’ to account for his findings.186 In another ascidian, Botryllus schlosseri, self/nonself recognition during a natural transplantation reaction is controlled by three loci and two of the polypeptides specified by these loci (fester and uncle fester) contain a Sushi domain.187,188 Such domains have also been detected in a protein found on mouse spermatozoa (sp56) which is thought to be involved in the interaction with the zona pellucida.189
We are referring to these systems, because they illustrate beyond any doubt that self/nonself discrimination mechanisms do not have to end once a spermatozoon has made its way into the vicinity of the mammalian oocyte. Even in the absence of MHC antigens from both of these cells,158–160 there are many other proteins that might enable a highly selective Self/Nonself perception during sperm—oocyte interaction. It is known since several decades that the acrosome reaction in mammalian spermatozoa can be triggered by binding to the zona pellucida (ZP) protein 3,190,191 and mice lacking the ZP3 protein are infertile.192,193 Together with ZP2, ZP3 belongs to the 10% of the most polymorphic proteins in mammals and parallels between these polypeptides and gamete recognition proteins in invertebrates have been pointed out.194 Several low-molecular weight substances are also able to induce the acrosome reaction in vitro, including γ-amino butyric acid, although the relevance of these observations for the situation in vivo has been questioned (reviewed in ref. 195). Presently, it must remain an open question whether xMHC-encoded proteins participate in the direct encounter of male and female gametes, although we have already highlighted some candidates (Table 2).
For a number of reasons, our knowledge of the events that directly follow fertilization is fragmentary, particularly so in humans due to ethical considerations. However, the outcome of the second meiotic division in the oocyte, which occurs in many vertebrates after the sperm has penetrated the vitelline membrane, is influenced by the MHC type carried by the spermatozoon and in vitro fertilization (IVF) experiments in mice have provided evidence that MHC-different mice exhibit female choice for distinct male MHC haplotypes also at this stage.196 In addition, when MHC-heterozygous and -homozygous embryos should have been produced in approximately equal numbers, females carried more heterozygous embryos when the parents were infected with hepatitis virus. This might indicate that parental infection exerts an effect on the degree of MHC heterozygosity in the offspring.197 At least in mice, MHC-influenced cryptic mate choice during fertilization or even at a later stage should therefore be taken into account.
How could this be accomplished in molecular terms? The time until a zygote is formed, can vary considerably between species, but takes ∼18 hours in case of humans. During this period, the condensed sperm chromatin is unwrapped,198 spermatozoan transcripts199 are released and transcription begins. Remarkably, a spermatozoon does not only deliver male transcripts, but also certain transcription factors.200 Among these is the product of the highly polymorphic POU5F1 gene (Table 2). By forming heterodimeric complexes with proteins specified by other transcription factor genes (SOX2, NANOG), both of which are not part of the xMHC, POU5F1 critically influences embryonic differentiation.201,202 The developmental target loci of POU5F1 and NANOG are hypomethylated in sperm DNA, but acquire methylation during development.75 The authors describe also the localization of certain modified histones to developmental promoters, but we will not further refer to these, because histone genes are not part of the xMHC in all vertebrates. It is unknown whether naturally occurring polymorphisms of POU5F1,101 or of the two other transcription factor genes affect these interactions. If so, this could be taken as an indication that the similarity between fungal and mammalian reproductive barriers does not only comprise the polymorphic pheromone receptor/pheromone system which we have previously mentioned (products of clustered OR genes, MHC molecules), but might also include transcriptional control mechanisms. In Coprinopsis cinerea, a successful completion of the mating process is dependent also on a transcription factor heterodimer to which both potential mates have to contribute distinct components.27
It has been estimated that a single sperm cell contains ∼18,000 transcripts that are delivered to the oocyte upon fertilization, and some of these may have a role in the developing zygote.199 For example, HSPA1L transcripts may participate in stress responses and other transcripts have been postulated to be involved in several other processes, including essential functions in early embryonic development,203 although paternal transcripts have not been observed beyond the four-cell stage.204 Even if the latter observation would be generally valid, paternal RNA molecules expressed in fertilized oocytes could have a function also as epigenetic modifiers of early embryonic development, by altering the phenotype of the offspring while preserving a wild-type genotype through paramutation.205–207 Paramutation takes place when two alleles of a single locus interact such that one allele influences the other, yielding a change that can be inherited for more than one generation, even if the allele causing the change is not directly transmitted.203 It appears possible that the product of the ZFP57 gene (Table 2) is involved in this process.89,90 The basic prerequisites for a substantial paternal contribution to embryonic development, apart from donating half of the zygote's nuclear chromosomes, do therefore seem to be fulfilled also in mammals, and there are abundant opportunities for executing MHC-dependent female choice as observed by Wedekind's group during their IVF experiments.196
A particularly striking case of post-fertilization, but pre-zygotic cryptic female choice is shown by a marine invertebrate, the ctenophore Beroë ovata. This organism has very large oocytes (∼1 mm), in which a female pronucleus can migrate considerable distances at a speed of ∼0.2 µm/sec. Fertilization occurs usually not only by a single, but by several male gametes. The male pronuclei are stationary within the egg's cytoplasm, but are ‘visited’ and ‘evaluated’ by the female pronucleus which seems to travel along microtubules that are provided by the male gamete. This inspection-like process may take several hours before fusion between the female and one of the male pronuclei eventually occurs (http://biodev.obs-vlfr.fr/recherche/biomarcell/ctenophores/beroe.htm).208
Female Choice Following Zygote Formation
In this short review, it is impossible to provide a comprehensive and critical summary of embryonic and foetal development and the participation of xMHC genes. However, some of these loci (Table 2) deserve particular, though brief, mention.
We have already pointed out that POU5F1 is a master regulatory transcription factor on whose activity early embryonic development is crucially dependent.102 The POU5F1 gene is downregulated during gastrulation and in cells of the trilaminar embryo, except in undifferentiated embryonic stem cells and in primordial germ cells. Its level of expression appears to be important for proper function,209 and the shut-down of its transcription in non-germ cell embryonic tissue seems mainly carried out through methylation.101 In this context, it is important that there are differences between vertebrate species as to how particular steps during embryonic development are accomplished210 and which transcription factors are involved.106
On the other hand, we have already mentioned the ZFP57 locus whose expression is essential in the earliest stages of embryonic development to ensure proper DNA methylation within specific genes. Instead of giving a detailed account of the function of the ZFP57 protein, we would like to refer the reader to the excellent review by X. Li.89 As in case of ZFP57, the function of the DDR1 gene is totally different from that of POU5F1 and a defect does not lead to global problems as in case of the latter. However, its effects on blastocyst implantation and mammary gland development100 demonstrate that the xMHC is also involved in these processes. With regard to HSPA1A, we have already drawn attention to the function of this nearly ubiquitously expressed gene in very early embryonic development.114
Finally, five further xMHC loci deserve mention, because they are expressed in placental or uterine tissue. One of these is KIFC1, which specifies a motor protein that is mainly found within the syncytiotrophoblast, both in early and term placentas and in even larger amounts in placentas from women suffering from preeclampsia or diabetes.211 As the name implies, the syncytiotrophoblast is a multinucleated syncytium with a barrier-like function between the foetus and its mother. Remarkably, HLA class I or II antigens cannot be found in this tissue, possibly due to the immunological incompatibility between mother and child. The function of KIFC1 within the syncytiotrophoblast has not been investigated.
