Abstract
Background
In adequately resourced clinical environments, diagnosis of hypoxemia via pulse oximetry is routine. Unfortunately, pulse oximetry is rarely utilized in under-resourced hospitals in developing countries.
Aim
The prevalence of undiagnosed hypoxemia among adults and children with illnesses other than pneumonia in these environments remains poorly described.
Methods
This cross-sectional analysis of the prevalence of hypoxemia was conducted in Kapiri Mposhi, Zambia, at the 60-bed District Hospital, which serves a population of 320,000. The resting room air oxygen saturations of two consecutive samples of all adult and pediatric inpatients were measured in December 2008 and March 2009 using handheld pulse oximetry. Hypoxemia was defined as resting room air SpO2 less than 90%.
Results
A total of 192 patients were enrolled: 68 young children (<5 years old), 15 older children (5–17 years old), and 109 adults (≥18 years old). Five young children (7%), 0 older children (0%), and 10 adults (9%) were hypoxemic. No hypoxemic patients were receiving oxygen therapy at the time of diagnosis. Pneumonia, tuberculosis, and malnutrition were the most common conditions among those with hypoxemia. Oximetry data changed clinical management in all observed cases of hypoxemia and several cases of normoxemia, leading to application of supplemental oxygen, initiation of further diagnostic testing, prolongation of inpatient stay, or expedited discharge home.
Conclusions
Undiagnosed hypoxemia is present among inpatients at this district hospital in rural Zambia with high prevalence in both adults and young children. These results support routine screening for hypoxemia in similar facilities in both age groups. Further investigation is warranted into the clinical impact and cost-effectiveness of pulse oximetry, provision of oxygen concentrators, and training on their use in developing countries.
Keywords: Hypoxemia, Developing countries, Respiratory infections, Public health, Pediatrics, Pulse-oximetry
Introduction
Pulse oximetry is a technology that is used routinely for diagnosis of hypoxemia in high-income countries [1]. Pulse oximetry has been shown in large trials to be more effective than clinical judgment in the detection of hypoxemia [2] and is well established in developed countries as the fifth vital sign in patients of all ages [3, 4].
Unfortunately, pulse oximetry is often unavailable in resource-limited clinical settings. The potential benefit of introducing the technology has been recognized by the field of anesthesiology, which has recently launched the Global Oximetry Initiative to promote oximetry utilization and reduce oximetry costs in low-income countries [5]. This effort focuses on increasing the availability of oximetry in perioperative settings, though randomized controlled trials in high-income countries have yet to demonstrate that oximetry reduces morbidity or mortality in this setting [6, 7].
Retrospective analysis has suggested that oximetry might improve the ability to predict clinical failure of oral amoxicillin therapy for children with WHO-defined severe pneumonia [8]. Preliminary studies in Egypt and Papua New Guinea have also shown that implementation of pulse oximeters, oxygen concentrators, and a training program on their use can reduce mortality in pediatric pneumonia [9–11]. Pulse oximetry in emergency triage has also been shown to save costs in a hospital in Kenya by enabling staff to target oxygen therapy more accurately [12]. However, overall, the literature evaluating the impact of pulse oximetry in resource-limited settings is sparse, and the prevalence of hypoxemia among adults in low-income countries remains largely unknown.
To date, most data collected on the clinical impact of pulse oximetry have been collected in high-income countries. For example, utilization of oximetry at patient triage has been shown to significantly reduce pediatric emergency department length of stay in the US, by expediting the identification and treatment of patients with hypoxemia [13]. Of those studies conducted in low-income countries, most report the prevalence of hypoxemia exclusively among children with pneumonia (acute lower respiratory infection) [14]. The few published studies that report data on other disease processes have found high prevalence of hypoxemia among pediatric patients with malaria, meningitis, diarrhea, malnutrition, anemia, tuberculosis, and neonatal illnesses [15–17]. To our knowledge, few data have been reported on the prevalence of hypoxemia among adults in resource-limited settings, and no data have been reported on the prevalence of hypoxemia at any clinical site in the country of Zambia [14].
