Abstract
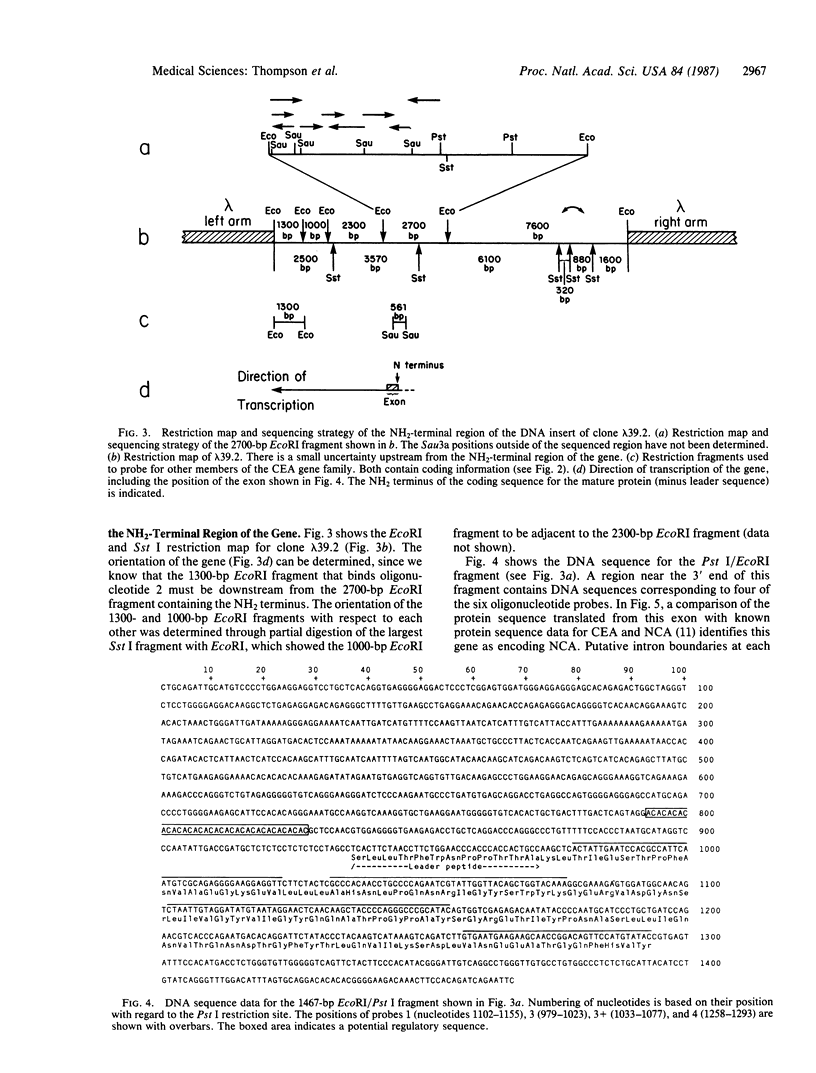
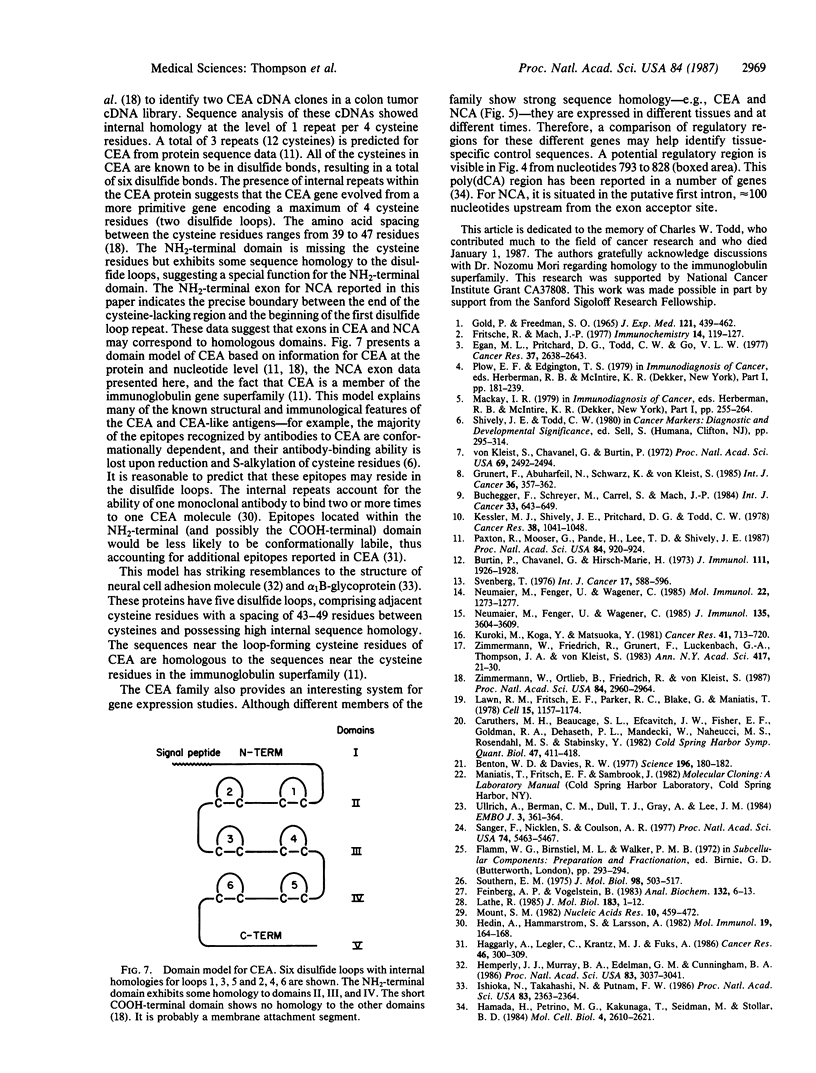
Carcinoembryonic antigen (CEA) is a glycoprotein important as a tumor marker for colonic cancer. Immunological and biochemical studies have shown it to be closely related to a number of other glycoproteins, which together make up a gene family. We have cloned a member of this gene family by using long oligonucleotide probes (42-54 nucleotides) based on our protein sequence data for CEA and NCA (nonspecific cross-reacting antigen) and on human codon usage. The clone obtained (lambda 39.2) hybridizes with six probes and has a 15-kilobase insert. The 5' end of the gene is contained within a 2700-base-pair EcoRI fragment, which hybridizes with five of the six synthetic probes. Sequencing of the 5' end region revealed the location and structure of one exon and two putative intron boundaries. The exon encodes part of the leader sequence and the NH2-terminal 107 amino acids of NCA. Southern blot analysis of human normal and tumor DNA, using as probes two lambda 39.2 fragments that contain coding sequences, suggests the existence of 9-11 genes for the CEA family. One of the restriction fragments described here has been used by Zimmermann et al. [Zimmermann, W., Ortlieb, B., Friedrich, R. & von Kleist, S. (1987) Proc. Natl. Acad. Sci. USA 84, 2960-2964] to isolate partial cDNA clones for CEA. The identity of this clone was verified with our protein sequence data [Paxton, R., Mooser, G., Pande, H., Lee, T.D. & Shively, J.E. (1987) Proc. Natl. Acad. Sci. USA 84, 920-924]. We discuss a domain structure for CEA based on the CEA sequence data and the NCA exon sequence data. It is likely that this gene family evolved from a common ancestor shared with neural cell adhesion molecule and alpha 1 B-glycoprotein and is perhaps a family within the immunoglobulin superfamily.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Buchegger F., Schreyer M., Carrel S., Mach J. P. Monoclonal antibodies identify a CEA crossreacting antigen of 95 kD (NCA-95) distinct in antigenicity and tissue distribution from the previously described NCA of 55 kD. Int J Cancer. 1984 May 15;33(5):643–649. doi: 10.1002/ijc.2910330515. [DOI] [PubMed] [Google Scholar]
