Abstract
The process of regeneration is most readily studied in species of sponge, hydra, planarian and salamander (i.e., newt and axolotl). The closure of MRL mouse ear pinna through-and-through holes provides a mammalian model of unusual wound healing/regeneration in which a blastema-like structure closes the ear hole and cartilage and hair follicles are replaced. Recent studies, based on a broad level of DNA damage and a cell cycle pattern of G2/M “arrest,” showed that p21Cip1/Waf1 was missing from the MRL mouse ear and that a p21-null mouse could close its ear holes. Given the p53/p21 axis of control of DNA damage, cell cycle arrest, apoptosis and senescence, we tested the role of p53 in the ear hole regenerative response. Using backcross mice, we found that loss of p53 in MRL mice did not show reduced healing. Furthermore, cross sections of MRL. p53−/− mouse ears at 6 weeks post-injury showed an increased level of adipocytes and chondrocytes in the region of healing whereas MRL or p21−/− mice showed chondrogenesis alone in this same region, though at later time points. In addition, we also investigated other cell cyclerelated mutant mice to determine how p21 was being regulated. We demonstrate that p16 and Gadd45 null mice show little healing capacity. Interestingly, a partial healing phenotype in mice with a dual Tgfβ/Rag2 knockout mutation was seen. These data demonstrate an independence of p53 signaling for mouse appendage regeneration and suggest that the role of p21 in this process is possibly through the abrogation of the Tgfβ/Smad pathway.
Key words: mouse, regeneration, p53, p21, MRL, ear-hole, Tgfβ
Introduction
A large number of species are capable of regeneration in some form and degree with different structures being regenerated. The most efficient regenerators include hydra and planaria, which can regenerate their whole body from only a small part of it. Vertebrates also include potent regenerators such as the urodele amphibians or newts and salamanders, which can regenerate limbs and other structures after amputation. Examples of mammalian regeneration are not common; although, many mammalian tissues possess the ability to regenerate as individual cell populations. These include bone, immune tissue, peripheral nerve, skeletal muscle and liver.1–3
The response to traumatic injury in tissues of higher organisms can proceed through either the process of wound repair and scar formation or through a poorly understood mechanism involving the formation of a blastema. Tissue regeneration through blastema formation is referred to as “epimorphic regeneration”. Blastema cells proliferate until the replacement and restoration of correct cellular architecture and differentiation into multiple cell types is achieved.4
Examples of mammalian epimorphic regeneration include the regrowth of antlers of deer5 and moose3 and punched ear hole closure in rabbits.6 Among these examples is the MRL mouse, first identified in 1996 as a mouse model of regeneration, which exhibits closure of punched ear holes with the formation of a blastema-like structures. This results in the perfect replacement of cartilage, hair follicles and sebaceous glands, as well as proliferating cells.7 Classifying a regenerative process as epimorphic regeneration is usually accomplished by comparing the process to that of limb regeneration in the amphibian. MRL mouse ear hole closure does exhibit such processes including wound epidermal proliferation, basement membrane breakdown,8 and dermal proliferation leading to hole closure.7
We have recently reported that the p21Cip1/Waf1 protein provides a possible link between cell cycle control and appendage regeneration in mice.9 This finding is derived from an in vitro study of cells from the MRL ear pinna, which demonstrated a higher proliferative rate than cells from non-regenerating mouse ears and a different cell cycle pattern with a significantly higher number of cells in G2 “arrest” than cells from non-regenerating mouse ears. We also found a DNA damage response (DDR) and widespread DNA damage demonstrated by almost 90% of healer cells being cometpositive and with increased p53 levels. Examination of these cells for defects in G1 checkpoint genes showed that the p21Cip1/Waf1 protein was lacking in healer cells. Using Cdkn1atmi/Tyj/J p21−/− mice, deficient in the cyclin-dependent kinase inhibitor protein p21Cip1/Waf1 for wounding experiments, we showed similar regenerative competency as seen in MRL mice, which provided a new transgenic mouse model of regeneration.
Consistent with the increased DDR in cells derived from regeneration-competent hosts, we found that the p53 gene was also upregulated in MRL regenerative cells both pre- and post-injury. It is generally considered that p21 is a major downstream effector of p53.10 Therefore, we investigated the role of p53 in the regenerative response.