The goal of this study was to determine the prevalence of hypoxemia at Kapiri District Hospital (KDH) in Kapiri Mposhi, Zambia, among both adults and pediatric inpatients. Characterization of the epidemiology of hypoxemia there is an important step in the design of subsequent interventions that aim to improve health outcomes. We hypothesized that a significant burden of hypoxemia was present and regularly remained undiagnosed at this hospital among both adults and children, and across a variety of illnesses.
Methods
Study design
A cross-sectional study was conducted to determine the prevalence of hypoxemia at this rural district hospital in Zambia. Prior to initiation of data collection, IRB approval was obtained from the Partners Human Research Committee and the Kapiri Mposhi District Health Office. There are no relevant financial disclosures for any of the authors involved in this project.
Study setting and population
KDH is a 60-bed, government-run district hospital serving the Kapiri District population of 320,000 [18]. Kapiri District is located in Zambia’s Central Province. At the time of this study, the hospital did not regularly measure patient oxygen saturations, and used therapeutic oxygen sparingly. Two consecutive samples of all adult and pediatric inpatients at KDH were measured in December 2008 and March 2009. All patients older than 30 days were eligible for enrollment. All patients seen during daily rounds with the local physician were considered for enrollment. Outpatients did not meet inclusion criteria. There were no exclusion criteria.
Study protocol
Resting oxygen saturations were measured by an emergency physician from the US, who rounded with the local hospital physician each day. At the end of each patient evaluation, the local physician asked the patient whether he or she would like to participate, and if the patient did wish to participate, obtained verbal consent, explaining the purpose of the study and potential risks. Because risks were felt to be minimal, the requirement for written consent was waived by the IRB. If the patient was too young or ill to consent, a family member was asked regarding desire to participate. Oxygen saturation was then measured by placing a handheld pulse oximeter (Masimo Corporation, Irvine, CA) on the right index finger and allowing the signal to equilibrate for 5 sec. When the waveform was high quality and the saturation reading had been stable for 5 s, the level was recorded. If the saturation was less than 70% SpO2, a value of <70% was recorded given known decreased accuracy below these levels. All measurements were obtained at rest while breathing room air. Measurement of the oxygen saturation level of the physician obtaining the measurement was taken at the beginning of each day of data collection to serve as a control and confirm that the oximeter was functioning appropriately.
Data collection was performed Monday through Saturday, when the local physician rounded and was available to translate and obtain verbal consent. After the oxygen saturation was measured, the local physician and visiting physician used the data to adjust clinical plans as appropriate. Patient demographic data obtained from the medical record was limited to age, gender, bed number, and presumed diagnosis. No personal identifiers were recorded. Data were initially written on paper datasheets, which were kept locked at all times and later transferred to an electronic database on a secure laptop computer.
Data analysis
The study hypothesis was that clinically significant hypoxemia (SpO2 <90%) was present among the inpatient population at this hospital. For statistical analysis, the null hypothesis was prevalence of hypoxemia at this hospital is less than 1%. The prevalence of hypoxemia was first calculated and analyzed for the entire sample population. Patients were then divided into three groups: age less than 5 years (a standard in the literature), age 5–17 years, and age 18 or older. Data were entered into Excel 2008 (Microsoft Corporation, Redmond, WA) and analyzed using Stata/IC 10.0 (Stata Corp., College Station, TX). Hypoxemia was defined as SpO2 less than 90%, the accepted threshold in the existing literature [14]. Descriptive statistics were performed. The proportion of patients who met criteria for hypoxemia from the entire sample population and for each age group was calculated and reported with 95% confidence intervals of the proportion. One-sided, one-sample tests of proportion were performed at α level 0.05 to assess whether the observed prevalences of hypoxemia for the entire population and each age group were statistically higher than 1%. A one-sided test was necessary because negative values of oxygen saturation are not possible, and there was only one alternate hypothesis, prevalence is greater than 1%. The Kruskal-Wallis equality-of-populations rank test was performed using an α level 0.05 to assess whether there was a statistically significant difference between the distribution of oxygen saturation levels among the three age groups. This test was chosen based on the single independent variable with three categories (age groups) and a non-parametric continuous outcome variable (saturation levels).
