Abstract
The endometrium has a remarkable capacity for efficient repair; however, factors involved remain undefined. Premenstrual progesterone withdrawal leads to increased prostaglandin (PG) production and local hypoxia. Here we determined human endometrial expression of interleukin-8 (IL-8) and the roles of PGE2 and hypoxia in its regulation. Endometrial biopsy specimens (n = 51) were collected. Endometrial cells and explants were exposed to 100 nmol/L of PGE2 or 0.5% O2. The endometrial IL-8 concentration peaked during menstruation (P < 0.001) and had a significant proangiogenic effect. IL-8 was increased by PGE2 and hypoxia in secretory but not proliferative explants, which suggests that exposure to progesterone is essential. In vitro progesterone withdrawal induced significant IL-8 up-regulation in proliferative explants primed with progestins, but only in the presence of hypoxia. Epithelial cells treated simultaneously with PGE2 and hypoxia demonstrated synergistic increases in IL-8. Inhibition of HIF-1 by short hairpin RNA abolished hypoxic IL-8 induction, and inhibition of NF-κB by an adenoviral dominant negative inhibitor decreased PGE2-induced IL-8 expression (P > 0.05). Increased menstrual IL-8 is consistent with a role in repair. Progesterone withdrawal, hypoxia, and PGE2 regulate endometrial IL-8 by acting via HIF-1 and NF-κB. Hence, progesterone withdrawal may activate two distinct pathways to initiate endometrial repair.
Menstruation exhibits many of the classic hallmarks of inflammation. The withdrawal of progesterone in the late secretory phase of the cycle triggers a cascade of inflammatory mediators, leading to a dramatic influx of leukocytes into the premenstrual endometrium.1 After shedding, the human endometrium exhibits a remarkable and immediate regenerative capacity. This cyclical injury and repair is tightly controlled and, unlike resolution of inflammation at other sites in the body, does not involve loss of function or scarring. However, the precise local mechanisms involved in this efficient repair have not yet been fully elucidated. Aberrations may lead to menstrual disorders including heavy menstrual bleeding and dysmenorrhea. Delineation of the physiologic processes of the endometrium could result in new therapeutic targets for these common debilitating conditions. In addition, the efficient endometrial model may provide an informative comparator for other tissue sites associated with problematic scarring or persistent inflammation.
Withdrawal of progesterone occurs in the late secretory endometrium as the corpus luteum regresses. Progesterone withdrawal leads to up-regulation of endometrial cyclooxygenase-2 (COX-2) and subsequent increased levels of prostaglandins (PGs), namely, PGE2 and PGF2α.1, 2 PGF2α induces myometrial contractions and vasoconstriction of the endometrial spiral arterioles. Consequently, it is believed that there is an episode of transient hypoxia in the uppermost endometrial zones. The existence of hypoxia was confirmed in a murine model of menstruation using pimonidazole, a marker of pO2 less than 10 mm Hg.3 The luminal portion of the endometrial functional layer was demonstrated to be intensely hypoxic during simulated menstruation, with negligible detection of pimonidazole by day 5. It was hypothesized that PGF2α along with other endometrial vasoconstrictors induces hypoxic conditions in the human perimenstrual endometrium to increase repair gene expression. The role of the other major prostaglandin present during the premenstrual phase, PGE2, is not fully understood. It was proposed, therefore, that PGE2 may also independently increase expression of genes responsible for endometrial repair.
Interleukin-8 (IL-8, CXCL8) is a CXC chemokine, best known for its role as a potent chemoattractant for neutrophils and T cells.4 In addition, it has mitogenic properties and a key role in angiogenesis in vivo.5 These processes are fundamental for endometrial shedding and repair. The present study demonstrated significant changes in IL-8 mRNA and protein expression during the menstrual cycle, with maximal expression at menstruation. Concentrations of IL-8 secreted by menstrual endometrium exhibited significantly greater angiogenic potential in vitro than did concentrations secreted by mid-secretory endometrium. IL-8 expression is up-regulated in endometrial epithelial cells by hypoxic conditions and by PGE2, with a synergistic increase observed in the presence of both factors. An in vitro model of progesterone withdrawal also increased IL-8 expression in human endometrial tissue, but only with the addition of hypoxic conditions. The presence of indomethacin, a COX enzyme inhibitor, attenuated the increase in IL-8 expression in this model. These observations suggest a role for progesterone withdrawal in the initiation of endometrial repair and indicate that subsequent hypoxia and PGE2 are necessary for increased expression of IL-8, an angiogenic factor with a putative role in the repair process.
Materials and Methods
Human Endometrial Tissue Collection and Culture
Human endometrial biopsy specimens were collected from women undergoing hysterectomy or investigation in the gynecologic outpatient setting (n = 51). Ethical approval was obtained from the Lothian Research Ethics Committee, and written informed consent was obtained from all participants before tissue collection. Participants were aged 31 to 52 years (median, 41 years; mean, 41 years). All women reported regular menstrual cycles (duration, 21 to 35 days) and had not taken exogenous hormones or used an intrauterine device during the 3 months before endometrial biopsy. Women with known uterine disease such as large myomas (>3 cm) and endometriosis were excluded. Endometrial biopsy specimens were collected using an endometrial suction curette (Pipelle; Laboratoire CCD, Paris, France). Immediately after collection, tissue was divided and i) placed in RNA stabilization solution (RNA Later; Ambion (Europe) Ltd., Warrington, UK), ii) stored at −70°C for RNA extraction, iii) fixed in neutral buffered formalin for wax embedding or iv) placed in PBS for in vitro culture. The specimens were dated according to the criteria of Noyes et al6 based on histologic appearance, which was consistent with the participants' reported last menstrual period. In addition, serum samples were collected from each woman at biopsy to determine circulating serum progesterone and estradiol concentrations, and were consistent for both last menstrual period and histologic assessment. For analysis, biopsy specimens were classified as proliferative, early secretory, mid secretory, late secretory, or menstrual (Table 1). Seven women consented to undergo a second endometrial biopsy, and returned for this procedure three to six months after insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS) for treatment of subjective report of heavy menstrual bleeding.
Table 1.
Circulating Estradiol and Progesterone Concentrations at Endometrial Biopsy
| Histologic stage of cycle | Age, mean, years | E2, mean (range), pmol/L | P4, mean (range), nmol/L |
|---|---|---|---|
| Menstrual (n = 8) | 41 | 192.25 (55–514) | 3.71 (1.24–10.59) |
| Proliferative (n = 16) | 42 | 441.18 (79–1105) | 2.81 (0.97–7.1) |
| Early secretory (n = 10) | 42 | 497.50 (289–841) | 59.60 (23.2–112.91) |
| Mid secretory (n = 11) | 40 | 638.00 (242–1949) | 64.30 (25.47–114.53) |
| Late secretory (n = 6) | 42 | 318.22 (59.09–819) | 8.22 (1.06–16.95) |
In Vitro Culture of Endometrial Tissue
Endometrial biopsy specimens (secretory phase, n = 7; proliferative phase, n = 3) were divided into three equal explants and incubated for at least 16 hours on raised platforms in 24-well plates just covered with serum-free RPMI 1640 medium plus 50 μg/ml of penicillin, 50 μg/ml of streptomycin, and 5 μg/ml of gentamicin (all from Sigma Aldrich, St. Louis, MO), and 8.4 μmol/L of indomethacin. The next day, two explants were treated with vehicle under normoxic conditions, 1 with 21% O2, 5% CO2, and 37°C, and one with 100 nmol/L of PGE2. The last explant was placed in a sealed hypoxic chamber (Coy Laboratory Products Inc., Grass Lake, MI) set at 0.5%O2, 5% CO2, and 37°C for 24 hours.
Five endometrial biopsy specimens from the proliferative phase were divided into 8 equal-sized explants and placed on raised platforms in four wells of 2 × 24-well plates. All explants were treated with 1 μmol/L of medroxyprogesterone acetate (MPA) for 24 hours. Explants were then treated with either 1 μmol/L of MPA plus vehicle, 1 μmol/L of MPA plus 8.4 μmol/L of indomethacin (a COX enzyme inhibitor), 1 μmol/L of MPA and 1 μmol/L of RU486 (a progesterone-receptor antagonist) plus vehicle, or 1 μmol/L of MPA and RU486 plus 8.4 μmol/L of indomethacin. One plate was placed in normoxic conditions, and the other in hypoxic conditions, for 48 hours.
Culture of Endometrial Cells
Human Ishikawa endometrial adenocarcinoma cells (European Collection of Cell Cultures, Centre for Applied Microbiology, Wiltshire, UK) stably expressing the EP2 receptor (EP2S)7 were maintained in Dulbecco modified Eagle medium nutrient mixture F-12 with glutamax-1 and pyridoxine, supplemented with 10% fetal calf serum, 1% antibiotic (stock 500 IU/ml of penicillin and 500 μg/ml of streptomycin), and 200 μg/ml of G418 at 37°C. Primary human endometrial stromal cells were isolated from mid-secretory endometrial tissue (n = 3) via enzymatic digestion as previously described,8 and were maintained in RPMI 1640 medium plus 50 μg/ml of penicillin, 50 μg/ml of streptomycin, and 5 μg/ml of gentamicin (all from Sigma Aldrich).