The Role of p53 in the Regenerative Response
p53 is a tumor suppressor protein that is central to genomic stability and is mutated in over 50% of all cancers.11 This molecule plays an important role in the cellular response to multiple types of stress including nucleotide depletion, hypoxia, oncogene activation or exposure to DNA damaging agents.11,12
Specific signaling pathways cope with genotoxic stress by initiating pauses in cell cycle progression to allow cells to survive and maintain themselves until the damage has been resolved or the stress has been removed.13 This is accomplished by cell cycle arrest, DNA repair, inhibition of ROS, angiogenesis through metabolic changes and autophagy. If the damage cannot be repaired, then multiple mechanisms can remove such cells via senescence, innate immune responses, apoptosis and tissue renewal. The activation and stabilization of p53 due to these multiple stress signals, depending on the amount of damage and tissue type, demonstrates the importance of p53 in many cellular functions. p53 has been directly implicated in activating these genotoxic pathways.14–18 All of these processes create a very complicated view of the functions of p53 in particular physiological contexts and specifically the pathways involving p21Waf1/Cip1.
The p53/p21 pathway functions in determining cell arrest, apoptosis or senescence in response to genotoxic stress.15 p21Waf1/Cip1 was originally identified as an inhibitor of cyclin/cyclin-dependent kinases, a molecular complex necessary to proceed through the cell cycle. As a mediator of p53 in growth suppression, it is a marker for senescence in fibroblasts.19 p21Waf1/Cip1 is a member of the CIP/KIP family of CDK inhibitors that also include p27 and p57 that can inhibit a wide range of cyclin/CDKs to control progression through the cell cycle. The canonical function of the p21 protein is to direct cell cycle arrest in response to DNA damage in a p53-dependent manner.20,21 The process involves the binding of p21 to cyclin-CDKs, specifically cyclin D-CDK4/6 in G0, preventing this complex from phosphorylating the retinoblastoma protein (pRb). Normally, hypophosphorylated Rb forms a transcriptional repressive complex with E2F that prevents expression of S phase-specific genes. Phosphorylation of pRb relieves transcription repression of E2F target genes, promoting cell cycle progression. p21 prevents Rb phosphorylation and maintains G1 arrest. The function of p21 in cell cycle arrest has also been extended to include S and G2 checkpoints through the interaction with PCNA and 14-3-3σ, respectively.22–24
In addition to the cell cycle checkpoint function of p21 in a p53-dependent manner, p21 also plays a direct p53-independent role in cellular senescence.25–27 Cellular senescence is defined as a permanent cell cycle arrest that can be triggered by an increase in reactive oxygen species, telomere shortening or by upregulation of an oncogene resulting in replicative stress.28 The function of senescence in cells appears to be a way of providing an obstacle to the progression of cancer by preventing damaged cells from undergoing aberrant proliferation.29–35 The two major pathways for activating senescence are controlled by p16Ink or p53/p21, both of which lead to Rb hypophosphorylation. The p53-dependent senescence is directly modulated by p21 through detection of cellular stress; however, there have been other reports demonstrating that p21 can also elicit senescence in a p53-independent manner.36,37 For example, p21 was found to be essential in upregulating senescence-specific markers in cells that did not express p53.
A recent study18 examining hair follicle regeneration relates to our studies of ear hole regeneration. It was shown that p53 is an important component in the renewal of adult tissues that have “increased genomic instability phenotypes.”18,34,38 The activity of p53 is thought to be involved in clearing out cells that have accumulated DNA damage. This results in the induction of senescence and immune-mediated clearance.33 It is proposed that the accumulation of damaged cells that persist in adult tissue that fail to be cleared by p53-mediated mechanisms act as an obstruction to stem cell proliferation and tissue renewal.39 Thus, p53-induced senescence is required for tissue regeneration and the removal of p53 causes tissue renewal to become delayed due to the accumulation of damaged cells.