Results
One hundred ninety-two patients were enrolled: 68 young children (<5 years old), 15 older children (5–17 years old), and 109 adults (≥18 years old). Six patients declined consent and were not enrolled. The mean age of the participants was 24.4 years, with an interquartile age range of 2 to 38.5 years and absolute range of 1 month to 75 years. There were 92 male and 100 female participants (see Table 1).
Table 1.
Study sample population demographics
| Characteristics of study participants | |
|---|---|
| Number enrolled | 192 |
| Declined to participate | 6 |
| Mean age | 24.3 years |
| Interquartile age range | 2–38.5 years |
| Age range | 1 month–75 years |
| Male | 92 (48%) |
| Female | 100 (52%) |
| <5 years old | 68 (35.4%) |
| 5–17 years old | 15 (7.8%) |
| ≥18 years old | 109 (56.8%) |
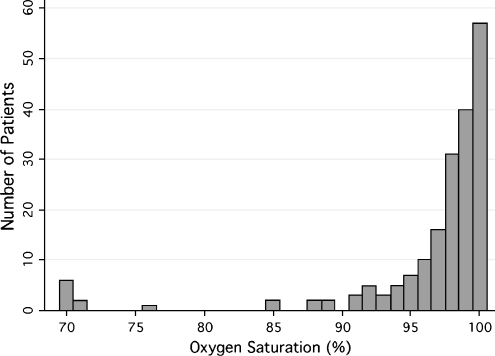
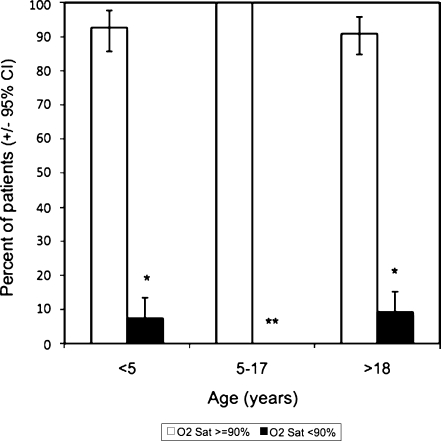
The majority of patients had measured oxygen saturation levels of greater than 90% (see Fig. 1). Five young children (7%), 0 older children (0%), and 10 adults (9%) were hypoxemic at time of measurement of saturation level. The one-sided, one-sample tests of proportion indicated that the prevalence of hypoxemia was greater than 1% for the entire population, for young children, and for adults, though not for older children (see Table 2). The Kruskal-Wallis equality-of-populations rank test indicated that there was a statistically significant difference between the oxygen saturation levels of the three age groups (p = 0.018). The data suggest that the age group 5–17 years, which had a measured sample prevalence of zero, likely has a significantly lower prevalence of hypoxemia than the other two age groups (see Fig. 2).
Fig. 1.
Histogram of inpatient resting room air oxygen saturation levels at Kapiri District Hospital (all ages)
Table 2.
Prevalence of hypoxemia (resting room air SpO2 <90%) at Kapiri District Hospital. P-value represents probability of obtaining the observed value due to random chance under the null hypothesis that prevalence of hypoxemia is less than 1%, using a one-sided, one-sample test of proportions at α = 0.05
| Age group | Prevalence of hypoxemia (%) | 95% confidence interval of proportion | P-value |
|---|---|---|---|
| All ages | 7.8 | (3.8, 14.6) | <0.0001 |
| <5 years | 7.4 | (1.1, 13.5) | <0.0001 |
| 5–17 years | 0.0 | (0.0, 0.0) | 0.652 |
| ≥18 years | 9.2 | (3.8, 14.5) | <0.001 |
Fig. 2.