Approximately 4 × 105 EP2S or 3 × 105 human endometrial stromal cells were seeded in 6-well plates. The following day, cells were incubated for at least 16 hours in serum-free culture medium containing antibiotics and 8.4 μmol/L of indomethacin. Cells were then treated with either vehicle or 100 nmol/L of PGE2 and placed at 37°C, 21% O2, and 5% CO2 for 2, 4, 8, 24, and 48 hours or placed in hypoxic conditions (0.5%O2 and 5% CO2) in a sealed chamber (Coy Laboratory Products Inc.) for the same amount of time. Alternatively, EP2S cells were pretreated with vehicle or 5 nmol/L of echinomycin (a specific inhibitor of HIF-1 DNA binding activity).9 After 1 hour, cells were stimulated for 6 hours with vehicle, 100 nmol/L of PGE2 with or without 5 nmol/L of echinomycin,or hypoxia with or without 5 nmol/L of echinomycin. A short-hairpin RNA (shRNA) sequence against human HIF-1α and scrambled control oligonucleotide (TIB MOLBIOL) were donated by Prof. T. Cramer (Charité-Universitätsmedizin Berlin, Berlin, Germany). A 19-nucleotide sequence derived from human HIF-1α mRNA (U22431; bp 1470 to 1489) was used and was termed HIF-1α/shRNA.10, 11 Cells were transiently transfected with lentivirus at a multiplicity of infection of 10 for 24 hours. Cells were incubated in serum-free medium overnight before treatment with 100 nmol/L of PGE2 or placed in the hypoxic chamber for 8 hours. Cells were washed with PBS and harvested, and RNA or protein was extracted for PCR or Western blot analysis. To determine the role of NF-κB in IL-8 up-regulation, EP2S cells were seeded at a density of 1 × 105. The following day, cells were infected with an adenovirus containing a dominant-negative Iκ-Bα mutant, which maintains NF-κB in a cytoplasmic location, or control adenovirus (Ad-d1703) at a total multiplicity of infection of 50 for 8 hours. Ad-d1703 and Ad–Iκ-Bα have been described previously.12, 13 Cells were serum-starved with 8.4 μmol/L of indomethacin for at least 16 hours before treatment with 100 nmol/L of PGE2 or hypoxic conditions for 6 hours.
Nuclear Protein Extraction
Protein was extracted from endometrial cells with a cytoplasmic protein lysis buffer (10 mmol/L of HEPES, pH 7.8), 10 nmol/L of KCl, 2 mmol/L of MgCl2, 1 mmol/L of dithiothreitol, 0.1 mmol/L of EDTA, and 10% Nonident P-40) containing protease inhibitors (Complete Mini Protease Inhibitor Cocktail; Roche Diagnostics, Ltd., Lewes, UK). After centrifugation at 13,000 rpm for 1 minute at 4°C, the cytoplasmic fraction supernatant was removed and stored at −80°C. The nuclear fraction was extracted using a nuclear protein lysis buffer (50 mmol/L of HEPES [pH 7.8], 50 nmol/L of KCl, 300 mmol/L of NaCl, 0.1 mmol/L of EDTA, 1 mmol/L of dithiothreitol, and 10% glycerol) containing protease inhibitors (Roche Diagnostics, Ltd), followed by agitation for 20 minutes at 4°C and centrifugation at 13,000 rpm for 5 minutes at 4°C. The nuclear fraction supernatant was removed and stored at −80°C. Protein content was determined using protein assay kits (Bio-Rad; Hemel Hempstead, UK).
HIF-1α Western Blot Analysis
For detection of HIF-1α and β-actin, 10 μg of nuclear protein was resuspended in a 2:1 ratio with Laemmli buffer (125 mmol/L Tris-HCl [pH 6.8], 4% SDS, 5% 2-mercaptoethanol, 20% glycerol, and 0.05% bromophenol blue) and denatured for 5 minutes at 90°C. Proteins were separated on 4% to 12% Bis-Tris gels (NuPAGE Novex; Invitrogen Corp., Carlsbad, CA) and transferred onto polyvinylidene difluoride membrane (Millipore Corp., Billerica, MA). Membranes were blocked overnight in 5% milk solution in Tris-buffered saline solution and Tween 20 (50 mmol/L of Tris HCl, 150 mmol/L of NaCl, and 0.05% v/v of Tween 20). After washing with Tris-buffered saline solution and Tween 20, the membranes were incubated with mouse monoclonal anti–HIF-1α antibody (BD Biosciences, Oxford, UK) (1:250) and rabbit polyclonal anti–β-actin (Abcam, Cambridge, UK) (1:5000). After washing, the membrane was incubated with horseradish peroxidase–conjugated goat anti-mouse IgG (DAKO Corp, Carpinteria, CA) or horseradish peroxidase–conjugated mouse anti-rabbit IgG (Sigma Aldrich) at 1:20,000. The chemiluminescent horseradish perioxidase substrate (Immobilon; Millipore Corp.) was used for immunoreactive protein detection according to the manufacturer's instructions.
Quantitative RT-PCR
Expression of IL-8 mRNA in endometrial tissue and Ishikawa cells was determined using quantitative RT-PCR (Taqman) analysis. Total RNA from cells and endometrial biopsy specimens was extracted using a kit (RNeasy Mini Kit; Qiagen Ltd, Sussex, UK) according to the manufacturer's instructions. Samples were treated for DNA contamination via DNA digestion during RNA purification. After extraction, RNA was quantified using a spectrophotometer (NanoDrop 1000, version 3.7; ThermoScientific, Wilmington, DE) and stored at −80°C. Quality of the RNA was assessed using a bioanalyzer (Agilent 2100 Bioanalyser System) in combination with RNA 6000 nano chips (Agilent Technologies, Palo Alto, CA).
RNA samples were reverse transcribed using 5.5 mmol/L of MgCl2, 0.5 mmol/L each of deoxynucleotide triphosphates, 2.5 μmol/L of random hexamers, 0.4 U/μlL of RNA inhibitor, and 1.25 U/μL of multiscribe reverse transcriptase (all from PE Biosystems, Warrington, UK). The mix was aliquoted into individual tubes, and 200 to 400 ng of RNA was added. A tube with no reverse transcriptase and a further tube with water were included to control for DNA contamination. After mixing, samples were incubated for 20 minutes at 25°C, 60 minutes at 42°C, and 5 minutes at 95°C. cDNA samples were subsequently stored at −20°C.
To measure cDNA expression, a reaction mix was prepared containing Taqman buffer (5.5 mmol/L of MgCl2, 200 μmol/L of deoxyadenosine triphosphate, 200 μmol/L of deoxycytidine, 200 μmol/L of deoxyguanosine, and 400 μmol/L of deoxyuridine triphosphate), ribosomal 18S primers and probe (Applied Biosystems, Warrington, UK), and specific forward and reverse primers and probe for IL-8 and EP2: IL-8 forward primer, 5′-CTGGCCGTGGCTCTCTTG-3′; reverse primer, 5′-TTAGCACTCCTTGGAAAACTG-3′; and probe, 5′-CCTTCCTGATTTCTGCAGCTCTGTGTGAA-3′; and EP2 forward primer, 5′-TGAAGTTGCAGGCGAGCA-3′; reverse primer, 5′-GACCGCTTACCTGCAGCT-3′; and probe, 5′-CCACCCTGCTGCTGCTGCTTCT-3′. After mixing, 36-μL aliquots were placed in separate tubes, and 1.5 μL of cDNA was added. Into one aliquot, 1.5 μL of water was added as a no template control. Triplicate 12-μL samples were placed in a PCR plate. PCR was performed using ABI Prism 7900 (Applied Biosystems). Data were analyzed and processed using Sequence Detector version 2.3 (PE Biosystems). Expression of target mRNA was normalized to RNA loading for each sample using the 18S ribosomal RNA as an internal standard.
IL-8 Enzyme-Linked Immunosorbent Assay
Endometrial tissue from women at each stage of the menstrual cycle was collected in PBS (n = 20), weighed, and incubated for 24 hours on raised platforms in 1 ml of serum-free RPMI 1640 medium with 50 μg/ml of penicillin, 50 μg/ml of streptomycin, and 5 μg/ml of gentamicin (all from Sigma Aldrich).
IL-8 protein secretion into the culture medium by EP2S cells and endometrial biopsy specimens after 24 hours was quantified using an in-house enzyme-linked immunosorbent assay as described previously.14 A mouse monoclonal anti-human IL-8 capture antibody and a biotinylated polyclonal goat anti-human IL-8 detection antibody were used (R&D Systems, Oxford, UK). Protein concentrations in the conditioned medium were normalized to tissue weight.