Given the discussion above, we asked several questions:
If DNA damage and a DDR are seen and p53 is upregulated while p21 is downregulated as seen in the MRL mouse, what response should we expect? DNA damage should lead to p53 activation and an apoptotic response10,21 and not to senescence40 in the absence of p21. We actually found an increase in TUNEL-positive cells in regenerative MRL tissue, indicating that DNA damage is tolerated in these cells. We have not seen evidence for an increase in apoptosis in regenerative cells in culture.9
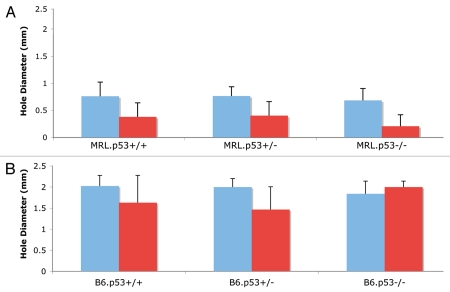
upregulated in the MRL tissue, is p53 necessary to accomplish an ear hole closure regenerative response? To answer this, we crossed a p53−/− mouse41 to MRL producing first (MRLxp53+/−) F1 mutant mice, then MRLx(MRLxp53+/−) BC1 mutant mice, and then IC1 intercross mice producing WT, heterozygous and homozygous mutants. As seen in Fig. 1A, 30 days after ear-punching (2 mm punch) of 8 week old mice, all female mice healed with a mean ear hole diameter ranging from 0.2−0.4 mm as is normally seen in parental MRL female mice.7 There were no statistically significant differences between WT (n = 11), heterozygous (n = 18) and homozygous p53 nulls (n = 5) even though the average homozygous null female hole size appeared smaller than the other two groups. The males showed larger hole sizes just on the border of the healing phenotype42 but, again, we found no significant differences in healing between any of the groups. Although more mice need to be analyzed, these early studies indicate that p53 is not essential for ear hole closure. It is clear from these results that lack of p53 does not have a negative effect on the regenerative ear hole closure response and may even have a beneficial effect. As discussed below (Fig. 3B), MRL.p53−/− healing ear tissue displays interesting differences histologically from the MRL/MpJ healing ear tissue.
Since p53 is a key activator of p21, one question concerns why p21 is down in the MRL regenerator even though p53 is up. This would suggest that the MRL p53/p21 interaction is defective. Would elimination of p53 then lead to a regenerative response similar to that seen with the elimination of p21? To approach this question, we crossed heterozygous B6.p53 mutant mice43 and then ear-punched all offspring at 8–10 weeks of age. As shown below (Fig. 1B), no significant differences were seen between the WT, heterozygous and homozygous p53 mutant mice with ear holes closing between 1.5–2.0 mm after 30 days, considered to be a non-healer phenotype.42 Interestingly, female mice, which are usually better healers than males, showed no significant differences.42
Figure 1.
Mice were ear-punched using a 2 mm hole punch and hole diameter was read 2 and 4 weeks post-injury. Red columns = female mice, the blue columns = male mice; error bars show standard deviations. (A) MRL/MpJ mice were bred to p53−/− mice41 and IC1 intercrosses were tested as WT, heterozygous and homozygous null for the p53 allele. (B) B6.p53−/− mice43 were bred from B6.p53+/− litters and WT, heterozygous and homozygous p53 null mice were examined.
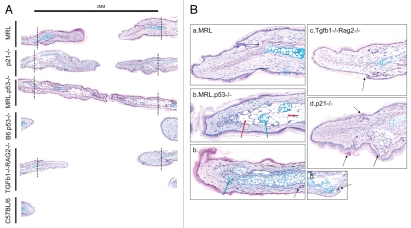
Figure 3.
Histological analysis of ear sections from ear-punched mice 42 days post-injury. Ears were fixed and embedded,and sections through the hole were stained with Alcian blue (cartilage). In (A) sections at low magnification (4X) show the degree of healing with hatched lines indicating the likely original hole cut. Above, there is a marker showing 2 mm (the original size of the hole). In (B) there are higher magnifications (20X) of the selected healing/regenerating ear tissue showing sections from (a) an MRL/MpJ mouse, (b) 3 different MRL.p53−/− mice, (c) a TgfB1−/−Rag2−/− mouse and (d) a p21−/− mouse. Arrows indicate adipocytes (red), chondrocytes (blue-green) and hair follicles (black).