Prevalence of hypoxemia (resting room air SpO2 < 90%) at Kapiri District Hospital by age group with 95% confidence intervals of proportions. *Denotes statistical significance for prevalence ≥1% via one-sided, one-sample test of proportion at α = 0.05. **Denotes statistical significance for difference in distribution of oxygen saturation levels among the three groups via Kruskal-Wallis equality-of-populations rank test at α = 0.05
None of the 15 hypoxemic patients were receiving oxygen therapy at the time they were identified as hypoxemic. Six of the 15 patients had oxygen saturations less than 70%, all of whom subsequently died during their hospitalization. These patients were generally ill-appearing, though not all were in acute distress—some were terminally ill-appearing and had been declining steadily for days or weeks.
Pneumonia, tuberculosis, and malnutrition were the most common diagnoses among those with hypoxemia. Other diagnoses included dilated cardiomyopathy of unclear etiology in a 26-year-old male, and presentations of fever and dyspnea of unclear etiologies. (see Table 3).
Table 3.
Etiologies of hypoxemia at Kapiri District Hospital
| Age | Gender | Diagnosis | O2 Saturation (room air) |
|---|---|---|---|
| 3 months | Female | Pneumonia | 88% |
| 4 months | Male | Fever | <70% |
| 13 months | Female | Pneumonia | 85% |
| 16 months | Female | Malnutrition | 89% |
| 3 years | Female | Malnutrition | 89% |
| 26 years | Male | Cardiomyopathy | 88% |
| 27 years | Female | Tuberculosis | 71% |
| 32 years | Male | Tuberculosis | <70% |
| 34 years | Male | Pneumonia | 76% |
| 39 years | Male | Tuberculosis | <70% |
| 45 years | Male | Tuberculosis | 85% |
| 52 years | Female | Dyspnea | <70% |
| 62 years | Male | Pneumonia | <70% |
| 69 years | Male | Tuberculosis | <70% |
| 79 years | Male | Dyspnea | 71% |
Oximetry data changed management in all cases of hypoxemia, leading to application of supplemental oxygen, initiation of further diagnostic testing such as x-rays, or prolongation of inpatient stay. Oximetry data also changed management in several cases where the saturation was normal, leading to the safe discontinuation of oxygen therapy (e.g., in pediatric respiratory illness) or expedited discharge home earlier than planned (e.g., transition to outpatient care in adult pulmonary tuberculosis). The proportion of cases in which oxymetry data changed management or any difference in outcomes was not recorded as part of this preliminary observational study.
Discussion
Hypoxemia was present in this under-resourced district hospital in Zambia with alarming prevalence. Numerous patients (n = 15, 8%) were found to be hypoxemic, despite a conservative definition for hypoxemia of SpO2 < 90% and measuring saturation at rest. Many more patients with resting SpO2 90–94% (n = 15, 8%) would have met criteria for treatment with therapeutic oxygen in developed countries, where exposure to saturations of 90–94% has been associated with adverse cognitive and behavioral outcomes [20, 21]. Hypoxemia was found among patients with a variety of diagnoses, though pulmonary infections accounted for the majority of cases. The prevalence of hypoxemia in adults (≥18 years) was at least as high as in young children (<5 years), indicating a need for more research and interventions aimed at understanding, diagnosing, and treating hypoxia in adults in resource-limited settings. Although the literature has previously focused on children with pneumonia, these data suggest that both adults and young children may benefit from routine screening for hypoxemia in similar under-resourced settings. The groups most likely to benefit from targeted screening include adults and young children with presumptive diagnoses of pneumonia, tuberculosis, and malnutrition.
Pneumonia (acute lower respiratory infection) currently accounts for more deaths in children under 5 worldwide than HIV, tuberculosis, malaria, and measles combined [22]. At Kapiri District Hospital, pneumonia was the leading cause of death in children under 5 and the third highest cause of death among adults [23]. Given the potential for impact on mortality, hospital length of stay, and cost-effective utilization of limited resources such as therapeutic oxygen, further implementation and evaluation are warranted in programs that provide under-resourced facilities with pulse oximeters, oxygen concentrators, and training in their use.