IL-8 Immunohistochemistry
IL-8 was immunolocalized in endometrial tissue sections as previously described.15 In brief, slides were dewaxed and rehydrated before antigen retrieval in 0.01mmol/L of sodium citrate on high power in a pressure cooker for 5 minutes. Primary antibody (rabbit polyclonal, 1:100) was added overnight at 4°C. After incubation with secondary antibody (goat anti-rabbit, 1:200) and avidin biotin peroxidise complex (ABC Elite; Vector Laboratories, Peterborough, UK), staining was detected with liquid biaminobenzidine (DAB kit; Zymed Laboratories, Inc., South San Francisco, CA). Localization and intensity of immunostaining were evaluated blindly by two independent observers using a previously validated semiquantitative scoring system (J.A.M.). Intensity was graded using a three-point scale (0 = no staining, 1 = mild staining, and 2 = strong staining). The percentage of cells stained at each of these intensities was assessed in each cellular compartment. A value was derived for each compartment using the sum of these percentages after multiplication by the intensity of staining.
Capillary Tube Formation Assay
Matrigel, 100 μL (BD Biosciences, Bedford, MA), was added in each well of a 48-well plate and allowed to polymerize for 1 hour at 37°C. Human umbilical vascular endothelial cells were seeded at a density of 2 × 104 in 200 μL of EBM-2 medium (Lonza, Walkersville, MD) supplemented with GA1000 and ascorbic acid SingleQuots (Lonza). Cells were then treated with 250 μL of culture supernatant from menstrual and mid-secretory tissue explants incubated in vitro for 24 hours (40 mg of tissue per milliliter of RPMI medium) or 0.5 or 20 ng of recombinant human IL-8 (R&D Systems) in 250 μL of medium. Each dose of IL-8 was assessed in triplicate in three separate experiments. Capillary tube formations were visualized after 8 hours. Images were captured in the same position in each well using an inverted microscope at ×5 magnification. Branch points of the formed tubes were counted by an observer (J.A.M.) blinded to the sample origin, and an average of the replicates was determined after unblinding.
Statistical Analysis
For mRNA expression in explants and cell culture, results are given as fold increase where relative expression of mRNA in cells treated with PGE2 was divided by the relative expression in vehicle-treated cells. Data are given as mean (SEM). Significant difference was determined using one-way analysis of variance of delta cycle threshold values using Tukey posttest analysis. For endometrial biopsy specimens from across the menstrual cycle, results are given as quantity relative to a comparator, a sample of RNA from the liver. Significant difference was determined using the Kruskal-Wallis nonparametric test with the Dunn multiple comparison posttest (Instat; GraphPad Software, Inc., San Diego, CA).
Results
Expression of IL-8 mRNA and Protein in Human Endometrium
IL-8 mRNA was present at low levels in endometrium from the proliferative, early secretory, and mid secretory stages of the menstrual cycle. A nonsignificant increase in IL-8 mRNA expression was observed in the late secretory phase. By the menstrual stage, IL-8 concentrations had increased significantly compared with endometrium from the proliferative (P < 0.01), early secretory (P < 0.001), and mid secretory (P < 0.05) stages (Figure 1A. The amount of IL-8 protein secreted from endometrial biopsy specimens cultured in vitro for 24 hours demonstrated a similar pattern (Figure 1B). Endometrium from the menstrual stage secreted significantly higher concentrations of IL-8 protein than did tissue from the early and mid secretory phases (P < 0.05). There was no significant decrease in IL-8 protein between the menstrual and proliferative stages. Immunolocalization of IL-8 demonstrated positive cytoplasmic staining in glandular epithelial, surface epithelial, stromal, and perivascular cells in endometrium from the menstrual phase of the cycle (Figure 1, C and D). In contrast, during the mid secretory phase of the cycle, stromal staining was negligible and glandular epithelial cells were only faintly positive (Figure 1, E and F). Semiquantitative scoring of staining intensity revealed that the strongest staining was in the glandular epithelial and perivascular cells (Figure 1G). IL-8 perivascular staining was significantly increased during the menstrual phase of the cycle when compared with the proliferative (P < 0.05), early secretory (P < 0.01), and mid secretory (P < 0.05) stages (Figure 1G). There was a nonsignificant increase in IL-8 staining in glandular epithelial and stromal cells during the menstrual phase (Figure 1G).
Figure 1.
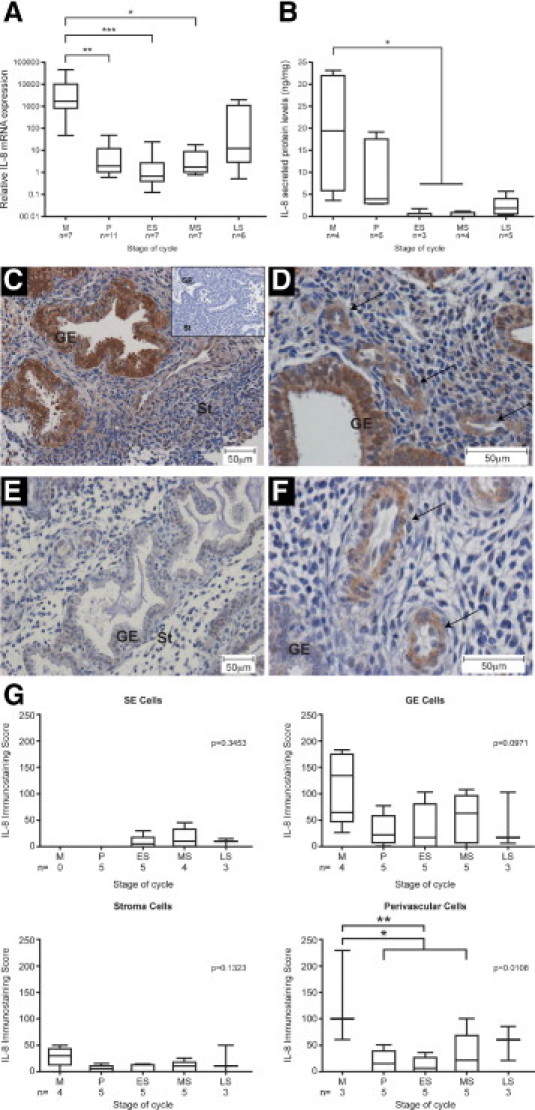
IL-8 is increased in endometrium from the menstrual phase of the cycle. A: Relative IL-8 mRNA expression in endometrium from across the menstrual cycle. Note logarithmic scale on the y axis. B: Secreted IL-8 protein levels by endometrial explants from different stages of the menstrual cycle cultured in vitro for 24 hours. Immunohistochemical staining for IL-8 in menstrual (C and D) and mid secretory (E and F) endometrium. Inset: Negative control menstrual endometrium. Scale bar = 50 μm. Arrows indicate perivascular cells. GE, glandular epithelial cells; St, stromal compartment. G: Semiquantitative scoring of IL-8 staining in endometrial surface epithelial (SE) cells, glandular epithelial (GE) cells, stromal compartment, and perivascular cells. Each box represents the 25th and 75th percentiles, and the whiskers the 10th and 90th percentiles. Horizontal lines represent the median. M, menstrual; P, proliferative; ES, early secretory; MS, mid secretory; LS, late secretory. *P < 0.05. **P < 0.01. ***P < 0.001. KW, Kruskal-Wallis statistical test.
Increased IL-8 Secretion by Menstrual Compared with Mid Secretory Endometrium Translates into Enhanced Angiogenic Activity
To assess the angiogenic potential of IL-8 produced by the endometrium, branching of human umbilical vascular endothelial cells (HUVECs) was quantified after various treatments. Compared with cells treated with unconditioned medium, cells treated with conditioned medium from menstrual tissue incubated for 24 hours in vitro demonstrated a significant increase in HUVEC capillary branch point formation (Figure 2A). No significant increase in angiogenesis was observed with conditioned medium from mid secretory phase explants. These endometrial explants are likely to produce several angiogenic factors. To assess the contribution of IL-8 alone, HUVECs were also treated with recombinant human IL-8. The mean (SEM; median) amount of IL-8 secreted by menstrual endometrial explants was 18.94 (7.57; 19.4) ng. Mid secretory endometrium secreted the lowest levels of IL-8: 0.53 (0.13; 0.44) ng. Therefore, HUVECs were treated with control medium, 20 ng or 0.5 ng of human recombinant IL-8. Compared with cells treated with 0.5 ng of IL-8 or control medium, treatment of HUVECs with 20 ng of IL-8 resulted in a significantly higher number of capillary tube branch points (Figure 2B). Mid-secretory levels of IL-8 had no significant effect on branch points when compared with control medium.
Figure 2.