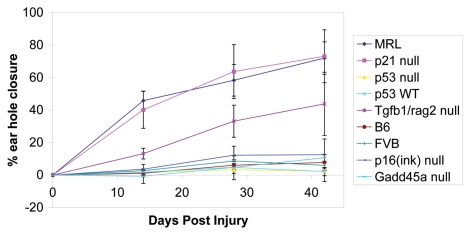
These results clearly demonstrate that p53 is not required for tissue and appendage (ear hole closure) epimorphic regeneration even in the case of high background levels of DNA damage as seen in the MRL mouse. This also suggests that p53-induced senescence and subsequent removal by the immune response is not essential for regeneration in the ear hole closure model. We have not ruled out p16-induced senescence, which is independent of p53 and p21. However, this typically is associated with the activation of an oncogene and we expect that this pathway is not involved in regeneration. We have shown that the p16-null transgenic mouse does not close ear holes (Fig. 2) and we are breeding p21/p16−/− mice to further investigate the role of senescence in regeneration.
Figure 2.
Preliminary analysis of mutant mice derived from MMHCC for regeneration capability. The ear pinnae of mice (n = 2 to 5) were wounded by hole punching and followed for 6 weeks. Healer (regeneration-competent) controls (MRL/MpJ and p21−/−) and non-healer (regeneration-incompetent) controls (B6, FVB) are included in this study. The experimental mice tested include GADD45−/−, p16−/− and tgfβ1, rag2−/−.
Evidence for a p53-Independent p21-Activation Pathway
An interesting feature of primary mouse ear fibroblasts from MRL mice is that approximately 50% of the cells appear to be in the G2 phase of the cell cycle.9 This is similar to the G2/M bias observed in regenerative tissue from hydra, embryonic stem cells and in the liver.44–50 To determine if our regeneration phenotype is due to a lack of cell cycle checkpoint controls, we screened various transgenic mice that are deficient in cell cycle checkpoint proteins. In collaboration with the MMHCC mouse repository in Frederick, Maryland, we were able to carry out a preliminary screen of transgenic and targeted-mutant mice for regeneration phenotypes as demonstrated by mean ear hole closure over 6 weeks as seen in Figure 2. The specific cell cycle checkpoint proteins examined were p16 and GADD45, which are proteins that have been shown to function in the G1 and/or G2 checkpoints of the cell cycle, respectively.51,52
Ear wounds in p16−/− mice on an FVB background53 and in GADD45−/− mice on a B6/129 background54 showed no hole closure. Figure 2 shows that neither targeted mutant knockouts healed differently than the negative controls. Other proteins such as p27 and p15 are also under investigation to determine whether the regeneration phenotype depends on an intact cell cycle checkpoint response. If DNA damage and senescence is at the heart of the regenerative response, then a p16−/− mouse should lead to no healing and that is what we see. However, the elimination of p16 in the context of a healing MRL or p21−/− mouse showed no healing beyond the negative controls. As mentioned above, crosses of p21 and p16 mutant null mice are being generated since these are the two key molecules important in senescence.31,55–57
If senescence is not involved, what p53-independent control mechanisms of p21 might be involved in enabling the regeneration phenotype? The p21 protein has been shown to regulate cell cycle progression through the control of the Rb protein phosphorylation.58 To this end, mice, which are deficient in the molecules downstream of p21, are being tested for their ability to regenerate tissue. Given that Rb knockout mice die in utero,59 Rb heterozygous knockout mice show no ear hole closure capacity. p21 acts through Rb indirectly to control E2F transcription factors and allow for progression through the cell cycle.10
One major p53-independent regulatory mechanism of p21, which is involved in cell growth or inhibition and differentiation, is the Tgfβ1/Smad pathway.60 Tgfβ1 directs multiple cellular activities in embryonic and adult tissues including proliferation, differentiation, migration and apoptosis, all of which play a role in regeneration.61–63 The regulation of p21 by Tgfβ1 has been shown to be mediated by Smad2 and 3.64,65
Is the Tgfβ/Smad pathway involved in the regeneration phenotype of the transgenic p21 knockout mouse? Various transgenic mouse studies have investigated the role of Tgfβ family members in wound repair.66 Specifically, Tgfβ1-deficient mice with full-thickness excisional back skin wounds show a severe delay in late-stage wound repair due to increased inflammation and, even when crossed onto a Scid background to compensate for lethality, healing is delayed due to multifocal inflammatory disease.67,68
There is also data showing that Smad3-null mice present accelerated wound healing with an increase in tissue renewal through re-epithelialization as well as reduced inflammation.69,70 This pathway is of particular interest, because Smad3 has been implicated as a candidate gene in our genetic mapping studies of healer MRL and parental LG mice.71 A notable experiment using liver transplantation in rats shows that Ad-Smad7, which inhibits Smad3, enhances liver regeneration and shows normal levels of p27 and p15, but no expression of p21, which suggests that there is a Smad3/p21 specific pathway.72
During our screen for healer mice, we tested a Tgfβ1 knockout mouse. Tgfβ1-null mice are lethal due to an early inflammatory response so they were crossed onto a Rag2-null B6/129 background.73 Preliminary results indicate that there is partial healing more so than the negative control WT mice (Fig. 2). Rag2−/− mice alone are non-healers (data not shown). Further testing of these mice showed unreadable and inflamed ear wounds, which were extensively torn, suggesting that the mice were still pro-inflammatory. This may also explain the unusual results seen in Smad3−/− mouse ear wounds,70 which showed large and highly irregular holes.