Limitations
This study was cross-sectional and therefore cannot provide longitudinal data about hypoxemia at this hospital. There may be seasonal variation in prevalence in this area, though no significant differences were observed between the data collected in December and March. All data were collected in a single district hospital in a single country, which may limit the generalizability of our findings. In order to estimate hypoxia, hypoxemia alone was measured with no determinations of hemoglobin concentration, cardiac output, or mixed central venous O2 saturation to truly calculate oxygen delivery to tissues. This use of hypoxemia as a surrogate marker of hypoxia is well established in both clinical and research settings [19]. The study does not specifically demonstrate improvement in patient outcomes due to identification and treatment of hypoxemia, which must be investigated further in subsequent research.
Conclusion
Undiagnosed and untreated hypoxemia (resting room air SpO2 <90%) was found in both adults and young children (<5 years) and in a variety of illnesses at this under-resourced district hospital. The most common diagnoses of those with hypoxemia were pneumonia, tuberculosis, and malnutrition. Measurement of O2 saturation altered the management of all hypoxemic and several normoxemic patients. Diagnosis of hypoxemia via pulse oximetry should be expanded in similar under-resourced settings. Further investigation is warranted into the clinical impact and cost-effectiveness of pulse oximetry, provision of oxygen concentrators, and training on their use in developing countries.
Acknowledgments
The authors would like to thank Calum McGregor and Humphrey Chibilika of the Maternal and Infant Health Initiative (MIHI) and The John and Katie Hansen Family Foundation for their assistance in Zambia. Two pulse oximeters were donated to Kapiri District Hospital in conjunction with this project by the Masimo Corporation.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Mark Foran
is an attending emergency physician at Brigham & Women’s Hospital in Boston, MA. After a Fulbright fellowship in South Korea and medical school at Johns Hopkins University, he completed residency in emergency medicine at the Harvard Affiliated Emergency Medicine Residency at Brigham & Women’s and Massachusetts General Hospitals. He is currently pursuing a Master of Public Health degree at the Harvard School of Public Health. He has worked clinically and conducted research in Peru, South Africa, India, Zambia, Sri Lanka, and Switzerland. His research interests include humanitarian outcomes and accountability, provision of care in resource-limited settings, and development of the sub-specialty of international emergency medicine.
Footnotes
Prior Presentations
Mediterranean Emergency Medicine Congress V, Valencia, Spain, September 2009 and American Public Health Association Annual Meeting, Philadelphia, November 2009.
Funding Sources
Massachusetts College of Emergency Physicians EM Resident Research Grant and MGH Division of Global Health and Human Rights.
Disclaimer
The views expressed in this paper are those of the author(s) and not those of the editors, editorial board or publisher.