The expression of two MHC class I genes, in man HLA-G and HLA-C, has been observed on placental cells as well. Human oocytes and preimplantation embryos lack expression of these class I loci,159,160 but, following implantation, both are strongly expressed on cells of the extravillous cytotrophoblast,212–215 a site of intense interaction between foetal and maternal tissues. On the maternal side, both molecules appear to be recognized by NK cell receptors and downregulation of the nearly invariant HLA-G molecules on the surface of a trophoblast cell line in RNAi inhibition experiments leads to an abolishment of the resistance to NK cell lysis.216 HLA-G is also thought to induce local tolerance of maternal immune cells towards the foetus (reviewed in ref. 217). The recent finding that there are HLA-G promoter variants that can be grouped into distinct haplotypes revealed that patients with recurrent spontaneous abortions had a significantly higher chance to carry a particular haplotype, while the healthy controls carried another. These results permit to conclude that variants of the HLA-G promoter may be associated with the risk to suffer from a spontaneous abortion.93
The other HLA class I molecule found on the surface of the extravillous cytotrophoblast, HLA-C, exhibits an intermediate level of polymorphism. Nevertheless, this poses a potential problem, since it opens the possibility for the mother to reject the foetal semi-allogeneic ‘transplant’. HLA-C molecules react with polymorphic 2-domain killer immunoglobulin-like receptor (KIR) molecules on maternal cells (reviewed in ref. 217) and it has been found that this interaction is dependent on the expression of suitable foetal and maternal haplotypes whose products recognize each other. Since certain HLA-C/KIR combinations might favor the remodelling of maternal blood vessels, the placenta and the foetus will profit in selected cases through an optimized blood supply.218 Therefore, even at this stage, self/nonself perception mechanisms appear to be at work.
The product of the EHMT2 gene, a histone methyltransferase, is crucial for the developing embryo as well, since mouse embryos with a defect of this locus die in utero at ∼day 10.115,219 EHMT2 forms a functional heterodimer and acts to repress imprinted genes in the trophoblast. A better understanding of these results would possibly also be of interest for human reproduction, since the culture of preimplantation embryos is accompanied by a preferential loss of placental imprinting.220,221 In addition, we have already referred to the DDAH2 locus which specifies an enzyme that regulates intracellular levels of nitric oxide synthase inhibitors. At least in the rat, this enzyme seems to have an important function in maintaining myometrial quiescence during gestation and is also involved in controlling delivery at term.108 It is unknown whether it is only of anecdotal relevance or truly important, but it has been reported that the DDAH2 activity is inhibited by tumor necrosis factor-α,222 whose gene (TNF) is located close to DDAH2, within the class III region of the xMHC (not shown in Fig. 2).52
Conclusions
We are aware that these considerations are in most cases still incomplete, often speculative, and certainly influenced by our own preferences and interests. Furthermore, there are several additional genes within the xMHC which we have not mentioned with a single word, although they fulfil indispensable functions in reproduction. For example, the expression of the TUBB locus (the centromeric neighbor of MDC1) is essential for all cells, as mitosis and meiosis cannot work without microtubules. Our selection of xMHC genes (Table 2) is therefore highly biased, and one can take it for granted that the list will grow over the next years. The EHMT2 locus provides a typical example. It has only recently been found that this protein carries out a crucial role during embryonic development,115,219 but it has now been observed that it contributes also to proper differentiation and function of CD4+ T helper cells.223 EHMT2 can thus be added to those xMHC genes that act in totally distinct developmental stages. MHC class I genes, OR loci or GABBR1 are well known further examples for the versatility of members of this interesting chromosomal segment. A particular hallmark of several of the encoded proteins is, however, their involvement in self/nonself discrimination processes that are fundamental not only for the functioning of the immune system, but also for the selection of appropriate mates.
Acknowledgements
We thank Alexander Ziegler for discussions and constructive criticisms regarding the manuscript and we express our gratitude to the VolkswagenStiftung, Hannover (I75/196; I72/740), the Monika-Kutzner-Stiftung, Berlin, and the Berliner Krebsgesllschaft, Berlin for supporting our work on HLA-linked odorant receptor genes as well as the products of the MHC class I loci.
Footnotes
Previously published online: www.landesbioscience.com/journals/selfnonself/article/12736
References
- 1.Darwin C. The descent of man and selection in relation to sex. London UK: John Murray; 1871. [Google Scholar]
- 2.Bittles AH, Mason WM, Greene J, Rao NA. Reproductive behavior and health in consanguineous marriages. Science. 1991;252:789–794. doi: 10.1126/science.2028254. [DOI] [PubMed] [Google Scholar]
- 3.Thornhill R, Gangestad SW. Facial attractiveness. Trends Cogn Sci. 1999;3:452–460. doi: 10.1016/s1364-6613(99)01403-5. [DOI] [PubMed] [Google Scholar]
- 4.Little AC, Perrett DI. Putting beauty back in the eye of the beholder: evolution and individual differences in face preference. Psychologist. 2002;15:28–32. [Google Scholar]
- 5.Santos PSC, Schinemann JA, Gabardo J, Bicalho MG. New evidence that the MHC influences odor perception in humans: a study with 58 Southern Brazilian students. Horm Behav. 2005;47:384–388. doi: 10.1016/j.yhbeh.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Haselton MG, Mortezaie M, Pillsworth EG, Bleske-Rechek A, Frederick DA. Ovulatory shifts in human female ornamentation: near ovulation, women dress to impress. Horm Behav. 2006;51:40–45. doi: 10.1016/j.yhbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Garver-Apgar CE, Gangestad SW, Thornhill R, Miller RD, Olp JJ. Major histocompatibility complex alleles, sexual responsivity and unfaithfulness in romantic couples. Psychol Sci. 2006;17:830–835. doi: 10.1111/j.1467-9280.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg DR, DeBruine LM, Jones BC, Little AC. Correlated preferences for men's facial and vocal masculinity. Evol Hum Behav. 2008;29:233–241. [Google Scholar]
- 9.Pipitone RN, Gallup GGJ. Women's voice attractiveness varies across the menstrual cycle. Evol Hum Behav. 2008;29:268–274. [Google Scholar]