- Burtin P., Chavanel G., Hirsch-Marie H. Characterization of a second normal antigen that cross-reacts with CEA. J Immunol. 1973 Dec;111(6):1926–1928. [PubMed] [Google Scholar]
- Caruthers M. H., Beaucage S. L., Efcavitch J. W., Fisher E. F., Goldman R. A., deHaseth P. L., Mandecki W., Matteucci M. D., Rosendahl M. S., Stabinsky Y. Chemical synthesis and biological studies on mutated gene-control regions. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):411–418. doi: 10.1101/sqb.1983.047.01.048. [DOI] [PubMed] [Google Scholar]
- Egan M. L., Pritchard D. G., Todd C. W., Go V. L. Isolation and immunochemical and chemical characterization of carcinoembryonic antigen-like substances in colon lavages of healthy individuals. Cancer Res. 1977 Aug;37(8 Pt 1):2638–2643. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fritsche R., Mach J. P. Isolation and characterization of carcinoembryonic antigen (CEA) extracted from normal human colon mucosa. Immunochemistry. 1977 Feb;14(2):119–127. doi: 10.1016/0019-2791(77)90290-7. [DOI] [PubMed] [Google Scholar]
- GOLD P., FREEDMAN S. O. DEMONSTRATION OF TUMOR-SPECIFIC ANTIGENS IN HUMAN COLONIC CARCINOMATA BY IMMUNOLOGICAL TOLERANCE AND ABSORPTION TECHNIQUES. J Exp Med. 1965 Mar 1;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert F., AbuHarfeil N., Schwarz K., von Kleist S. Two CEA and three NCA species, although distinguishable by monoclonal antibodies, have nearly identical peptide patterns. Int J Cancer. 1985 Sep 15;36(3):357–362. [PubMed] [Google Scholar]
- Haggarty A., Legler C., Krantz M. J., Fuks A. Epitopes of carcinoembryonic antigen defined by monoclonal antibodies prepared from mice immunized with purified carcinoembryonic antigen or HCT-8R cells. Cancer Res. 1986 Jan;46(1):300–309. [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T., Seidman M., Stollar B. D. Characterization of genomic poly(dT-dG).poly(dC-dA) sequences: structure, organization, and conformation. Mol Cell Biol. 1984 Dec;4(12):2610–2621. doi: 10.1128/mcb.4.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Murray B. A., Edelman G. M., Cunningham B. A. Sequence of a cDNA clone encoding the polysialic acid-rich and cytoplasmic domains of the neural cell adhesion molecule N-CAM. Proc Natl Acad Sci U S A. 1986 May;83(9):3037–3041. doi: 10.1073/pnas.83.9.