Differentiation in Regenerating Tissue
Examination of the histology of ears from ear-punched mice shows that the B6.p53-null mice do not exhibit newly developed tissue and seem to have gone through normal healing and scarring as seen in B6 mice (Fig. 3A). However, analysis of ear tissue from the p21-null mice displays newly formed dermal tissue, limited chondrogenesis and newly formed hair follicles at 6 weeks after wounding, similar to MRL.7 Previous reports have shown a dependence on p53 for hair follicle regeneration.18 Here, we report that the formation of new hair follicles is through a p21-independent mechanism. On the other hand, MRL.p53−/− ears show unusual responses. At 6 weeks, the re-grown ear tissue not only has new dermal tissue but also extensive adipogenesis and/or chondrogenesis indicating more rapid differentiation to adipocytes and chondrocytes in new tissue. This supports a recent study showing that p53 inhibits adipogenesis.74 It is possible that this response lacking p53 would lead to enhanced ear-hole closure which is supported by our ear hole closure results above (Fig. 1A). Also, this relates to recent studies showing that IPS induction is enhanced by the lack of both p53 and p21.75
Taken together, we show that in the MRL ear hole closure model, an example of epimorphic regeneration which involves the replacement of multiple tissue types including cartilage, hair follicles and sebaceous glands, there is not a requirement for p53.
Acknowledgements
We would like to thank N. Dahmane for useful discussions and Keith Smith, Dawn Crummkitt, Nicole Roberts for their help with the MMHCC studies. Support for these studies came from the F.M. Kirby Foundation, Inc., the G. Harold and Leila Y. Mathers Foundation, an NIH ARRA grant from NIGMS, NCI Cancer Center Grant (P30 CA10815) and from the Lab Animal Sciences Program, Frederick. L.M.A. was supported by the Training Program Grant in Basic Cancer Biology 5T32CA09171.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13119
References
- 1.Rao N, Jhamb D, Milner D, Li B, Song F, Wang M, et al. Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biology. 2009;7:83. doi: 10.1186/1741-7007-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocum DL. The urodele limb regeneration blastema. Determination and organization of the morphogenetic field. Differentiation. 1984;27:55–57. doi: 10.1111/j.1432-0436.1984.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 3.Carlson BM. Some principles of regeneration in mammalian systems. 2010:4–13. doi: 10.1002/ar.b.20079. [DOI] [PubMed] [Google Scholar]
- 4.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 5.Kierdorf U, Kierdorf H. Deer antlers—a model of mammalian appendage regeneration: An extensive review. Gerontology. 2010 doi: 10.1159/000300565. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.ten Koppel PGJ, van Osch GJVM, Verwoerd CDA, Verwoerd-Verhoef HL. A new in vivo model for testing cartilage grafts and biomaterials: the ‘rabbit pinna punch-hole’ model. Biomaterials. 2001;22:1407–1414. doi: 10.1016/s0142-9612(00)00298-2. [DOI] [PubMed] [Google Scholar]
- 7.Clark LD, Clark RK, Heber-Katz E. A New Murine Model for Mammalian Wound Repair and Regeneration. Clinical Immunology and Immunopathology. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 8.Gourevitch D, Clark L, Chen P, Seitz A, Samulewicz SJ, Heber-Katz E. Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Developmental Dynamics. 2003;226:377–387. doi: 10.1002/dvdy.10243. [DOI] [PubMed] [Google Scholar]
- 9.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, et al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Nat Acad Sci USA. 2010;107:5845–5850. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Deiry WS. p21/p53, cellular growth control and genomic integrity. Curr Top Microbiol Immunol. 1998;227:121–137. doi: 10.1007/978-3-642-71941-7_6. [DOI] [PubMed] [Google Scholar]
- 11.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 Mutations in Human Cancers. Science. 91 A.D.;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 12.Vousden KH. p53: Death Star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 13.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 15.El Deiry WS. Regulation of p53downstream genes. Semin Cancer Biol. 1998;8:345–357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 16.Kuribayashi K, El Deiry WS. Regulation of programmed cell death by the p53 pathway. Adv Exp Med Biol. 2008;615:201–221. doi: 10.1007/978-1-4020-6554-5_10. [DOI] [PubMed] [Google Scholar]