- 10.Havlicek J, Roberts SC. MHC-correlated mate choice in humans: a review. Psychoneuroendocrinol. 2009;34:497–512. doi: 10.1016/j.psyneuen.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Alvergne A, Lummaa V. Does the contraceptive pill alter mate choice in humans? Trends Ecol Evol. 2010;25:171–179. doi: 10.1016/j.tree.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Birkhead T, Moller A. Female control of paternity. Trends Ecol Evol. 1993;8:100–104. doi: 10.1016/0169-5347(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 13.von Schantz T, Wittzell H, Göransson G, Grahn M, Persson K. MHC genotype and male ornamentation: genetic evidence for the Hamilton-Zuk model. Proc R Soc London Ser B. 1996;263:265–271. doi: 10.1098/rspb.1996.0041. [DOI] [PubMed] [Google Scholar]
- 14.Neff BD, Pitcher TE. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. [DOI] [PubMed] [Google Scholar]
- 15.Sommer S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool. 2005;2:16. doi: 10.1186/1742-9994-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milinski M. The major histocompatibility complex, sexual selection and mate choice. Annu Rev Ecol Evol Syst. 2006;37:159–186. [Google Scholar]
- 17.Hoffman JI, Forcada J, Trathan PN, Amos W. Female fur seals show active choice for males that are heterozygous and unrelated. Nature. 2007;445:912–914. doi: 10.1038/nature05558. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg LA, Dannewitz J, Petersson E, Grahn M. Influence of genetic dissimilarity in the reproductive success and mate choice of brown trout—females fishing for optimal MHC dissimilarity. J Evol Biol. 2007;20:1859–1869. doi: 10.1111/j.1420-9101.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 19.Ilmonen P, Stundner G, Thoss M, Penn DJ. Females prefer the scent of outbred males: good-genes-as-heterozygosity? BMC Evol Biol. 2009;16:104. doi: 10.1186/1471-2148-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setchell JM, Charpentier MJ, Abbott KM, Wickings EJ, Knapp LA. Opposites attract: MHC-associated mate choice in a polygynous primate. J Evol Biol. 2010;23:136–148. doi: 10.1111/j.1420-9101.2009.01880.x. [DOI] [PubMed] [Google Scholar]
- 21.Marino R, de Santis R, Giuliano P, Pinto MR. Follicle cell proteasome activity and acid extract from the egg vitelline coat prompt the onset of self-sterility in Ciona intestinalis oocytes. Proc Natl Acad Sci USA. 1999;96:9633–9636. doi: 10.1073/pnas.96.17.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawniczak MKN, Barnes AI, Linklater JR, Boone JM, Wigby S, Chapman T. Mating and immunity in invertebrates. Trends Ecol Evol. 2006;22:48–55. doi: 10.1016/j.tree.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Harada Y, Takagaki Y, Sunagawa M, Saito T, Yamada L, Taniguchi H, et al. Mechanism of self-sterility in a hermaphroditic chordate. Science. 2008;320:548–550. doi: 10.1126/science.1152488. [DOI] [PubMed] [Google Scholar]
- 24.Palumbi SR. Speciation and the evolution of gamete recognition genes: pattern and process. Heredity. 2009;102:66–76. doi: 10.1038/hdy.2008.104. [DOI] [PubMed] [Google Scholar]
- 25.Morran LT, Parmentier MD, Phillips C. Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature. 2009;462:350–352. doi: 10.1038/nature08496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol. 2005;56:467–489. doi: 10.1146/annurev.arplant.56.032604.144249. [DOI] [PubMed] [Google Scholar]
- 27.Casselton LA. Mate recognition in fungi. Heredity. 2002;88:142–147. doi: 10.1038/sj.hdy.6800035. [DOI] [PubMed] [Google Scholar]
- 28.Garamszegi LZ, Eens M, Török J. Birds reveal their personality when singing. PLoS One. 2008;3:2647. doi: 10.1371/journal.pone.0002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy M, Heckel G, Voigt CC, Mayer F. Female-biased dispersal and patrilocal kin groups in a mammal with a resource-defense polygyny. Proc Biol Sci. 2007;274:3019–3024. doi: 10.1098/rspb.2007.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, Abbott J, et al. Control of mating preference in mice by genes in the major histocompatibility complex. J Exp Med. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RE, Singh PB, Roser B. The major histocompatibility complex and the chemosensory recognition of individuality in rats. Physiol Behav. 1987;40:65–73. doi: 10.1016/0031-9384(87)90186-7. [DOI] [PubMed] [Google Scholar]
- 32.Wedekind C, Seebeck T, Bettens F, Paepke AJ. MHC-dependent mate preferences in humans. Proc R Soc Ser B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 33.Reusch TBH, Häberli MA, Aeschlimann PB, Milinski M. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature. 2001;414:300–302. doi: 10.1038/35104547. [DOI] [PubMed] [Google Scholar]
- 34.Olsson M, Madsen T, Nordby J, Wapstra E, Ujwari B, Wittsell H. Major histocompatibility complex and mate choice in sand lizards. Proc R Soc Ser B. 2003;270:254–256. doi: 10.1098/rsbl.2003.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman-Gallant CR, Meguerdichian M, Wheelwright NT, Sollecito SV. Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Mol Ecol. 2003;12:3077–3083. doi: 10.1046/j.1365-294x.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler A, Kentenich H, Uchanska-Ziegler B. Female choice and the MHC. Trends Immunol. 2005;26:496–502. doi: 10.1016/j.it.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan LC, Clements CS, Rossjohn J, Brooks AG. The major histocompatibility complex class Ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens. 2008;72:415–424. doi: 10.1111/j.1399-0039.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 39.McKitrick TR, De Tomaso AW. Molecular mechanisms of allorecognition in a basal chordate. Sem Immunol. 2010;22:34–38. doi: 10.1016/j.smim.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crites TJ, Varma R. On the issue of peptide recognition in T cell development. Self/Nonself. 2010;1:55–61. doi: 10.4161/self.1.1.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madden DR. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 42.Boyington JC, Sun PD. A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Mol Immunol. 2002;38:1007–1021. doi: 10.1016/s0161-5890(02)00030-5. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 44.Nowak MA, Tarczy-Hornoch K, Austyn JM. The optimal number of major histocompatibility complex molecules in an individual. Proc Natl Acad Sci USA. 1992;89:10896–10899. doi: 10.1073/pnas.89.22.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woelfing B, Traulsen A, Milinski M, Boehm T. Does intra-individual major histocompatibility complex diversity keep a golden mean? Phil Trans R Soc B. 2009;364:117–128. doi: 10.1098/rstb.2008.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekblom R, Saether SA, Grahn M, Fiske P, Kalas JA, Höglund J. Major histocompatibility complex variation and mate choice in a lekking bird, the great snipe (Gallinago media) Mol Ecol. 2004;13:3821–3828. doi: 10.1111/j.1365-294X.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- 47.Bos DH, Williams RN, Gopurenko D, Bulut Z, DeWoody JA. Condition-dependent mate choice and a reproductive disadvantage for MHC-divergent male tiger salamanders. Mol Ecol. 2009;18:3307–3315. doi: 10.1111/j.1365-294X.2009.04242.x. [DOI] [PubMed] [Google Scholar]