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka N., Takahashi N., Putnam F. W. Amino acid sequence of human plasma alpha 1B-glycoprotein: homology to the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2363–2367. doi: 10.1073/pnas.83.8.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. J., Shively J. E., Pritchard D. G., Todd C. W. Isolation, immunological characterization, and structural studies of a tumor antigen related to carcinoembryonic antigen. Cancer Res. 1978 Apr;38(4):1041–1048. [PubMed] [Google Scholar]
- Kuroki M., Koga Y., Matsuoka Y. Purification and characterization of carcinoembryonic antigen-related antigens in normal adult feces. Cancer Res. 1981 Feb;41(2):713–720. [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier M., Fenger U., Wagener C. Delineation of four carcinoembryonic antigen (CEA) related antigens in normal plasma by transblot studies using monoclonal anti-CEA antibodies with different epitope specificities. Mol Immunol. 1985 Nov;22(11):1273–1277. doi: 10.1016/0161-5890(85)90046-x. [DOI] [PubMed] [Google Scholar]
- Neumaier M., Fenger U., Wagener C. Monoclonal antibodies for carcinoembryonic antigen (CEA) as a model system: identification of two novel CEA-related antigens in meconium and colorectal carcinoma tissue by Western blots and differential immunoaffinity chromatography. J Immunol. 1985 Nov;135(5):3604–3609. [PubMed] [Google Scholar]
- Paxton R. J., Mooser G., Pande H., Lee T. D., Shively J. E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976 May 15;17(5):588–596. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Friedrich R., Grunert F., Luckenbach G. A., Thompson J., von Kleist S. Characterization of messenger RNA specific for carcinoembryonic antigen. Ann N Y Acad Sci. 1983;417:21–30. doi: 10.1111/j.1749-6632.1983.tb32844.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Ortlieb B., Friedrich R., von Kleist S. Isolation and characterization of cDNA clones encoding the human carcinoembryonic antigen reveal a highly conserved repeating structure. Proc Natl Acad Sci U S A. 1987 May;84(9):2960–2964. doi: 10.1073/pnas.84.9.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kleist S., Chavanel G., Burtin P. Identification of an antigen from normal human tissue that crossreacts with the carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2492–2494. doi: 10.1073/pnas.69.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]