References
- 1.McMorrow RC, Mythen MG. Pulse oximetry. Curr Opin Crit Care. 2006;12(3):269–271. doi: 10.1097/01.ccx.0000224873.16700.78. [DOI] [PubMed] [Google Scholar]
- 2.Moller JT, Pedersen T, Rasmussen LS, et al. Randomized evaluation of pulse oximetry in 20,802 patients: I. Design, demography, pulse oximetry failure rate, and overall complication rate. Anesthesiology. 1993;78(3):436–444. doi: 10.1097/00000542-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Mower WR, Myers G, Nicklin EL, Kearin KT, Baraff LJ, Sachs C. Pulse oximetry as a fifth vital sign in emergency geriatric assessment. Acad Emerg Med. 1998;5(9):858–865. doi: 10.1111/j.1553-2712.1998.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 4.Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics. 1997;99(5):681–686. doi: 10.1542/peds.99.5.681. [DOI] [PubMed] [Google Scholar]
- 5.Thoms GM, McHugh GA, O'Sullivan E. The global oximetry initiative. Anaesthesia. 2007;62(Suppl 1):75–77. doi: 10.1111/j.1365-2044.2007.05305.x. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen T, Moller AM, Pedersen BD. Pulse oximetry for perioperative monitoring: systematic review of randomized, controlled trials. Anesth Analg. 2003;96(2):426–431. doi: 10.1097/00000539-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen T, Moller AM, Hovhannisyan K (2009) Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev (4):CD002013 [DOI] [PubMed]
- 8.Fu LY, Ruthazer R, Wilson I, et al. Brief hospitalization and pulse oximetry for predicting amoxicillin treatment failure in children with severe pneumonia. Pediatrics. 2006;118(6):e1822–1830. doi: 10.1542/peds.2005-2673. [DOI] [PubMed] [Google Scholar]
- 9.Dobson M, Peel D, Khallaf N. Field trial of oxygen concentrators in upper Egypt. Lancet. 1996;347(9015):1597–1599. doi: 10.1016/S0140-6736(96)91080-6. [DOI] [PubMed] [Google Scholar]
- 10.Matai S, Peel D, Wandi F, Jonathan M, Subhi R, Duke T. Implementing an oxygen programme in hospitals in Papua New Guinea. Ann Trop Paediatr. 2008;28(1):71–78. doi: 10.1179/146532808X270716. [DOI] [PubMed] [Google Scholar]
- 11.Duke T, Wandi F, Jonathan M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372(9646):1328–1333. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 12.Mwaniki MK, Nokes DJ, Ignas J, et al. Emergency triage assessment for hypoxaemia in neonates and young children in a Kenyan hospital: an observational study. Bull World Health Organ. 2009;87(4):263–270. doi: 10.2471/BLT.07.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J, Claudius I. Decrease in emergency department length of stay as a result of triage pulse oximetry. Pediatr Emerg Care. 2006;22(6):412–414. doi: 10.1097/01.pec.0000221340.26873.2f. [DOI] [PubMed] [Google Scholar]
- 14.Subhi R, Adamson M, Campbell H, Weber M, Smith K, Duke T. The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis. 2009;9(4):219–227. doi: 10.1016/S1473-3099(09)70071-4. [DOI] [PubMed] [Google Scholar]
- 15.Wandi F, Peel D, Duke T. Hypoxaemia among children in rural hospitals in Papua New Guinea: epidemiology and resource availability–a study to support a national oxygen programme. Ann Trop Paediatr. 2006;26(4):277–284. doi: 10.1179/146532806X152791. [DOI] [PubMed] [Google Scholar]
- 16.Junge S, Palmer A, Greenwood BM, Kim Mulholland E, Weber MW. The spectrum of hypoxaemia in children admitted to hospital in The Gambia, West Africa. Trop Med Int Health. 2006;11(3):367–372. doi: 10.1111/j.1365-3156.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- 17.Maitland K, Levin M, English M, et al. Severe P. falciparum malaria in Kenyan children: evidence for hypovolaemia. QJM. 2003;96(6):427–434. doi: 10.1093/qjmed/hcg077. [DOI] [PubMed] [Google Scholar]
- 18.Central Statistical Office of Zambia. Census Data from Zambia. www.zamstats.gov.zm/census.php. Accessed December 29, 2009
- 19.Duke T, Subhi R, Peel D, Frey B. Pulse oximetry: technology to reduce child mortality in developing countries. Ann Trop Paediatr. 2009;29(3):165–175. doi: 10.1179/027249309X12467994190011. [DOI] [PubMed] [Google Scholar]
- 20.Bass JL, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114(3):805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 21.Bass JL, Gozal D. Oxygen therapy for bronchiolitis. Pediatrics. 2007;119(3):611. doi: 10.1542/peds.2006-3002. [DOI] [PubMed] [Google Scholar]
- 22.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet. 2006;368(9541):1048–1050. doi: 10.1016/S0140-6736(06)69334-3. [DOI] [PubMed] [Google Scholar]
- 23.Kapiri District Hospital. Unpublished data. 2008