- 17.Fei P, El Deiry WS. P53 and radiation responses. Oncogene. 2003;22:5774–5783. doi: 10.1038/sj.onc.1206677. [DOI] [PubMed] [Google Scholar]
- 18.Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ. Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat Genet. 2009;41:1144–1149. doi: 10.1038/ng.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Child ES, Mann DJ. The intricacies of p21 phosphorylation: Protein/protein interactions, subcellular localization and stability. Cell Cycle. 2006;5:1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- 20.El Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 21.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 2010;22:1003–1012. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niculescu AB, III, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on Serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 24.Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Cooperative effects of genes controlling the G2/M checkpoint. Genes Dev. 2000;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 25.Afshari CA, Nichols MA, Xiong Y, Mudryj M. A role for a p21-E2F interaction during senescence arrest of normal human fibroblasts. Cell Growth Differ. 1996;7:979–988. [PubMed] [Google Scholar]
- 26.Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999;18:4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- 27.Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA. 1997;94:14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozturk M, Arslan-Ergul A, Bagislar S, Senturk S, Yuzugullu H. Senescence and immortality in hepatocellular carcinoma. Cancer Lett. 2009;286:103–113. doi: 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 29.Dimri GP. What has senescence got to do with cancer? Cancer Cell. 2005;7:505–512. doi: 10.1016/j.ccr.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta. 2007;1775:5–20. doi: 10.1016/j.bbcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begus-Nahrmann Y, Lechel A, Obenauf AC, Nalapareddy K, Peit E, Hoffmann E, et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet. 2009;41:1138–1143. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA. p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene. 1999;18:2789–2797. doi: 10.1038/sj.onc.1202615. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Blandino G, Givol D. Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene. 1999;18:2643–2649. doi: 10.1038/sj.onc.1202632. [DOI] [PubMed] [Google Scholar]
- 38.Yazinski SA, Westcott PMK, Ong K, Pinkas J, Peters RM, Weiss RS. Dual inactivation of Hus1 and p53 in the mouse mammary gland results in accumulation of damaged cells and impaired tissue regeneration. Proc Nat Acad Sci. 2009;106:21282–21287. doi: 10.1073/pnas.0904965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoppy DW, Ruzankina Y, Brown EJ. Removing all obstacles: A critical role for p53 in promoting tissue renewal. Cell Cycle. 2010;9:1313–1319. doi: 10.4161/cc.9.7.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21Cip1/Waf1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 41.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 42.Blankenhorn EP, Troutman S, Clark LD, Zhang XM, Chen P, Heber-Katz E. Sexually dimorphic genes regulate healing and regeneration in MRL mice. Mamm Genome. 2003;14:250–260. doi: 10.1007/s00335-002-2222-3. [DOI] [PubMed] [Google Scholar]
- 43.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 44.Dubel S, Schaller HC. Terminal differentiation of ectodermal epithelial stem cells of Hydra can occur in G2 without requiring mitosis or S phase. J Cell Biol. 1990;110:939–945. doi: 10.1083/jcb.110.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt T, David CN. Gland cells in Hydra: cell cycle kinetics and development. J Cell Sci. 1986;85:197–215. doi: 10.1242/jcs.85.1.197. [DOI] [PubMed] [Google Scholar]
- 46.Chuykin IA, Lianguzova MS, Pospelova TV, Pospelov VA. Activation of DNA damage response signaling in mouse embryonic stem cells. Cell Cycle. 2008;7:2922–2928. doi: 10.4161/cc.7.18.6699. [DOI] [PubMed] [Google Scholar]
- 47.Salo E, Baguna J. Regeneration and pattern formation in planarians: I. The pattern of mitosis in anterior and posterior regeneration in Dugesia (G) tigrina and a new proposal for blastema formation. J Embryol Exper Morphol. 1984;83:63–80. [PubMed] [Google Scholar]