- 48.Roberts SC. Complexity and context of MHC-correlated mating preferences in wild populations. Mol Ecol. 2009;18:3121–3123. doi: 10.1111/j.1365-294X.2009.04244.x. [DOI] [PubMed] [Google Scholar]
- 49.McCallum H. Tasmanian devil facial tumour disease: lessons for conservation biology. Trends Ecol Evol. 2008;23:631–637. doi: 10.1016/j.tree.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Siddle HV, Kreiss A, Eldridge MDB, Noonan E, Clarke CJ, Pyecroft S, et al. Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proc Natl Acad Sci USA. 2007;104:16221–16226. doi: 10.1073/pnas.0704580104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddle HV, Marzec J, Cheng Y, Jones M, Belov K. MHC gene copy number variation in Tasmanian devils: implications for the spread of a contagious cancer. Proc Biol Sci. 2010;277:2001–2006. doi: 10.1098/rspb.2009.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 53.Malfroy L, Roth MP, Carrington M, Borot N, Volz A, Ziegler A, et al. Heterogeneity in rates of recombination in the 6-Mb region telomeric to the human major histocompatibility complex. Genomics. 1997;43:226–231. doi: 10.1006/geno.1997.4800. [DOI] [PubMed] [Google Scholar]
- 54.Fan W, Liu YC, Parimoo S, Weissman SM. Olfactory receptor-like genes are located in the human major histocompatibility complex. Genomics. 1995;27:119–123. doi: 10.1006/geno.1995.1013. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler A, Ehlers A, Forbes S, Trowsdale J, Uchanska-Ziegler B, Volz A, et al. Polymorphic olfactory receptor genes and HLA loci constitute extended haplotypes. In: Kasahara M, editor. Major Histocompatibility Complex—Evolution, Structure and Function. Tokyo: Springer Verlag; 2000. pp. 110–130. [Google Scholar]
- 56.Eklund AC, Belchak MM, Lapidos K, Raha-Chowdhury R, Ober C. Polymorphisms in the HLA-linked olfactory receptor genes in Hutterites. Hum Immunol. 2000;61:711–717. doi: 10.1016/s0198-8859(00)00132-4. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler A, Ehlers A, Forbes S, Trowsdale J, Volz A, Younger R, et al. Polymorphisms in olfactory receptor genes: A cautionary note. Hum Immunol. 2000;61:1281–1284. doi: 10.1016/s0198-8859(00)00219-6. [DOI] [PubMed] [Google Scholar]
- 58.Ehlers A, Beck S, Forbes S, Trowsdale J, Volz A, Younger R, et al. MHC-linked olfactory receptor loci exhibit polymorphism and contribute to extended HLA/OR haplotypes. Genome Res. 2000;10:1968–1978. doi: 10.1101/gr.10.12.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Younger RM, Amadou C, Bethe G, Ehlers A, Fischer-Lindahl K, Forbes S, et al. Characterisation of clustered MHC-linked olfactory receptor genes in human and mouse. Genome Res. 2001;11:519–530. doi: 10.1101/gr.160301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amadou C, Younger RM, Sims S, Matthews LH, Rogers J, Kumánovics A, et al. Co-duplication of olfactory receptor and MHC class I genes in the mouse major histocompatibility complex. Hum Molec Genet. 2003;12:3025–3040. doi: 10.1093/hmg/ddg317. [DOI] [PubMed] [Google Scholar]
- 61.Ziegler A. Biology of chromosome 6. DNA Sequence. 1997;8:189–202. doi: 10.3109/10425179709034072. [DOI] [PubMed] [Google Scholar]
- 62.Brown JL, Eklund A. Kin recognition and the major histocompatibility complex—an integrative view. Am Nat. 1994;143:435–461. [Google Scholar]
- 63.Brown JL. A theory of mate choice based on heterozygosity. Behav Ecol. 1997;8:60–65. [Google Scholar]
- 64.Penn D, Potts WK. How do major histocompatibility complex genes influence odor and mating preferences? Adv Immunol. 1998;69:411–436. doi: 10.1016/s0065-2776(08)60612-4. [DOI] [PubMed] [Google Scholar]
- 65.Piertney SB, Oliver MK. The evolutionary ecology of the major histocompatibility complex. Heredity. 2006;96:7–21. doi: 10.1038/sj.hdy.6800724. [DOI] [PubMed] [Google Scholar]
- 66.Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Phil Trans R Soc B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuntner M, Coddington JA, Schneider JM. Intersexual arms race? Genital coevolution in nephilid spiders (Araneae, Nephilidae) Evolution. 2009;63:1451–1463. doi: 10.1111/j.1558-5646.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- 68.Brennan PL, Prum RO, McCracken KG, Sorenson MD, Wilson RE, Birkhead TR. Coevolution of male and female genital morphology in waterfowl. PLoS One. 2007;2:418. doi: 10.1371/journal.pone.0000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dixson A, Anderson M. Sexual selection and the comparative anatomy of reproduction in monkeys, apes and human beings. Annu Rev Sex Res. 2001;12:121–144. [PubMed] [Google Scholar]
- 70.Price T, Lewis Z, Wedell N. Sperm dumping as a defense against meiotic drive. J Biol. 2009;8:6. doi: 10.1186/jbiol109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillingham MAF, Richardson DS, Lovlie H, Moynihan A, Worley K, Pizzari T. Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proc R Soc B. 2009;276:1083–1092. doi: 10.1098/rspb.2008.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin PA, Reimers TJ, Lodge JR, Dziuk PJ. The effect of ratios and numbers of spermatozoa mixed from two males on proportion of offspring. J Reprod Fert. 1974;39:251–258. doi: 10.1530/jrf.0.0390251. [DOI] [PubMed] [Google Scholar]
- 73.International Chicken Genome Sequencing Consortium, author. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 74.Gaucher J, Reynoird N, Motellier E, Boussouar F, Rousseaux S, Khochbin S. From meiosis to postmeiotic events: The secrets of histone disappearance. FEBS J. 2010;277:599–604. doi: 10.1111/j.1742-4658.2009.07504.x. [DOI] [PubMed] [Google Scholar]
- 75.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Tirosvoutis KN, Divane A, Jones M, Affara N. Characterization of a novel zinc finger gene (ZNF165) mapping to 6p21 that is expressed specifically in testis. Genomics. 1995;28:485–490. doi: 10.1006/geno.1995.1178. [DOI] [PubMed] [Google Scholar]
- 78.Cao T, Shannon M, Handel MA, Etkin LD. Mouse ret finger protein (rfp) proto-oncogene is expressed at specific stages of mouse spermatogenesis. Dev Genet. 1996;19:309–320. doi: 10.1002/(SICI)1520-6408(1996)19:4<309::AID-DVG4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 79.Parmentier M, Libert F, Schurmans S, Schiffermann S, Lefort A, Eggerinckx D, et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature. 1992;355:453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- 80.Santos PSC, Uehara CJS, Ziegler A, Uchanska-Ziegler B, Bicalho MG. Variation and linkage disequilibrium within odorant receptor gene clusters linked to the human major histocompatibility complex. Hum Immunol. 2010 doi: 10.1016/j.humimm.2010.06.011. In press. [DOI] [PubMed] [Google Scholar]