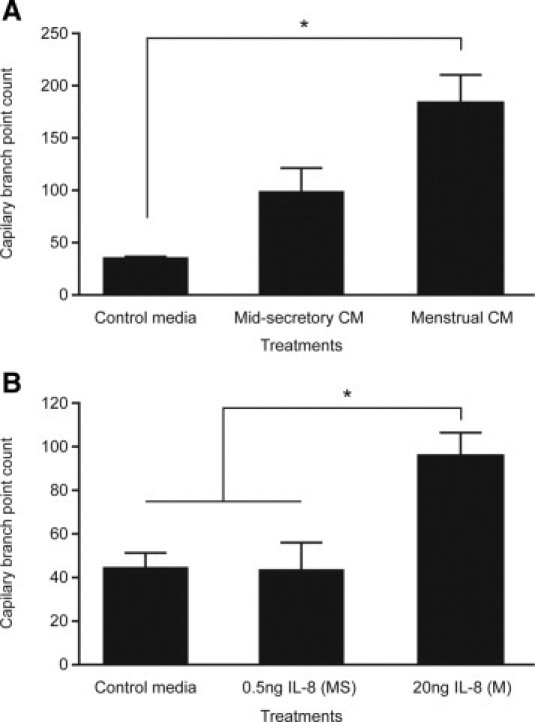
Menstrual levels of IL-8 can up-regulate network formation in HUVECs. A: The number of capillary tube branch points formed by HUVECs significantly increased when treated for 8 hours with conditioned medium (CM) from menstrual endometrial biopsy specimens cultured for 24 hours in vitro versus control medium. No such increase was seen with conditioned medium from mid secretory explants (n = 4 or 5). B: The number of capillary tube branch points formed by HUVECs treated with 20 ng of human recombinant IL-8 (M, menstrual levels) was significantly greater that those formed when treated with control medium or 0.5 ng of IL-8 (MS, mid secretory levels) (n = 3). *P < 0.05.
IL-8 mRNA Expression Is Increased by PGE2 and Hypoxia in Secretory Endometrial Tissue
To investigate the regulation of endometrial IL-8, human endometrial explants were cultured for 24 hours with vehicle, 100 nmol/L of PGE2, or hypoxic conditions. Secretory endometrium from seven women demonstrated a nonsignificant increase in IL-8 expression with PGE2 treatment under normoxic conditions. Culture of endometrial explants under hypoxic conditions significantly elevated IL-8 mRNA expression (P < 0.05) (Figure 3A). In contrast, neither treatment induced up-regulation of IL-8 in endometrium from the proliferative phase (n = 3) (Figure 3B). This suggests that previous exposure to progesterone is essential for up-regulation of IL-8 by PGE2 and hypoxia. There was no significant difference in EP2 receptor mRNA expression in response to PGE2 or hypoxia between explants from the proliferative and secretory phases of the cycle (data not shown).
Figure 3.
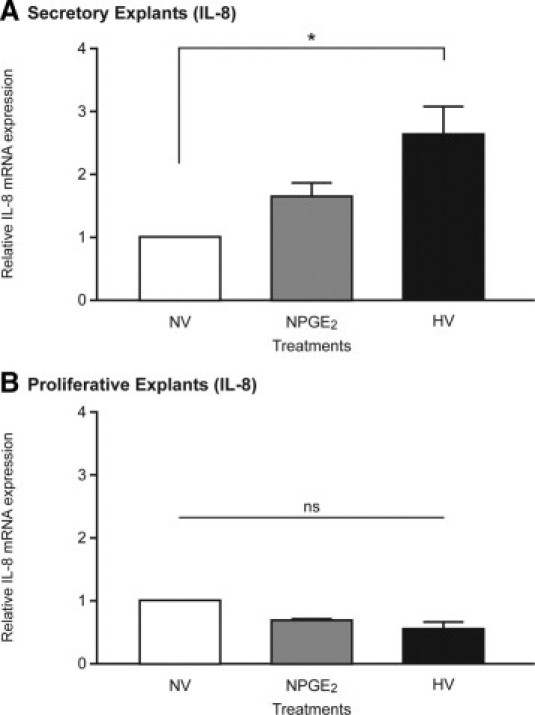
IL-8 mRNA up-regulation by PGE2 and hypoxia depends on cycle stage. A: IL-8 mRNA expression in early-mid-secretory endometrium (n = 7) after in vitro culture for 24 hours under normoxic conditions with vehicle (NV), normoxic conditions (21% O2) with 100 nmol/L PGE2 (NPGE2), or hypoxic conditions (0.5% O2) plus vehicle (HV). B: IL-8 mRNA expression in proliferative endometrium cultured in identical conditions (n = 3). *P < 0.05. ns, not significant.
In Vitro and in Vivo Models of Progesterone Withdrawal Increase IL-8 mRNA Expression
To establish whether progesterone withdrawal induces IL-8 mRNA expression, proliferative endometrial biopsy specimens were divided into 8 explants (n = 5). All explants were treated with MPA for 24 hours. After progesterone exposure, progesterone withdrawal was simulated in four of the explants by co-treating with RU486, a progesterone-receptor antagonist. Progesterone withdrawal under normoxic conditions did not significantly up-regulate IL-8 mRNA expression (Figure 4).
Figure 4.
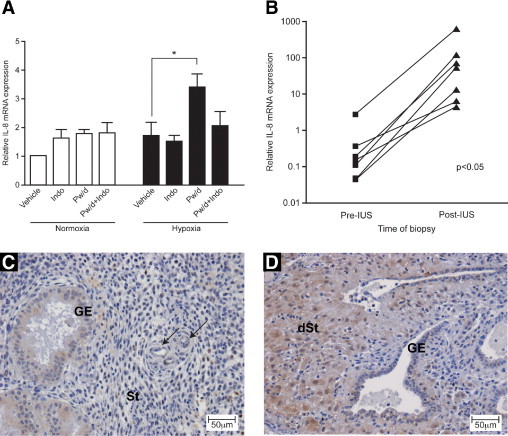
Progesterone withdrawal results in elevated IL-8 mRNA expression but only under hypoxic conditions. A: Significant up-regulation of IL-8 mRNA expression was observed in proliferative endometrium (n = 5, five different women) subjected to in vitro progesterone withdrawal (Pw/d) in the presence of hypoxia (*P < 0.05). This effect was abrogated by the addition of 8.4 μmol/L of indomethacin (Indo), a COX inhibitor. B: Insertion of the LNG-IUS, with consequent local progesterone deprivation analogous to local progesterone withdrawal, resulted in a significant increase in IL-8 mRNA (P < 0.05). Note logarithmic scale on the y axis. Immunohistochemical staining of IL-8 in mid secretory phase endometrium before IUS insertion (C) and in endometrium from the same woman four months after IUS insertion (D). GE, glandular epithelial cells; St, stromal compartment; dSt, decidualized stromal cells. Arrows indicate perivascular cells.
It was postulated that in vivo, progesterone withdrawal in the late secretory phase induces synthesis of prostaglandins and constriction of spiral arterioles, resulting in an episode of transient hypoxia. Therefore, to mimic the in vivo condition more accurately, two endometrial explants were exposed to hypoxic conditions at simulated progesterone withdrawal. Addition of hypoxic conditions induced significant induction of IL-8 mRNA expression 48 hours after progesterone withdrawal (P < 0.05) (Figure 4A).
To assess the contribution of prostaglandins after progesterone withdrawal, explants were concomitantly treated with MPA (progestogen), RU486 (progesterone-receptor antagonist), and indomethacin (a COX enzyme inhibitor). Addition of indomethacin attenuated up-regulation of IL-8 mRNA after progesterone withdrawal under hypoxic conditions (Figure 4A).
To further investigate the role of progesterone and hypoxia in regulating endometrial IL-8 expression, endometrial biopsy specimens from seven women obtained before and 3 to 6 months after LNG-IUS insertion were examined. The LNG-IUS markedly down-regulated the progesterone receptor in all components of the endometrium,16 resulting in a human model of progesterone deficiency. At comparison of endometrium obtained during the proliferative, early secretory, and mid secretory stages with paired samples obtained after 3- to 6-month exposure to LNG-IUS (n = 7), significant up-regulation of IL-8 mRNA expression was observed after LNG-IUS exposure (P < 0.05) (Figure 4B). This increase in endometrial IL-8 after LNG-IUS insertion was also identified at the protein level. Increased IL-8 immunohistochemical staining was visible in the decidualized stromal cells present after LNG-IUS exposure (Figure 4, C and D). Endometrial biopsy specimens obtained during the late secretory and menstrual phases (n = 2) demonstrated no significant change in IL-8 mRNA expression on exposure to the LNG-IUS (data not shown). This suggests that endometrium already exposed to progesterone withdrawal in vivo has no further capacity for IL-8 induction on insertion of LNG-IUS.
PGE2 and Hypoxia Increase IL-8 mRNA and Protein Expression in Endometrial Epithelial Cells and Together Result in a Synergistic Increase
To delineate the mechanisms by which PGE2 and hypoxia induce IL-8 expression, an Ishikawa endometrial epithelial cell line stably expressing the EP2 receptor was used. This cell line was used to mimic primary endometrial epithelial cells, which express receptors for PGE2.17 Cells were exposed to treatment with vehicle or 100 nmol/L of PGE2 for up to 48 hours under normoxic and hypoxic conditions. Treatment with PGE2 under normoxic conditions (Figure 5A) demonstrated a significant increase in IL-8 mRNA expression, with maximal up-regulation after 8 hours (P < 0.01). Hypoxic conditions also significantly increased IL-8 mRNA expression (Figure 5B) but exhibited a more delayed induction, reaching maximum up-regulation after 8 to 24 hours (P < 0.01). When cells were exposed to both PGE2 and hypoxic conditions for 24 hours (Figure 5C), there was a synergistic increase in IL-8 mRNA expression that was significantly greater than with treatment with PGE2 in normoxia (P < 0.05) or hypoxia (P < 0.05) alone. Levels of secreted IL-8 protein demonstrated a similar pattern, with a synergistic increase in IL-8 protein secretion with PGE2 treatment under hypoxic conditions (Figure 5D). In contrast, in human endometrial stromal cells, hypoxic conditions had no significant effect on IL-8 mRNA expression or protein levels at any time examined (data not shown). Treatment with 100 nmol/L of PGE2 resulted in a significant increase in IL-8 mRNA expression after 48 hours (P < 0.05) and a nonsignificant increase in secreted protein levels at the same time point (data not shown).