- 48.Tassava RA, Mescher AL. Mitotic activity and nucleic acid precursor incorporation in denervated and innervated limb stumps of axolotl larvae. J Exper Zool. 1976;195:253–262. doi: 10.1002/jez.1401950210. [DOI] [PubMed] [Google Scholar]
- 49.Van Bezooijen CF, Van Noord MJ, Knook DL. The viability of parenchymal liver cells isolated from young and old rats. Mech Ageing Dev. 1974;2:107–119. doi: 10.1016/0047-6374(74)90009-8. [DOI] [PubMed] [Google Scholar]
- 50.Guidotti JE, Bregerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: A pivotal role for binuclear hepatocytes. J Biol Chem. 2003;278:19095–19101. doi: 10.1074/jbc.M300982200. [DOI] [PubMed] [Google Scholar]
- 51.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 52.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 53.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 54.Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 55.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 56.Herbig U, Sedivy JM. Regulation of growth arrest in senescence: telomere damage is not the end of the story. Mech Ageing Dev. 2006;127:16–24. doi: 10.1016/j.mad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Ben Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. J Clin Invest. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 59.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell M, Weinberg O. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 60.Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF[beta] signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 61.Bierie B, Moses HL. Tumour microenvironment: TGF[beta]: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 62.Pardali K, Moustakas A. Actions of TGF[beta] as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Massague J. TGF[beta] in cancer. Cell. 2008;134:215–230. [Google Scholar]
- 64.Moustakas A, Kardassis D. Regulation of the human p21/Waf1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci USA. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pardali K, Kurisaki A, Moren A, ten Dijke P, Kardassis D, Moustakas A. Role of Smad proteins and transcription factor Sp1 in p21Waf1/Cip1 regulation by transforming growth factor-Í2. J Biol Chem. 2000;275:29244–29256. doi: 10.1074/jbc.M909467199. [DOI] [PubMed] [Google Scholar]
- 66.Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol Biotechnol. 2004;28:147–166. doi: 10.1385/MB:28:2:147. [DOI] [PubMed] [Google Scholar]
- 67.O'Kane S, Ferguson MWJ. Transforming growth factor [beta]s and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 68.Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF[beta]1 knockout mice. J Invest Dermatol. 2000;115:3–11. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 69.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JJ, Mizel DE, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 70.Arany PR, Flanders KC, Kobayashi T, Kuo CK, Stuelten C, Desai KV, et al. Smad3 deficiency alters key structural elements of the extracellular matrix and mechanotransduction of wound closure. Proc Natl Acad Sci USA. 2006;103:9250–9255. doi: 10.1073/pnas.0602473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blankenhorn EP, Bryan G, Kossenkov AV, Desquenne Clark L, Zhang X-M, Chang C, et al. Loci that regulate healing and regeneration in LG/J and SW/J mice. Mamm Genome. 2009;20:720–733. doi: 10.1007/s00335-009-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong Z, Tsukada S, Rehman H, Parsons CJ, Theruvath TP, Rippe RA, et al. Inhibition of transforming growth factor-beta/Smad signaling improves regeneration of small-for-size rat liver grafts. Liver Transpl. 2010;16:181–190. doi: 10.1002/lt.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Engle SJ, Hoying JB, Boivin GP, Ormsby I, Gartside PS, Doetschman T. Transforming growth factor-beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 1999;59:3379–3386. [PubMed] [Google Scholar]
- 74.Hallenborg P, Feddersen S, Madsen L, Kristiansen K. The tumor suppressors pRB and p53 as regulators of adipocyte differentiation and function. Expert Opin Ther Targets. 2009;13:235–246. doi: 10.1517/14712590802680141. [DOI] [PubMed] [Google Scholar]
- 75.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, et al. Suppression of induced pluripotent stem cell generation by the p53/p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]