- 81.Volz A, Ehlers A, Younger RM, Forbes S, Trowsdale J, Schnorr D, et al. Complex transcription and splicing of odorant receptor genes. J Biol Chem. 2003;278:19691–19701. doi: 10.1074/jbc.M212424200. [DOI] [PubMed] [Google Scholar]
- 82.Peters HC, Kammer G, Volz A, Kaupmann K, Ziegler A, Bettler B, et al. Mapping, genomic structure and polymorphisms of the human GABABR1 receptor gene: evaluation of its involvement in idiopathic generalized epilepsy. Neurogenetics. 1998;2:47–54. doi: 10.1007/s100480050051. [DOI] [PubMed] [Google Scholar]
- 83.Pin JP, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, et al. Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol. 2004;68:1565–1572. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 84.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 85.Vidal RL, Ramirez A, Castro M, Concha II, Couve A. Marlin-1 is expressed in testis and associates to the cytoskeleton and GABAB receptors. J Cell Biochem. 2008;103:886–895. doi: 10.1002/jcb.21456. [DOI] [PubMed] [Google Scholar]
- 86.Burrello N, Vicari E, D'Amico L, Satta A, D'Agata R, Calogero AE. Human follicular fluid stimulates the sperm acrosome reaction by interacting with the γ-aminobutyric acid receptors. Fert Steril. 2004;82:1086–1090. doi: 10.1016/j.fertnstert.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 87.Nozawa M, Nei M. Evolutionary dynamics of olfactory receptor genes in Drosophila species. Proc Natl Acad Sci USA. 2007;104:7122–7127. doi: 10.1073/pnas.0702133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blair Hedges S, Kumar S. Genomic clocks and evolutionary timescales. Trends Genet. 2003;19:200–206. doi: 10.1016/S0168-9525(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 89.Li X. Extending the maternal-zygotic effect with genomic imprinting. Mol Hum Reprod. 2010 doi: 10.1093/molehr/gaq028. In press. [DOI] [PubMed] [Google Scholar]
- 90.Mackay DJ, Callaway JL, Marks SM, White HE, Acerini CL, Boonen SE, et al. Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet. 2008;40:949–951. doi: 10.1038/ng.187. [DOI] [PubMed] [Google Scholar]
- 91.Hutter H, Hammer A, Blaschitz A, Hartmann M, Ebbesen P, Dohr G, et al. Expression of HLA class I molecules in human first trimester and term placenta trophoblast. Cell Tissue Res. 1996;286:439–447. doi: 10.1007/s004410050713. [DOI] [PubMed] [Google Scholar]
- 92.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 93.Berger DS, Hogge WA, Barmada MM, Ferrell RE. Comprehensive analysis of HLA-G: implications for recurrent spontaneous abortion. Reprod Sci. 2010;17:331–338. doi: 10.1177/1933719109356802. [DOI] [PubMed] [Google Scholar]
- 94.Ziegler A, Dohr G, Uchanska-Ziegler B. Possible roles for products of polymorphic MHC and linked olfactory receptor genes during selection processes in reproduction. Am J Reprod Immunol. 2002;48:34–42. doi: 10.1034/j.1600-0897.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- 95.Cheng L, Pilder S, Nairn AC, Ramdas S, Vijayaraghavan S. PP1gamma2 and PPP1R11 are parts of a multimeric complex in developing testicular germ cells in which their steady state levels are reciprocally related. PLoS One. 2009;4:4861. doi: 10.1371/journal.pone.0004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han YB, Feng HL, Cheung CK, Lam PM, Wang CC, Haines CJ. Expression of a novel T-complex testis expressed 5 (Tctex5) in mouse testis, epididymis and spermatozoa. Mol Reprod Dev. 2007;74:1132–1140. doi: 10.1002/mrd.20631. [DOI] [PubMed] [Google Scholar]
- 97.Huang Z, Vijayaraghavan S. Increased phosphorylation of a distinct subcellular pool of protein phosphatase, PP1γ2, during epididymal sperm maturation. Biol Reprod. 2004;70:439–447. doi: 10.1095/biolreprod.103.020024. [DOI] [PubMed] [Google Scholar]
- 98.Pilder SH, Lu J, Han Y, Hui L, Samant SA, Olugbemiga OO, et al. The molecular basis of “curlicue”: a sperm motility abnormality linked to the sterility of t Haplotype homozygous male mice. Soc Reprod Fertil Suppl. 2007;63:123–133. [PubMed] [Google Scholar]
- 99.Ahmed EA, van der Vaart A, Barten A, Kal HB, Chen J, Lou Z, et al. Differences in DNA double strand breaks repair in male germ cell types: lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair. 2007;6:1243–1254. doi: 10.1016/j.dnarep.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 100.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hussain SK, Sequerra R, Bertucci C, Hastings NC, Rieder M, Schwartz SM. Sequence variation in the human transcription factor gene POU5F1. BMC Genet. 2008;9:15. doi: 10.1186/1471-2156-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 103.Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transciption factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 104.Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Bolani M, et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tenenhaus Dann C, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 106.Frankenberg S, Pask A, Renfree MB. The evolution of class V POU domain transcription factors in vertebrates and their characterization in a marsupial. Dev Biol. 2010;337:162–170. doi: 10.1016/j.ydbio.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 107.Sasaki T, Marcon E, McQuire T, Arai Y, Moens PB, Okada H. Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis. J Cell Biol. 2008;182:449–458. doi: 10.1083/jcb.200802113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ito E, Obayashi S, Nagai A, Imamura M, Azuma H. Regulation of myometrial contractivity during pregnancy of the rat: potential role for DDAH. Mol Hum Reprod. 2009;15:507–512. doi: 10.1093/molehr/gap041. [DOI] [PubMed] [Google Scholar]
- 109.Xu K, Zhou H, Bai L, Xiang Y. The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin Chim Acta. 2010;411:49–52. doi: 10.1016/j.cca.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 110.Terribas E, Bonache S, Garcia-Arevalo M, Sanchez J, Franco E, Bassas L, et al. Changes in the expression profile of the meiosis-involved mismatch repair (MMR) genes in impaired human spermatogenesis. J Androl. 2010 doi: 10.2164/jandrol.109.008805. In press. [DOI] [PubMed] [Google Scholar]
- 111.Ito Y, Ando A, Ando H, Ando J, Saijoh Y, Inoko H, et al. Genomic structure of the spermatid-specific hsp70 homolog gene located in the class III region of the major histocompatibility complex of mouse and man. J Biochem. 1998;124:347–353. doi: 10.1093/oxfordjournals.jbchem.a022118. [DOI] [PubMed] [Google Scholar]
- 112.Neuhaus EM, Mashukova A, Zhang W, Barbour J, Hatt H. A specific heat shock protein enhances the expression of mammalian olfactory receptor proteins. Chem Senses. 2006;31:445–452. doi: 10.1093/chemse/bjj049. [DOI] [PubMed] [Google Scholar]
- 113.Snoek M, Jansen M, Olavesen MG, Campbell RD, Teuscher C, van Vugt H. Three Hsp70 genes are located in the C4-H-2D region: possible candidates for the Orch-1 locus. Genomics. 1993;15:350–356. doi: 10.1006/geno.1993.1067. [DOI] [PubMed] [Google Scholar]