Figure 5.
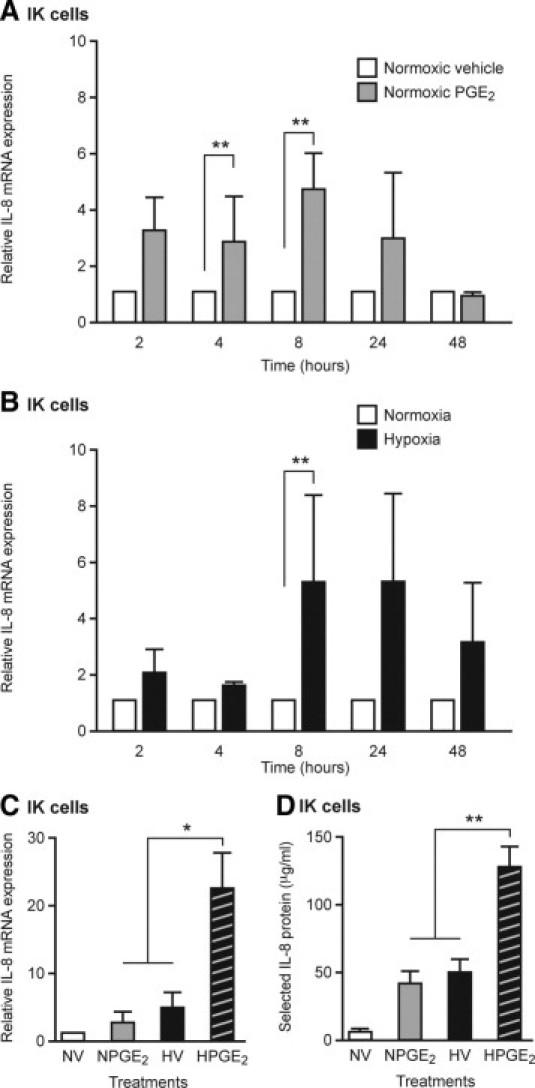
IL-8 mRNA and protein expression is up-regulated by PGE2 and hypoxic conditions (0.5% O2) in endometrial epithelial cells. A: An endometrial epithelial cell line (EP2S cells) treated with 100 nmol/L of PGE2 under normoxic conditions (21% O2) showed significantly increased levels of IL-8 mRNA at 4 and 8 hours, compared with cells treated with vehicle (V) at the same time point (n = 3). B: EP2S cells under hypoxic conditions showed a slower pattern of IL-8 mRNA induction, reaching significance after 8 hours (n = 3). C: EP2S cells treated simultaneously with PGE2 and hypoxia revealed a synergistic increase in IL-8 mRNA expression after 24 hours when compared with treatment with PGE2 in normoxia or hypoxia alone (n = 3). D: This synergistic increase was also observed when examining IL-8 secreted protein levels in EP2S-conditioned medium from the same experiments (n = 3). *P < 0.05. **P < 0.01.
IL-8 Up-Regulation by PGE2 Under Normoxic Conditions Is Inhibited by a Dominant-Negative of NF-κB
To determine the role of NF-κB in up-regulation of IL-8 in the endometrium, cells were infected with a dominant-negative inhibitor of NF-κB (Ad–Iκ-Bα) and cultured for 6 hours either in the presence of vehicle or PGE2 or under hypoxic conditions. Infection of cells with Ad–Iκ-Bα resulted in significant reduction of PGE2-induced IL-8 mRNA expression (P < 0.05) when compared with uninfected cells or cells infected with control Ad-d1730 (Figure 6B). Hypoxia-induced IL-8 mRNA expression was not significantly affected by inhibition of NF-κB (Figure 6C).
Figure 6.
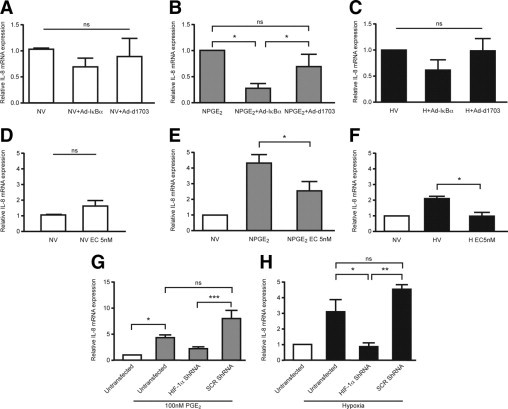
NF-κB and HIF-1 contribute to PGE2 and hypoxia-induced IL-8 mRNA expression. A: Infection of endometrial epithelial cells (EP2S cells) with a dominant-negative inhibitor of NF-κB (Ad–Iκ-Bα) or control adenovirus (Ad-d1703) had no significant effect on basal IL-8 levels. B: Cells infected with Ad–Iκ-Bα demonstrated significant attenuation of PGE2-induced IL-8 mRNA expression compared with uninfected cells or cells infected with Ad-d1703. C: Infection of cells with Ad–Iκ-Bα had no significant effect on the hypoxic induction of IL-8 expression. D: Treatment of cells with echinomycin alone for 8 hours did not significantly alter IL-8 mRNA expression. E: Concomitant treatment of cells with 100 nmol/L of PGE2 and 5 nmol/L of echinomycin (EC), an inhibitor of HIF-1 binding, showed a significant reduction in IL-8 mRNA expression. F: Echinomycin treatment under hypoxic conditions abolished hypoxia-induced IL-8 mRNA up-regulation. G: PGE2-induced IL-8 mRNA expression in EP2S cells was not significantly decreased by silencing of HIF-1α by shRNA. Transfection of a scrambled shRNA sequence (SCR) had no significant effect on IL-8 expression when compared with untransfected cells (n = 3). H: Hypoxic induction of IL-8 expression was significantly decreased when HIF-1α was silenced in cells before hypoxic incubation (n = 3–5). Hypoxia, 0.5% O2; normoxia, 21% O2; V, vehicle. *P < 0.05. **P < 0.01. ***P < 0.001).
IL-8 Up-Regulation by Hypoxia Is Inhibited by Echinomycin, a Pharmacologic Inhibitor of Hypoxia-Inducible Factor-1α Binding
Echinomycin is a small molecule that inhibits the DNA binding of hypoxia-inducible factor (HIF) to the hypoxic response element sequence but does not affect AP-1 or NF-κB binding9 (Figure 6D–F). Cells concomitantly treated with PGE2 and 5 nmol/L of echinomycin demonstrated a significant (P < 0.05) but not absolute reduction in IL-8 mRNA expression when compared with cells treated with 100 nmol/L of PGE2 alone (Figure 6E). Hypoxia-induced IL-8 mRNA expression was abolished when cells were concomitantly treated with 5 nmol/L of echinomycin (P < 0.05) (Figure 6F).
Silencing of HIF-1α with shRNA Confirms Involvement of HIF-1α in Upregulation of IL-8 by Hypoxia and PGE2
HIF-1α knockdown was confirmed at Western blot analysis (see Supplemental Figure S1A at http://ajp.amjpathol.org). There was a marked decrease in HIF-1α protein in cells transfected with shRNA against HIF-1α before hypoxic incubation versus untransfected cells or those transfected with a scrambled shRNA sequence. Specificity of the knockdown was confirmed by examination of lamin A/C mRNA expression, which was not significantly different with transfection of any construct (Figure S1B). IL-8 expression was increased with PGE2 or hypoxic incubation. Transfection of cells with a scrambled sequence did not significantly change IL-8 mRNA expression. In agreement with pharmacologic inhibition of HIF-1α binding, the hypoxic increase in IL-8 was significantly abrogated when HIF-1α was silenced before treatment (P < 0.05) (Figure 6H). PGE2-induced IL-8 mRNA expression was nonsignificantly decreased when HIF-1α was silenced, when compared with untransfected cells.
Discussion
In the present study, significant menstrual up-regulation of endometrial IL-8 mRNA and protein was observed. The timing of this elevation in IL-8 expression is consistent with the onset of endometrial repair. The data support the hypothesis that progesterone withdrawal followed by increased PGE2 and hypoxic conditions up-regulates endometrial repair factor expression. Furthermore, NF-κB and HIF-1 are two transcription factors that have a role in the induction of IL-8 for menstrual repair. Cross-talk between these factors presents a mechanism for the synergistic increase in IL-8 observed when PGE2 and hypoxia are present simultaneously, as occurs in the perimenstrual endometrium.