- 114.Fiorenza MT, Bevilacqua A, Canterini S, Torcia S, Pontecorvi M, Mangia F. Early transcriptional activation of the hsp70.1 gene by osmotic stress in one-cell embryos of the mouse. Biol Reprod. 2004;70:1606–1613. doi: 10.1095/biolreprod.103.024877. [DOI] [PubMed] [Google Scholar]
- 115.Wagschal A, Sutherland HG, Woodfine K, Henckel A, Chebli K, Schulz R, et al. G9a Histone methyltransferase contributes to imprinting in the mouse placenta. Mol Cell Biol. 2008;28:1104–1113. doi: 10.1128/MCB.01111-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Selleri L, DiMartino J, van Deursen J, Brendolan A, Sanyal M, Boon E, et al. The TALE homeodomain protein Pbx2 is not essential for development and long-term survival. Mol Cell Biol. 2004;24:5324–5331. doi: 10.1128/MCB.24.12.5324-5331.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang F, Liu H, Blanton WP, Belkina A, Lebrasseur NK, Denis GV. Brd2 disruption in mice causes severe obesity without Type 2 diabetes. Biochem J. 2010;425:71–83. doi: 10.1042/BJ20090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thorpe KL, Beck S. DNA sequence and structure of the mouse RING3 gene: identification of variant RING3 transcripts. Immunogenetics. 1998;48:82–86. doi: 10.1007/s002510050406. [DOI] [PubMed] [Google Scholar]
- 120.Pal DK, Evgrafov OV, Tabares P, Zhang F, Durner M, Greenberg DA. BRD2 (RING3) is a probable major susceptibility gene for common juvenile myoclonic epilepsy. Am J Hum Genet. 2003;73:261–270. doi: 10.1086/377006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pivot-Pajot C, Caron C, Govin J, Vion A, Rousseaux S, Khochbin S. Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol Cell Biol. 2003;23:5354–5365. doi: 10.1128/MCB.23.15.5354-5365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sperry AO, Zhao LP. Kinesin-related proteins in the mammalian testes: candidate motors for meiosis and morphogenesis. Mol Biol Cell. 1996;7:289–305. doi: 10.1091/mbc.7.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang WX, Sperry AO. C-terminal kinesinmotor KIFC1 participates in acrosome biogenesis and vesicle transport. Biol Reprod. 2003;69:1719–1729. doi: 10.1095/biolreprod.102.014878. [DOI] [PubMed] [Google Scholar]
- 124.Yang WX, Jefferson H, Sperry AO. The molecular motor KIFC1 associates with a complex containing nucleoporin NUP62 that is regulated during development and by the small GTPase RAN. Biol Reprod. 2006;74:684–690. doi: 10.1095/biolreprod.105.049312. [DOI] [PubMed] [Google Scholar]
- 125.Yu K, Hou L, Zhu JQ, Ying XP, Yang WX. KIFC1 participates in acrosomal biogenesis, with discussion of its importance for the perforatorium in the Chinese mitten crab Eriocheir sinensis. Cell Tissue Res. 2009;337:113–123. doi: 10.1007/s00441-009-0800-3. [DOI] [PubMed] [Google Scholar]
- 126.Kelley J, Walter L, Trowsdale J. Comparative genomics of major histocompatibility complexes. Immunogenetics. 2005;56:683–695. doi: 10.1007/s00251-004-0717-7. [DOI] [PubMed] [Google Scholar]
- 127.Ober C, Weitkamp LR, Cox N, Dytch H, Kostyu DD, Elias S. HLA and mate choice in humans. Am J Hum Genet. 1997;61:497–504. doi: 10.1086/515511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roberts SC, Little AC, Gosling LM, Jones BC, Perrett DI, Carter V, et al. MHC-assortative facial preferences in humans. Biol Lett. 2005;1:400–403. doi: 10.1098/rsbl.2005.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roberts SC, Little AC, Gosling LM, Perrett DI, Carter V, Jones BC, et al. MHC-heterozygosity and human facial attractiveness. Evol Hum Behav. 2005;26:213–226. [Google Scholar]
- 130.Snead MP, Yates JRW. Clinical and molecular genetics of Stickler syndrome. J Med Genet. 1999;36:353–359. [PMC free article] [PubMed] [Google Scholar]
- 131.Milinski M, Wedekind C. Evidence for MHC-correlated perfume preferences in humans. Behav Ecol. 2001;12:140–149. [Google Scholar]
- 132.Jacob S, McClintock MK, Zelano B, Ober C. Paternally inherited HLA alleles are associated with women's choice of male odor. Nat Genet. 2002;30:175–179. doi: 10.1038/ng830. [DOI] [PubMed] [Google Scholar]
- 133.Potts WK. Wisdom through immunogenetics. Nat Genet. 2002;30:130–131. doi: 10.1038/ng0202-130. [DOI] [PubMed] [Google Scholar]
- 134.Carroll LS, Penn DJ, Potts WK. Discrimination of MHC-derived odors by untrained mice is consistent with divergence in peptide-binding region residues. Proc Natl Acad Sci USA. 2002;99:2187–2192. doi: 10.1073/pnas.042244899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors: II. Relationship among odor maps, genetics, odor composition and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beauchamp GK, Yamazaki K. Chemical signaling in mice. Biochem Soc Trans. 2003;31:147–151. doi: 10.1042/bst0310147. [DOI] [PubMed] [Google Scholar]
- 137.Shepherd GM. Smell images and the flavour system in the human brain. Nature. 2006;444:316–321. doi: 10.1038/nature05405. [DOI] [PubMed] [Google Scholar]
- 138.Penn DJ, Oberzaucher E, Grammer K, Fischer G, Soini HA, Wiesler D, et al. Individual and gender fingerprints in human body odour. J R Soc Interface. 2007;4:331–340. doi: 10.1098/rsif.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bard J, Yamazaki K, Curran M, Boyse EA, Beauchamp GK. Effect of B2m gene disruption on MHC-determined odortypes. Immunogenetics. 2000;51:514–518. doi: 10.1007/s002510000165. [DOI] [PubMed] [Google Scholar]
- 140.Madden DR, Gorga JC, Strominger JL, Wiley DC. The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC. Cell. 1992;70:1035–1048. doi: 10.1016/0092-8674(92)90252-8. [DOI] [PubMed] [Google Scholar]
- 141.Lopez de Castro JA, Alvarez I, Marcilla M, Paradela M, Ramos M, Sesma L, et al. HLA-B27: a registry of constitutive peptide ligands. Tissue Antigens. 2004;63:424–445. doi: 10.1111/j.0001-2815.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- 142.Ziegler A, Loll B, Misselwitz R, Uchanska-Ziegler B. Implications of structural and thermodynamic studies of HLA-B27 subtypes exhibiting differential association with ankylosing spondylitis. Adv Exp Med Biol. 2009;649:177–195. doi: 10.1007/978-1-4419-0298-6_13. [DOI] [PubMed] [Google Scholar]
- 143.Ziegler A, Muller CA, Bockmann RA, Uchanska-Ziegler B. Low-affinity peptides and T-cell selection. Trends Immunol. 2009;30:53–60. doi: 10.1016/j.it.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 145.Mombaerts P. Genes and ligands for odorant, vomeronasal and tast receptors. Nat Rev Neurosci. 2004;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 146.Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;29:100–107. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- 148.Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemoreceptors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 149.Munger SD. Noses within noses. Nature. 2009;459:521–522. doi: 10.1038/459521a. [DOI] [PubMed] [Google Scholar]