Previous studies have found an increase in IL-8 mRNA and protein expression during the late secretory phase of the menstrual cycle.18, 19 However, those studies did not examine tissue from the menstrual phase; thus, the maximal increase in IL-8 during this stage was not demonstrated. The finding of significant elevation of IL-8 protein during menstruation is in agreement with the findings of Jones et al,20 who reported undetectable levels of IL-8 mRNA during the menstrual cycle until a dramatic up-regulation at menstruation. As endometrial repair has been shown microscopically to commence on cycle day 2,21 the finding of maximal IL-8 levels during menstruation is consistent with a role in endometrial repair. A recent study of the menstrual endometrium revealed an increase in genes associated with extracellular matrix biosynthesis in stromal cells from the functional layer when compared with those from the basal layer.22 Overexpression of these genes, which includes IL8 (>4-fold increase), suggests that fragments of the functional layer of endometrium participate in endometrial repair.
IL-8 is a potent chemokine,4 and is reported to control the migration and activation of leukocytes during menstruation. A host of chemokines are present in the premenstrual endometrium, including monocyte chemotactic protein-3, eotaxin, fractaline, and 6Ckine (chemokine with 6 cysteines).20 By using a gene array approach and validation with RT-PCR, Jones et al20 demonstrated that of all of the chemokines assessed, only IL8 was significantly increased in menstrual phase endometrium. Inflammatory cells produce and secrete proteases, such as matrix metalloproteinases, that have the ability to break down the extracellular matrix.23 Therefore, the maximal expression of IL-8 at menstruation described herein is consistent with a role in chemotaxis and inflammatory cell accumulation in the endometrium, key events in the initiation of menstruation. In addition, leukocytes form an essential component of the endometrial repair process. Neutrophil depletion using the antibody RB6 8C5 markedly delayed endometrial repair in the mouse model of menstruation.24 In addition to its role in neutrophil chemotaxis, IL-8 has important angiogenic properties5 and induces mitogenesis of vascular smooth muscle cells.25 IL-8 interacts with two chemokine receptors, CXCR1 and CXCR2. Both are expressed in the endometrium throughout the menstrual cycle.26 Therefore, it was postulated that IL-8 has a functional role in human endometrial angiogenesis and repair. The present study demonstrated that menstrual phase endometrial explants have the ability to produce factors with significant angiogenic potential. In addition, the elevated levels of IL-8 present during menstruation have increased angiogenic potential when compared with levels secreted during the mid secretory phase. Numerous angiogenic factors are present in the endometrium during menstruation, including vascular endothelial growth factor,3 the angiopoietins,27 and platelet-derived growth factor.28 All likely have a role in vascular proliferation and differentiation, enabling rapid repair of damaged blood vessels. An element of functional redundancy of these factors is to be expected to ensure efficient endometrial repair. Although IL-8 may not be essential for angiogenesis during endometrial repair, the IL-8 protein levels present during menstruation are sufficient for an active contribution to this physiologic process.
Postmenstrual repair was traditionally considered estrogen-dependent. However, using scanning electron microscopy, Ludwig and Spornitz21 demonstrated that epithelial cell proliferation and migration commenced on day 2 of the menstrual cycle and that full coverage of the uterine lumen was achieved by day 6. Because estrogen levels remain low throughout the menstrual phase, these observations suggest that initiation of repair may be estrogen-independent. The murine model of menstruation also supports the hypothesis that estrogen is not essential for endometrial repair.29 Ovariectomized mice were maintained on a soy-free diet and treated with an aromatase inhibitor to remove all estrogenic influence. When assessed morphologically, no significant difference in the rate of endometrial repair was observed in the complete absence of estrogen. Notwithstanding the limitations of the mouse model of simulated menstruation, these results support findings in the human endometrium that suggest that estrogen is not necessary for repair, although it may contribute to the process. Therefore, it was postulated that progesterone withdrawal rather than an increase in estradiol is the stimulus for endometrial repair factor expression.
Progesterone withdrawal in vivo causes significant up-regulation of endometrial IL-8 mRNA expression after 48 hours.1, 19 However, the mechanisms by which progesterone withdrawal manifests this effect remain undefined. Progesterone withdrawal during the late secretory phase of the menstrual cycle results in up-regulation of COX-2, an enzyme responsible for prostaglandin synthesis.1, 30 PGF2α is a potent vasoconstrictor.31 Premenstrual increases in PGF2α and other vasoconstrictors such as endothelin-1 result in constriction of spiral arterioles. This causes a transient episode of hypoxia in the functional layer of the endometrium (Figure 7). The hypothesis that hypoxia exists during the perimenstrual phase was derived from classic experiments in the rhesus monkey.32 Direct observation of changes in intraocular endometrial implants demonstrated vasoconstriction of the spiral arterioles and a decrease in blood flow. Hypoxia has also been demonstrated in the mouse model of menstruation using pimonidazole.33 Furthermore, although some controversy remains about the presence of hypoxia in the human endometrium,34 late secretory and menstrual endometrium exhibits positive nuclear immunohistochemical staining for HIF-1α and CAIX, two markers of hypoxia.35, 36 Therefore, it is proposed that hypoxia is involved in the initiation of postmenstrual repair factor expression after progesterone withdrawal.
Figure 7.
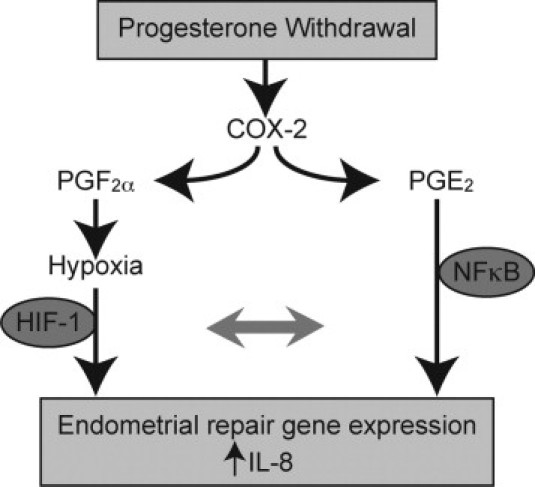
It was hypothesized that up-regulation of IL-8 in the perimenstrual endometrium after progesterone withdrawal occurs by two pathways. Elevation of COX-2 induces synthesis of PGE2 and PGF2α. PGF2α is a potent vasoconstrictor and, along with other vasoconstrictors, causes an episode of transient hypoxia in the superficial endometrial zones. It was demonstrated that both PGE2 and hypoxic conditions can increase endometrial IL-8 mRNA and protein levels, with synergistic increases in IL-8 observed in the presence of both treatments simultaneously. NF-κB and HIF-1α seem to mediate transcription of IL-8 for endometrial repair.
Herein, it has been demonstrated that PGE2 and hypoxia independently up-regulate IL-8 mRNA expression in endometrial epithelial cells and in endometrial explants that have had previous progesterone exposure. Endometrial tissue from the proliferative stage, that is, with no significant in vivo progesterone exposure, demonstrated no such increase in IL-8 expression with PGE2 or hypoxia. There was no significant difference in EP2 mRNA expression between explants from the proliferative and secretory phases of the cycle. In addition, previously published data on the endometrial expression of the EP2 receptor demonstrated no significant variation across the menstrual cycle.17 These data suggest that the variation observed in explants from various phases of the cycle in response to PGE2 and hypoxia is not due to differing levels of EP2 receptor expression. When proliferative explants were subjected to an in vitro model of progesterone withdrawal using the progesterone-receptor antagonist mifepristone, there was no up-regulation of IL-8 under normoxic conditions. Under in vitro conditions, endometrial architecture is disturbed, and up-regulation of COX-2 and subsequent synthesis of PGF2α are unlikely to result in vasoconstriction and local tissue hypoxia. To overcome the limitations of the in vitro culture system, explants were placed in a hypoxic chamber (0.5% O2) at the time of progesterone withdrawal to more accurately simulate the in vivo environment. The addition of hypoxic conditions induced a significant increase in IL-8 mRNA expression 48 hours after progesterone withdrawal, which suggests that hypoxia is necessary for the increase in endometrial repair factors at menstruation. To delineate the contribution of prostaglandins after progesterone withdrawal, the COX inhibitor indomethacin was added to the in vitro progesterone withdrawal system. This abrogated the up-regulation of IL-8 mRNA expression, indicating that both prostaglandins and hypoxia are required after progesterone withdrawal for up-regulation of repair factor expression.
To determine whether a similar human model of progesterone deprivation up-regulated IL-8 expression, endometrial biopsy specimens from women obtained before and after insertion of LNG-IUS were examined. This IUS markedly down-regulates the progesterone receptor in all endometrial compartments,16 resulting in a progesterone-deficient environment that simulates the in vitro model used in the present study. The added advantage of this in vivo human model is that the endometrial architecture remains intact, enabling the physiologic processes of chemoattraction and vasoconstriction. Previous studies of long-term progestogen exposure have demonstrated reduced endometrial perfusion and profoundly decreased vasomotion, which may induce a relative endometrial hypoxia.37 The results demonstrated that IL-8 mRNA expression in normal endometrium during the proliferative, early, and mid secretory phases is low. Paired samples obtained four to six months after LNG-IUS insertion demonstrated significantly increased IL-8 mRNA expression in all seven women. Levels after IUS insertion were comparable to those observed during the normal menstrual phase. The increased IL-8 mRNA expression in this LNG-IUS human model of progesterone withdrawal is comparable to the finding of significantly elevated IL-8 mRNA expression in endometrial samples from women obtained 48 hours after withdrawal of vaginal progesterone administration compared with mid secretory control endometrium.1
After progesterone withdrawal during the late secretory phase, both PGE2 and hypoxia are present in the luminal portion of the endometrium. Therefore, the effect of both PGE2 plus hypoxic conditions on IL-8 expression in endometrial cells was examined. An Ishikawa endometrial epithelial cell line was used for these studies because primary human glandular endometrial epithelial cells have a limited capacity to proliferate in culture. Treatment with PGE2 and hypoxia induced a synergistic increase in IL-8 mRNA and protein compared with either treatment alone, which suggests an interaction between the two pathways of IL-8 stimulation. Another endometrial proangiogenic factor, CYR61, has a similar regulation pattern.38 Endometrial cells treated with hypoxia and PGE2 demonstrated a synergistic increase in CYR61 mRNA and protein levels. Mechanistic studies have described CYR61-mediated induction of IL-8 receptors CXCR1 and CXCR2.39 Hence, there is evidence that hypoxia and PGE2 initiate a perimenstrual angiogenic and tissue repair response by activation of CYR61- and IL-8–mediated signaling.
HIF-1 and NF-κB are two nuclear transcription factors present in the endometrium during the perimenstrual phase.35, 40 The hypoxic response element and the NF-κB binding site have both previously been identified in the IL-8 promoter.41, 42 Both HIF-1 and NF-κB up-regulate IL-8 mRNA expression in cells from other tissue sites in the body.41, 43, 44 An adenoviral dominant-negative inhibitor of NF-κB (Ad–Iκ-Bα) maintains NF-κB in a cytoplasmic location, preventing transcription of its target genes. On infection of endometrial epithelial cells with Ad–Iκ-Bα, there was a significant decrease in PGE2-mediated IL-8 mRNA up-regulation. Concomitant treatment with hypoxia and echinomycin revealed a significant reduction in hypoxia-mediated IL-8 mRNA expression. These results suggest that PGE2-mediated IL-8 up-regulation is NF-κB–dependent and that hypoxia-mediated IL-8 up-regulation is HIF-1–mediated. Echinomycin also reduces c-Myc and AP-1 binding by 30% and 50%, respectively,45 and these transcription factors may also contribute to the decrease in IL-8 production. However, specific inhibition of HIF-1α with shRNA also demonstrated a significant reduction in hypoxia-mediated IL-8 expression. This supports the presence of an interaction between NF-kB and HIF-1α to regulate IL-8 expression. There is mounting evidence for cross-talk between NF-κB and HIF-1 in other tissue sites.46, 47, 48, 49 Therefore, the presence of both of these transcription factors and possible cross-talk between them may explain the synergistic up-regulation of IL-8 mRNA observed in endometrial cells exposed to PGE2 and hypoxic conditions simultaneously.
Aberrations in endometrial repair factor expression may lead to prolonged heavy menstrual bleeding. In women with menstrual blood loss in excess of 90 ml the PGF2α-PGE2 ratio is significantly decreased50 and prostaglandin F2α receptor expression is also decreased.51 Excessive PGE2 production at the expense of PGF2α may result in less constriction of the spiral arterioles and an absent or decreased perimenstrual hypoxic insult. If endometrial repair factor expression depends on the interaction between PGE2 and hypoxia-induced pathways, it can be speculated that endometrial repair processes may be defective in these women as a result of an altered hypoxic episode.
In summary, IL-8 mRNA and protein are increased in the human endometrium at menstruation. The present data support the hypothesis that progesterone withdrawal, followed by increased PGE2 and hypoxic conditions, up-regulates endometrial repair factor expression. Endometrial IL-8 mRNA up-regulation may be mediated by NF-κB and HIF-1. Cross-talk between these two transcription factors presents a mechanism for the synergistic increases in IL-8 observed in endometrial cells when PGE2 and hypoxia are present together. Further studies are required to determine whether hypoxic conditions and subsequent repair factor expression are aberrant in women with heavy menstrual bleeding.
Acknowledgments
We thank all of the women who participated in the study and clinical research nurses Catherine Murray, Sharon McPherson, and Catherine Cairns for assistance with patient recruitment and tissue collection. The adenovirus Iκ-Bα was kindly donated by J.M. Sallenave, M.D., and the shRNA constructs by Prof. Thorston Cramer. We thank Paula Lourenco and Sarah McDonald for technical assistance, and Pamela Brown, M.D., for help and advice. In addition, we thank Ronnie Grant for help with illustrations and Sheila Milne with manuscript preparation.
Footnotes
Supported by grant G0600048 from the UK Medical Research Council.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi:10.1016/j.ajpath.2010.11.070.
Supplementary data
Short-hairpin silencing of HIF-1α. A: Confirmation of HIF-1α protein knockdown by a short-hairpin sequence against HIF-1α compared with a scrambled sequence (ShSCR) or untransfected cells after 8 hours under hypoxic conditions (0.5% O2). B: Specificity of the knockdown was confirmed by examining lamin A/C mRNA expression, which showed no significant changes with transfection of either construct (n = 3).
References
- 1.Critchley H.O., Jones R.L., Lea R.G., Drudy T.A., Kelly R.W., Williams A.R., Baird D.T. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J Clin Endocrinol Metab. 1999;84:240–248. doi: 10.1210/jcem.84.1.5380. [DOI] [PubMed] [Google Scholar]
- 2.Sugino N., Karube-Harada A., Taketani T., Sakata A., Nakamura Y. Withdrawal of ovarian steroids stimulates prostaglandin F2alpha production through nuclear factor-kappaB activation via oxygen radicals in human endometrial stromal cells: potential relevance to menstruation. J Reprod Dev. 2004;50:215–225. doi: 10.1262/jrd.50.215. [DOI] [PubMed] [Google Scholar]
- 3.Nayak N.R., Brenner R.M. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab. 2002;87:1845–1855. doi: 10.1210/jcem.87.4.8413. [DOI] [PubMed] [Google Scholar]
- 4.Larsen C.G., Anderson A.O., Appella E., Oppenheim J.J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 5.Koch A.E., Polverini P.J., Kunkel S.L., Harlow L.A., DiPietro L.A., Elner V.M., Elner S.G., Strieter R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 6.Noyes R.W., Hertig A.T., Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 7.Sales K.J., Maudsley S., Jabbour H.N. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3′,5′-adenosine monophosphate–mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol. 2004;18:1533–1545. doi: 10.1210/me.2004-0022. [DOI] [PubMed] [Google Scholar]
- 8.Kane N., Jones M., Brosens J.J., Saunders P.T., Kelly R.W., Critchley H.O. Transforming growth factor-{beta}1 attenuates expression of both the progesterone receptor and dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol. 2008;22:716–728. doi: 10.1210/me.2007-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong D., Park E.J., Stephen A.G., Calvani M., Cardellina J.H., Monks A., Fisher R.J., Shoemaker R.H., Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 10.Mizukami Y., Li J., Zhang X., Zimmer M.A., Iliopoulos O., Chung D.C. Hypoxia-inducible factor-1–independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004;64:1765–1772. doi: 10.1158/0008-5472.can-03-3017. [DOI] [PubMed] [Google Scholar]
- 11.Sowter H.M., Raval R.R., Moore J.W., Ratcliffe P.J., Harris A.L. Predominant role of hypoxia-inducible transcription factor (HIF)-1alpha versus HIF-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- 12.Jobin C., Haskill S., Mayer L., Panja A., Sartor R.B. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol. 1997;158:226–234. [PubMed] [Google Scholar]