- 150.Slev PR, Nelson AC, Potts WK. Sensory neurons with MHC-like peptide binding properties: disease consequences. Curr Opin Immunol. 2006;18:608–616. doi: 10.1016/j.coi.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 151.Leinders-Zufall T, Ishii T, Mombaerts P, Zufall F, Boehm T. Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nat Neurosci. 2009;12:1551–1558. doi: 10.1038/nn.2452. [DOI] [PubMed] [Google Scholar]
- 152.Alper CA, Larsen CE, Dubey DP, et al. The Haplotype structure of the human major histocompatibility complex. Hum Immunol. 2006;67:73–84. doi: 10.1016/j.humimm.2005.11.006. (2006) [DOI] [PubMed] [Google Scholar]
- 153.Santos PSC, Füst G, Prohaszka Z, Volz A, Horton R, Miretti M, et al. Association of smoking behavior with an odorant receptor allele telomeric to the human major histocompatibility complex. Genetic Testing. 2008;12:481–486. doi: 10.1089/gte.2008.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thompson E, Haller G, Pinto J, Sun Y, Zelano B, Jacob S, et al. Sequence variations at the human leukocyte antigen-linked olfactory receptor cluster do not influence female preferences for male odors. Hum Immunol. 2010;71:100–103. doi: 10.1016/j.humimm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romanes GJ. Experiments on the sense of smell in dogs. Nature. 1887;36:273–274. [Google Scholar]
- 156.Kalmus H. The discrimination by the nose of the dog of individual human odours and in particular of the odours of twins. Br J Anim Behav. 1955;3:25–31. [Google Scholar]
- 157.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 158.Kuhlmann D, Dohr G, Pusch HH, Scherbaum W, Schieferstein G, Uchanska-Ziegler B, et al. Absence of HLA class I and class II antigens as well as β2-microglobulin from normal and pathological human spermatozoa. Tissue Antigens. 1986;27:179–184. doi: 10.1111/j.1399-0039.1986.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 159.Dohr GA, Motter W, Leitinger S, Desoye G, Urdl W, Winter R, et al. Lack of expression of HLA class I and class II molecules on the human oocyte. J Immunol. 1987;138:3766–3770. [PubMed] [Google Scholar]
- 160.Desoye G, Dohr GA, Ziegler A. Expression of human major histocompatibility antigens on germ cells and early preimplantation embryos. Lab Invest. 1991;64:306–312. [PubMed] [Google Scholar]
- 161.Hecht NB. Molecular mechanisms of male germ cell differentiation. Bioessays. 1998;20:555–561. doi: 10.1002/(SICI)1521-1878(199807)20:7<555::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 162.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 163.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 164.Schaller CE, Wang CL, Beck-Engeser G, Goss L, Scott HS, Anderson MS, et al. Expression of Aire and the early wave of apoptosis in spermatogenesis. J Immunol. 2008;180:1338–1343. doi: 10.4049/jimmunol.180.3.1338. [DOI] [PubMed] [Google Scholar]
- 165.Fukuda N, Yomogida K, Okabe M, Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci. 2004;117:5835–5845. doi: 10.1242/jcs.01507. [DOI] [PubMed] [Google Scholar]
- 166.Branscomb A, Seger J, White RL. Evolution of odorant receptors expressed in mammalian testes. Genetics. 2000;156:785–797. doi: 10.1093/genetics/156.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tatsura H, Nagao H, Tamada A, Sasaki S, Kohri K, Mori K. Developing germ cells in mouse testis express pheromone receptors. FEBS Lett. 2001;488:139–144. doi: 10.1016/s0014-5793(00)02411-x. [DOI] [PubMed] [Google Scholar]
- 168.Walensky LD, Ruat M, Bakin RE, Blackshaw S, Ronnett GV, Snyder SH. Two novel odorant receptor families expressed in spermatids undergo 5′-splicing. J Biol Chem. 1998;273:9378–9387. doi: 10.1074/jbc.273.16.9378. [DOI] [PubMed] [Google Scholar]
- 169.Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species. Genomics. 1997;39:239–246. doi: 10.1006/geno.1996.4490. [DOI] [PubMed] [Google Scholar]
- 170.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 171.Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Olfactory receptors are displayed on dog mature sperm cells. J Cell Biol. 1993;123:1441–1452. doi: 10.1083/jcb.123.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fukuda N, Touhara K. Developmental expression patterns of testicular olfactory receptor genes during mouse spermatogenesis. Genes Cells. 2006;11:71–81. doi: 10.1111/j.1365-2443.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- 173.Villanueva-Diaz C, Vadillo-Ortega F, Kably-Ambe A, de los Angeles Diaz-Perez M, Krivitzky SK. Evidence that human follicular fluid contains a chemoattractant for spermatozoa. Fertil Steril. 1990;54:1180–1182. doi: 10.1016/s0015-0282(16)54027-8. [DOI] [PubMed] [Google Scholar]
- 174.Eisenbach M, Tur-Kaspa I. Do human eggs attract spermatozoa? BioEssays. 1999;21:203–210. doi: 10.1002/(SICI)1521-1878(199903)21:3<203::AID-BIES4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 175.Kaupp UB, Kashikar ND, Weyand I. Annu Rev Physiol. 2008;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- 176.Eisenbach M, Giojalas LC. Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 177.Stockley P. Sperm competition in mammals. Hum Fertil. 2004;7:91–97. doi: 10.1080/14647270410001699054. [DOI] [PubMed] [Google Scholar]
- 178.Pizzari T, Foster KR. Sperm sociality: cooperation, altruism and spite. PLoS Biol. 2008;6:130. doi: 10.1371/journal.pbio.0060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koelman CA, Coumans ABC, Nijman HW, Doxiadis IIN, Dekker GA, Claas FHJ. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. 2000;46:155–166. doi: 10.1016/s0165-0378(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 180.Lyon MF. Transmission ratio distortion in mice. Annu Rev Genet. 2003;37:393–408. doi: 10.1146/annurev.genet.37.110801.143030. [DOI] [PubMed] [Google Scholar]
- 181.Veron N, Bauer H, Weisse AY, Luder G, Werber M, Herrmann BG. Retention of gene products in syncytial spermatids promotes non-Mendelian inheritance as revealed by the t complex responder. Genes Dev. 2010;23:2705–2710. doi: 10.1101/gad.553009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schwensow N, Eberle M, Sommer S. Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proc R Soc B. 2008;275:555–564. doi: 10.1098/rspb.2007.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Willis BL, van Oppen MJH, Miller DJ, Vollmer SV, Ayre DJ. The role of hybridization in the evolution of reef corals. Ann Rev Ecol Evol Syst. 2006;37:489–517. [Google Scholar]
- 184.Lessios HA. Reproductive isolation between species of sea urchins. Bull Mar Sci. 2007;81:191–208. [Google Scholar]
- 185.Galindo BE, Vacquier VD, Swanson WJ. Positive selection in the egg receptor for abalone sperm lysin. Proc Natl Acad Sci USA. 2003;100:4639–4643. doi: 10.1073/pnas.0830022100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Morgan TH. The genetic and the physiological problems of self-sterility in Ciona VI. Theoretical discussion of genetic data. J Exp Zool. 1944;95:37–59. [Google Scholar]