- 13.Henriksen P.A., Hitt M., Xing Z., Wang J., Haslett C., Riemersma R.A., Webb D.J., Kotelevtsev Y.V., Sallenave J.M. Adenoviral gene delivery of elafin and secretory leukocyte protease inhibitor attenuates NF-kappa B–dependent inflammatory responses of human endothelial cells and macrophages to atherogenic stimuli. J Immunol. 2004;172:4535–4544. doi: 10.4049/jimmunol.172.7.4535. [DOI] [PubMed] [Google Scholar]
- 14.Denison F.C., Riley S.C., Wathen N.C., Chard T., Calder A.A., Kelly R.W. Differential concentrations of monocyte chemotactic protein-1 and interleukin-8 within the fluid compartments present during the first trimester of pregnancy. Hum Reprod. 1998;13:2292–2295. doi: 10.1093/humrep/13.8.2292. [DOI] [PubMed] [Google Scholar]
- 15.Critchley H.O., Kelly R.W., Kooy J. Perivascular location of a chemokine interleukin-8 in human endometrium: a preliminary report. Hum Reprod. 1994;9:1406–1409. doi: 10.1093/oxfordjournals.humrep.a138719. [DOI] [PubMed] [Google Scholar]
- 16.Critchley H.O., Wang H., Kelly R.W., Gebbie A.E., Glasier A.F. Progestin receptor isoforms and prostaglandin dehydrogenase in the endometrium of women using a levonorgestrel-releasing intrauterine system. Hum Reprod. 1998;13:1210–1217. doi: 10.1093/humrep/13.5.1210. [DOI] [PubMed] [Google Scholar]
- 17.Milne S.A., Perchick G.B., Boddy S.C., Jabbour H.N. Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2001;86:4453–4459. doi: 10.1210/jcem.86.9.7856. [DOI] [PubMed] [Google Scholar]
- 18.Arici A., Seli E., Senturk L.M., Gutierrez L.S., Oral E., Taylor H.S. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab. 1998;83:1783–1787. doi: 10.1210/jcem.83.5.4754. [DOI] [PubMed] [Google Scholar]
- 19.Milne S.A., Critchley H.O., Drudy T.A., Kelly R.W., Baird D.T. Perivascular interleukin-8 messenger ribonucleic acid expression in human endometrium varies across the menstrual cycle and in early pregnancy decidua. J Clin Endocrinol Metab. 1999;84:2563–2567. doi: 10.1210/jcem.84.7.5833. [DOI] [PubMed] [Google Scholar]
- 20.Jones R.L., Hannan N.J., Kaitu'u T.J., Zhang J., Salamonsen L.A. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–6167. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig H., Spornitz U.M. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann NY Acad Sci. 1991;622:28–46. doi: 10.1111/j.1749-6632.1991.tb37848.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaide Chevronnay H.P., Galant C., Lemoine P., Courtoy P.J., Marbaix E., Henriet P. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology. 2009;150:5094–5105. doi: 10.1210/en.2009-0750. [DOI] [PubMed] [Google Scholar]
- 23.Salamonsen L.A., Zhang J., Brasted M. Leukocyte networks and human endometrial remodelling. J Reprod Immunol. 2002;57:95–108. doi: 10.1016/s0165-0378(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 24.Kaitu'u-Lino T.J., Morison N.B., Salamonsen L.A. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007;328:197–206. doi: 10.1007/s00441-006-0358-2. [DOI] [PubMed] [Google Scholar]
- 25.Yue T.L., Wang X., Sung C.P., Olson B., McKenna P.J., Gu J.L., Feuerstein G.Z. Interleukin-8: a mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994;75:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Mulayim N., Palter S.F., Kayisli U.A., Senturk L., Arici A. Chemokine receptor expression in human endometrium. Biol Reprod. 2003;68:1491–1495. doi: 10.1095/biolreprod.102.009639. [DOI] [PubMed] [Google Scholar]
- 27.Hewett P., Nijjar S., Shams M., Morgan S., Gupta J., Ahmed A. Down-regulation of angiopoietin-1 expression in menorrhagia. Am J Pathol. 2002;160:773–780. doi: 10.1016/S0002-9440(10)64899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boehm K.D., Daimon M., Gorodeski I.G., Sheean L.A., Utian W.H., Ilan J. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27:93–101. doi: 10.1002/mrd.1080270203. [DOI] [PubMed] [Google Scholar]
- 29.Kaitu'u-Lino T.J., Morison N.B., Salamonsen L.A. Estrogen is not essential for full endometrial restoration after breakdown: lessons from a mouse model. Endocrinology. 2007;148:5105–5111. doi: 10.1210/en.2007-0716. [DOI] [PubMed] [Google Scholar]
- 30.Critchley H.O., Kelly R.W., Brenner R.M., Baird D.T. Antiprogestins as a model for progesterone withdrawal. Steroids. 2003;68:1061–1068. doi: 10.1016/j.steroids.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Baird D.T., Cameron S.T., Critchley H.O., Drudy T.A., Howe A., Jones R.L., Lea R.G., Kelly R.W. Prostaglandins and menstruation. Eur J Obstet Gynecol Reprod Biol. 1996;70:15–17. doi: 10.1016/s0301-2115(96)02568-7. [DOI] [PubMed] [Google Scholar]
- 32.Markee J.E. Menstruation in intraocular transplants in the rhesus monkey. Contr Embryol Carnegie Inst. 1940;28:219–308. [Google Scholar]
- 33.Fan X., Krieg S., Kuo C.J., Wiegand S.J., Rabinovitch M., Druzin M.L., Brenner R.M., Giudice L.C., Nayak N.R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Salamonsen L.A. Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum Reprod. 2002;17:265–274. doi: 10.1093/humrep/17.2.265. [DOI] [PubMed] [Google Scholar]
- 35.Critchley H.O., Osei J., Henderson T.A., Boswell L., Sales K.J., Jabbour H.N., Hirani N. Hypoxia-inducible factor-1alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2) Endocrinology. 2006;147:744–753. doi: 10.1210/en.2005-1153. [DOI] [PubMed] [Google Scholar]
- 36.Punyadeera C., Thijssen V.L., Tchaikovski S., Kamps R., Delvoux B., Dunselman G.A., de Goeij A.F., Griffioen A.W., Groothuis P.G. Expression and regulation of vascular endothelial growth factor ligands and receptors during menstruation and post-menstrual repair of human endometrium. Mol Hum Reprod. 2006;12:367–375. doi: 10.1093/molehr/gal027. [DOI] [PubMed] [Google Scholar]
- 37.Hickey M., Carati C., Manconi F., Gannon B.J., Dwarte D., Fraser I.S. The measurement of endometrial perfusion in Norplant users: a pilot study. Hum Reprod. 2000;15:1086–1091. doi: 10.1093/humrep/15.5.1086. [DOI] [PubMed] [Google Scholar]
- 38.Gashaw I., Stiller S., Boing C., Kimmig R., Winterhager E. Premenstrual regulation of the pro-angiogenic factor CYR61 in human endometrium. Endocrinology. 2008;149:2261–2269. doi: 10.1210/en.2007-1568. [DOI] [PubMed] [Google Scholar]
- 39.Lin B.R., Chang C.C., Chen L.R., Wu M.H., Wang M.Y., Kuo I.H., Chu C.Y., Chang K.J., Lee P.H., Chen W.J., Kuo M.L., Lin M.T. Cysteine-rich 61 (CCN1) enhances chemotactic migration, transendothelial cell migration, and intravasation by concomitantly up-regulating chemokine receptors 1 and 2. Mol Cancer Res. 2007;5:1111–1123. doi: 10.1158/1541-7786.MCR-06-0289. [DOI] [PubMed] [Google Scholar]
- 40.King A.E., Critchley H.O., Kelly R.W. The NF-kappaB pathway in human endometrium and first trimester decidua. Mol Hum Reprod. 2001;7:175–183. doi: 10.1093/molehr/7.2.175. [DOI] [PubMed] [Google Scholar]
- 41.Kim K.S., Rajagopal V., Gonsalves C., Johnson C., Kalra V.K. A novel role of hypoxia-inducible factor in cobalt chloride– and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 42.Kunsch C., Rosen C.A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn J.K., Koh E.M., Cha H.S., Lee Y.S., Kim J., Bae E.K., Ahn K.S. Role of hypoxia-inducible factor-1alpha in hypoxia-induced expressions of IL-8: MMP-1 and MMP-3 in rheumatoid fibroblast-like synoviocytes. Rheumatol (Oxford) 2008;47:834–839. doi: 10.1093/rheumatology/ken086. [DOI] [PubMed] [Google Scholar]
- 44.Mizukami Y., Jo W.S., Duerr E.M., Gala M., Li J., Zhang X., Zimmer M.A., Iliopoulos O., Zukerberg L.R., Kohgo Y., Lynch M.P., Rueda B.R., Chung D.C. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 45.Vlaminck B., Toffoli S., Ghislain B., Demazy C., Raes M., Michiels C. Dual effect of echinomycin on hypoxia-inducible factor-1 activity under normoxic and hypoxic conditions. FEBS J. 2007;274:5533–5542. doi: 10.1111/j.1742-4658.2007.06072.x. [DOI] [PubMed] [Google Scholar]
- 46.Frede S., Stockmann C., Freitag P., Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Uden P., Kenneth N.S., Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walmsley S.R., Print C., Farahi N., Peyssonnaux C., Johnson R.S., Cramer T., Sobolewski A., Condliffe A.M., Cowburn A.S., Johnson N., Chilvers E.R. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belaiba R.S., Bonello S., Zahringer C., Schmidt S., Hess J., Kietzmann T., Gorlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith S.K., Abel M.H., Kelly R.W., Baird D.T. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol. 1981;88:434–442. doi: 10.1111/j.1471-0528.1981.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 51.Smith O.P., Jabbour H.N., Critchley H.O. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22:1450–1456. doi: 10.1093/humrep/del503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Short-hairpin silencing of HIF-1α. A: Confirmation of HIF-1α protein knockdown by a short-hairpin sequence against HIF-1α compared with a scrambled sequence (ShSCR) or untransfected cells after 8 hours under hypoxic conditions (0.5% O2). B: Specificity of the knockdown was confirmed by examining lamin A/C mRNA expression, which showed no significant changes with transfection of either construct (n = 3).