- 187.De Tomaso AW, Nyholm SV, Palmeri KJ, Ishizuka KJ, Luddington WB, Mitchel K, et al. Isolation and characterization of a protochordate histocompatibility locus. Nature. 2005;438:454–459. doi: 10.1038/nature04150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McKitrick TR, De Tomaso AW. Molecular mechanisms of alloreceognition in a basal chordate. Semin Immunol. 2010;22:34–38. doi: 10.1016/j.smim.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bookbinder LH, Cheng A, Bleil JD. Tissue- and species-specific expression of sp56, a mouse sperm fertilization protein. Science. 1995;269:86–89. doi: 10.1126/science.7604284. [DOI] [PubMed] [Google Scholar]
- 190.Bleil JD, Wassarman PM. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980;20:873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- 191.Bleil JD, Wassarman PM. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 192.Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, et al. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucid and infertility in female mice. Proc Nat Acad Sci USA. 1996;93:5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucid and are infertile. Development. 1996;122:2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- 194.Monne M, Han L, Schwend T, Burendahl S, Jovine L. Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats. Nature. 2008;456:653–657. doi: 10.1038/nature07599. [DOI] [PubMed] [Google Scholar]
- 195.Yoshida M, Kawano N, Yoshida K. Control of sperm motility and fertility: Diverse factors and common mechanisms. Cell Mol Life Sci. 2008;65:3446–3457. doi: 10.1007/s00018-008-8230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wedekind C, Chapuisat M, Macas E, Rulicke T. Non-random fertilization in mice correlates with the MHC and something else. Heredity. 1996;77:400–409. doi: 10.1038/hdy.1996.160. [DOI] [PubMed] [Google Scholar]
- 197.Rulicke T, Chapuisat M, Homberger FR, Macas E, Wedekind C. MHC-genotype of progeny influenced by parental infection. Proc R Soc Lond B. 1998;265:711–716. doi: 10.1098/rspb.1998.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McLay DW, Clarke HJ. Remodelling the paternal chromatin at fertilization in mammals. Reproduction. 2003;125:625–633. doi: 10.1530/rep.0.1250625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- 200.Pittoggi C, Magnano AR, Sciamanna I, Giordano R, Lorenzini R, Spadafora C. Specific localization of transcription factors in the chromatin of mouse mature spermatozoa. Mol Reprod Dev. 2001;60:97–106. doi: 10.1002/mrd.1066. [DOI] [PubMed] [Google Scholar]
- 201.Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, et al. Transcriptional regulation of Nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 203.Lalancette C, Miller D, Li Y, Krawetz SA. Paternal contributions: new functional insights for spermatozoal RNA. J Cell Biochem. 2008;104:1570–1579. doi: 10.1002/jcb.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hayashi S, Yang J, Christenson L, Yanagimachi R, Hecht NB. Mouse preimplantation embryos developed from oocytes injected with round spermatids or spermatozoa have similar but distinct patterns of early messenger RNA expression. Biol Reprod. 2003;69:1170–1176. doi: 10.1095/biolreprod.103.016832. [DOI] [PubMed] [Google Scholar]
- 205.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. RNA-mediated non-Mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 206.Chandler VL. Paramutation: from maize to mice. Cell. 2007;128:641–645. doi: 10.1016/j.cell.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 207.Cuzin F, Grandjean V, Rassoulzadegan M. Inherited variation at the epigenetic level: paramutation from the plant to the mouse. Curr Opin Genet Dev. 2008;18:193–196. doi: 10.1016/j.gde.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 208.Rouviere C, Houliston E, Carre D, Chang P, Sardet C. Characteristics of pronuclear migration in Beroë ovata. Cell Mot Cytoskel. 1994;29:301–311. doi: 10.1002/cm.970290403. [DOI] [PubMed] [Google Scholar]
- 209.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;4:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 210.Xie D, Chen CC, Ptaszek LM, Xiao S, Cao X, Fang F, et al. Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species. Genome Res. 2010;20:804–815. doi: 10.1101/gr.100594.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sati L, Seval-Celik Y, Unek G, Korgun ET, Demir R. The presence of kinesin superfamilymotor proteins KIFC1 and KIF17 in normal and pathological human placenta. Placenta. 2009;30:848–854. doi: 10.1016/j.placenta.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 212.Blaschitz A, Juch H, Volz A, Hutter H, Daxboeck C, Desoye G, et al. The soluble pool of HLA-G produced by human trophoblasts does not include detectable levels of the intron 4-containing HLA-G5 and HLA-G6 isoforms. Mol Hum Reprod. 2005;11:699–710. doi: 10.1093/molehr/gah185. [DOI] [PubMed] [Google Scholar]
- 213.Gauster M, Blaschitz A, Dohr G. Monoclonal antibody HC10 does not bind HLA-G. Rheumatology. 2007;46:892–893. doi: 10.1093/rheumatology/kel440. [DOI] [PubMed] [Google Scholar]
- 214.James JL, Chamley LW. A caution on the use of HLA-G isoforms as markers of extravillous trophoblasts. Placenta. 2008;29:305–306. doi: 10.1016/j.placenta.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 215.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen LJ, Han ZQ, Zhou H, Zou L, Zou P. Inhibition of HLA-G expression via RNAi abolishes resistance of extravillous trophoblast cell line TEV-1 to NK lysis. Placenta. 2010;31:519–527. doi: 10.1016/j.placenta.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 217.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 218.Norman PJ, Abi-Rached L, Gendzekhadze K, Hammond JA, Moesta AK, Sharma D, et al. Meiotic recombination generates rich diversity in NK cell receptor genes, alleles and haplotypes. Genome Res. 2009;19:757–769. doi: 10.1101/gr.085738.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- 221.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- 222.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrlolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 223.Lehnertz B, Northrop JP, Antignano F, Burrows K, Hadidi S, Mullaly SC, et al. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med. 2010;207:915–922. doi: 10.1084/jem.20100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McCallum H, Jones M. To lose both would look like carelessness: Tasmanian devil facial tumour disease. PLoS Biol. 2006;4:342. doi: 10.1371/journal.pbio.0040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Santos PSC, Kellermann T, Uchanska-Ziegler B, Ziegler A. Genomic architecture of MHC-linked odorant receptor gene repertoires among 16 vertebrate species. Immunogenetics. 2010 doi: 10.1007/s00251-010-0468-6. in press. [DOI] [PubMed] [Google Scholar]